Summary
The neglected tropical diseases (NTDs) represent a group of parasitic and related infectious diseases such as amebiasis, Chagas disease, cysticercosis, echinococcosis, hookworm, leishmaniasis, and schistosomiasis. Together, these conditions are considered the most common infections in low- and middle-income countries, where they produce a level of global disability and human suffering equivalent to better known conditions such as human immunodeficiency virus/acquired immunodeficiency syndrome and malaria. Despite their global public health importance, progress on developing vaccines for NTD pathogens has lagged because of some key technical hurdles and the fact that these infections occur almost exclusively in the world’s poorest people living below the World Bank poverty line. In the absence of financial incentives for new products, the multinational pharmaceutical companies have not embarked on substantive research and development programs for the neglected tropical disease vaccines. Here, we review the current status of scientific and technical progress in the development of new neglected tropical disease vaccines, highlighting the successes that have been achieved (cysticercosis and echinococcosis) and identifying the challenges and opportunities for development of new vaccines for NTDs. Also highlighted are the contributions being made by non-profit product development partnerships that are working to overcome some of the economic challenges in vaccine manufacture, clinical testing, and global access.
Keywords: neglected tropical diseases, tropical diseases, vaccines, parasitic vaccines
Introduction
As the world’s population soon approaches 7 billion people, approximately 1.4 billion people will remain below the World Bank poverty line (1). These individuals, mostly the world’s subsistence farmers and their families as well as the urban poor, are referred to as ‘the bottom billion’ (2). There is geographic dimension to this global poverty, as most individuals of the bottom billion live in 58 low- and middle-income countries in Africa, Asia, and Latin America and the Caribbean (2). In 2000 Kofi Annan, the Secretary General of the United Nations, began an international effort to lift the world’s poorest people out of poverty, which resulted in the drafting of a set of eight Millennium Development Goals (MDGs) for sustainable poverty reduction. Included among these goals was one that was specifically devoted to infectious diseases in low-income countries. MDG 6 ‘to combat acquired immunodeficiency syndrome (AIDS), malaria, and other diseases, launched several international initiatives for human immunodeficiency virus (HIV)/AIDS and malaria in developing countries, including programs for large-scale interventions that employed available drugs and diagnostics. Included among the best-known programs is the United States (US) President’s Emergency Plan for AIDS Relief (PEPFAR), the US President’s Malaria Initiative (PMI), and the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM). Today these global health initiatives are placing tens of millions in Africa, Asia, and Latin America and the Caribbean on antiretrovirals as well as providing them with antimalarial drugs and insecticide-treated nets (1). In addition, MDG 6 helped to launch product development-public private partnerships (PD-PPPs) to develop and test new vaccines for HIV/AIDS and malaria such as the International AIDS Vaccine Initiative (IAVI) and the Malaria Vaccine Initiative of the Program for Appropriate Technology in Health (PATH-MVI), in addition to large-scale support for these partnerships from the Bill & Melinda Gates Foundation, the US National Institutes of Health, and the Wellcome Trust (1).
Unfortunately research and development (R&D) efforts for vaccines to combat many of the ‘other diseases’ outlined in MDG 6 have lagged behind AIDS and malaria vaccine efforts. Here, we outline the current progress in international R&D initiatives to develop new vaccines for one important group of such other diseases, known as the neglected tropical diseases (NTDs). This review emphasizes progress in NTD vaccine development since the launch of the MDGs a decade ago and since this topic was last reviewed in 2006 (3). Emphasis will be placed on parasitic NTDs as well as vaccines that target the arthropod vectors of some of these infections. Dengue and other viral NTDs, as well as vaccines for cholera and most of the other enteric bacteria are reviewed elsewhere.
Overview of the NTDs
The major clinical and epidemiological features of the NTDs were reviewed previously (1, 4–7). Briefly, the NTDs are chronic parasitic and other infections that represent the most common diseases of the world’s poorest people; most of the bottom billion suffers from at least one NTD (1, 8) (Table 1). The major NTDs are ranked by prevalence in Table 2. The most common are helminth infections such as hookworm, schistosomiasis, and liver fluke infections, as well as selected protozoan infections such as leishmaniasis and Chagas disease or bacterial infections such as trachoma. Other NTDs such as amebiasis and leptospirosis are also believed to be extremely common and have a high global prevalence, but there are insufficient data estimates for these conditions (9, 10).
Table 1.
What are the neglected tropical diseases (NTDs)?
The NTDs are a group of chronic parasitic and other infectious diseases with the following characteristics:
|
Table 2.
The major NTDs by prevalence
| Disease
|
Common name(s)
|
Global prevalence*
|
Sub-Saharan Africa
|
Southeast Asia and China
|
South Asia
|
LAC†
|
|---|---|---|---|---|---|---|
| Helminthic NTDs | ||||||
| Ascariasis | Roundworm | 800 | 170 | 300 | 250 | 80 |
| Hookworm | Hookworm | 600 | 200 | 180 | 170 | 50 |
| Trichuriasis | Whipworm | 600 | 160 | 220 | 120 | 100 |
| Schistosomiasis | Bilharzia | 200–400 | 200–400 | 1 | 0 | 2–7 |
| Lymphatic filariasis | Elephantiasis | 120 | 40–50 | <15 | 40–50 | 1 |
| Strongyloidiasis | Threadworm | 30–100 | ND | ND | ND | ND |
| Clonorchiasis/Opisthorchiasis | Asian liver fluke | 20 | 0 | 20 | 0 | 0 |
| Onchocerciasis | River blindness | 20 | 20 | 0 | 0 | <1 |
| Loiasis | African eyeworm | <13 | <13 | 0 | 0 | 0 |
| Dracunculiasis | Guinea worm | <0.01 | <0.01 | 0 | 0 | 0 |
| Cysticercosis/Echinococcosis | ND | ND | ND | ND | 0.4 | |
| Protozoan NTDs | ||||||
| Toxoplasmosis | – | ND | ND | ND | ND | ND |
| Amebiasis | Amebic dysentery | 500 | ND | ND | 2–55%‡ | ND |
| Leishmaniasis | Kala-azar | 12 | ND | ND | 4 | <1 |
| American trypanosomiasis | Chagas disease | 8–9 | 0 | 0 | 0 | 8–9 |
| African trypanosomiasis | Sleeping sickness | 0.05 | 0.05 | 0 | 0 | 0 |
| Giardiasis/Cryptosporidiosis | ND | ND | ND | ND | ND | |
| Bacterial NTDs | ||||||
| Trachoma | – | 60 | 30 | 30 | 1–2 | 1 |
| Leprosy | Hansen’s disease | 0.2§ | <0.05§ | <0.05§ | 0.15§ | <0.0§ |
| Buruli ulcer | – | <0.1 | <0.1 | ND | ND | ND |
| Leptospirosis | Weil’s disease | ND | ND | ND | ND | ND |
Prevalence is expressed in millions unless otherwise indicated.
Latin American and Caribbean (LAC) countries.
Refers to prevalence.
Refers to new cases annually.
NTDs, neglected tropical diseases; ND, not determined.
The NTDs exhibit a number of clinical and epidemiologic features that distinguish them from better known infectious diseases. For instance, oftentimes people are infected with NTD pathogens for decades or even their entire lives. Over this period the NTDs produce enormous amounts of disability including chronic anemia and inflammation, malnutrition, disfigurement, and blindness (1, 7). Another important distinguishing feature of NTDs is that they frequently elicit these chronic morbidities without causing death. The overall low mortality of the NTDs is considered a key reason why these conditions have been neglected so long. Without the large numbers of annual deaths, the international policy makers cannot rely on this traditional metric to express the global public health importance of the NTDs. However, using disability adjusted life years (DALYs), i.e. the number of life years lost from premature disability or deaths, some estimates indicate that the NTDs may be as important as malaria or HIV/AIDS as public health threats (11–13). Moreover, some economic analyses indicate that the NTDs not only occur in the setting of poverty but also can actually cause poverty (8). The term ‘antipoverty vaccine’ has been applied to new NTD vaccines under development because of the potential of such biologics to improve economic development as well as health (3).
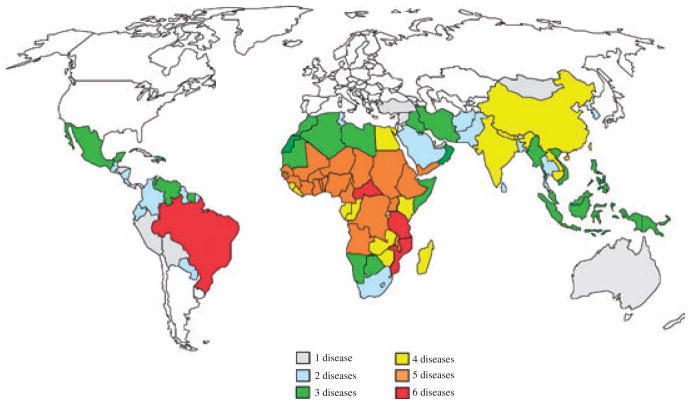
The poverty-promoting aspects of the NTDs reflect their disproportionate impact on selected vulnerable populations in developing countries. Growing and developing children for instance are susceptible to the anemia and malnutrition caused by the most common NTDs worldwide, especially hookworm and other soil-transmitted helminth infections (14) as well as schistosomiasis (13). As a result, such children experience growth stunting, loss of intelligence, and cognitive delays (15–17). Presumably through these mechanisms, chronic hookworm infection in childhood was determined to reduce future wage earning (18). Adolescent girls, young women, and especially pregnant women also represent a highly susceptible population (19). Anemia and inflammation from hookworm and schistosomiasis are two important examples that result in increased maternal morbidity and adverse pregnancy outcomes (20). In addition, some of the NTDs such as schistosomiasis in the genital tract and trichomoniasis can result in infertility, and there is even evidence that female genital schistosomiasis increases susceptibility to horizontal transmission from HIV/AIDS (21), while the stigma from disfigurement resulting from lymphatic filariasis, onchocerciasis, and other NTDs also disproportionately affects young women (19). Neurocysticercosis is recognized as a major cause of acquired epilepsy in most low-income countries (22). Finally, the NTDs promote poverty because of their documented impact on reducing agricultural worker productivity (8). The disproportionate impact of NTDs on subsistence farmers means that many are too disabled to go to work or work effectively, with demonstrable economic losses as a result (8). Through the mechanisms outlined above, the NTDs are key but often stealth reasons why the bottom billion cannot escape poverty or destitution (8). However, exactly how the NTDs exert their public health and economic impact often varies depending on geographic region (Fig. 1). Therefore, important to the framework for understanding the devastation wrought by the NTDs is to consider them separately by different regions of the world.
Fig. 1. The geographic overlap of the Neglected Tropical Diseases (NTDs).
Of the 56 nations with five or more co-endemic NTDs, 40 are found in Africa, nine in Asia, five in the Americas, and two in the Middle East. Map prepared Molly Brady, Emory University and reproduced in Molyneux et al. (287).
NTDs in sub-Saharan Africa
Of the 800 million people who live in this region, approximately one-half live below the World Bank poverty figure. Among these individuals, helminth infections are the most common NTDs accounting for about 85% of the NTD disease burden in the region (10). Overall, the NTD disease burden in sub-Saharan Africa has been estimated to be equivalent to roughly one-half the disease burden resulting from malaria and one-quarter that of HIV/AIDS (10). Hookworm infection (caused predominantly by Necator americanus) and schistosomiasis (Schistosoma haematobium and Schistosoma mansoni) are the most common African helminthiases, with approximately 200 million cases of each infection occurring at any given time (23, 24). However, King (12) recently determined that the actual number of cases of schistosomiasis in Africa could be two or more times higher. Sub-Saharan Africa accounts for approximately one-third of the world’s hookworm cases and more than 90% of the schistosomiasis cases. As a result of the anemia resulting from hookworm infection and the anemia as well as chronic inflammation, malnutrition, and end-organ pathology (including bladder cancer) resulting from schistosomiasis, some estimates indicate that these two helminthiases are also the most important NTDs in Africa terms of their overall morbidity and disease burden (10, 12). In addition, sub-Saharan Africa accounts for one-half of the world’s cases of 120 million cases of lymphatic filariasis occur in sub-Saharan Africa and virtually all of the cases of onchocerciasis (river blindness) and loiasis (Africa eye worm infection) (10). In contrast, guinea worm infection is close to being eradicated (25). Cysticercosis and/or hydatid disease are endemic in most sub-Saharan African countries, with some regions among the most endemic areas in the world (26, 27). Two important protozoan NTDs are vector-borne kinetoplastid infections. According to a new World Health Organization (WHO) report, the number of cases of human African trypanosomiasis (HAT) (28) has dropped below 10 000 for the first time in 50 years, but there are an unknown number of cases visceral leishmaniasis (VL) (Leishmania donovani) (29). Both HAT and leishmaniasis are found most commonly in conflict and postconflict areas in West and East Africa, respectively (10). Amebiasis (Entamoeba histolytica) is also believed to be extremely common, but there are no prevalence data available (30). Among the bacterial NTDs, approximately one-half of the world’s cases of active trachoma (Chlamydia trachomitis) occur in sub-Saharan Africa, especially in the Sahelian countries and in conflict and postconflict areas of East Africa (10), while most of the world’s cases of Buruli ulcer (Mycobacterium ulcerans) occur in West Africa. Two tick-borne bacterial NTDs, tick-borne relapsing fever and African tick-bite fever, are common in Africa, as is both typhoidal and non-typhoidal salmonellosis and yaws; however, no disease burden estimates are available for these conditions (10).
NTDs in East Asia
Despite the impressive economic growth and urbanization in parts of this region, pockets of extreme poverty remain. As a result, the soil-transmitted helminth infections are still widely prevalent. Up to 40% of the world’s cases of ascariasis and trichuriasis and one third of the hookworm cases occur in Southeast Asia and China, with the largest number in Indonesia, Philippines, Myanmar, and the Southwestern provinces of China (31). In many of these same areas, lymphatic filariasis is still endemic. Food-borne trematode infections are also highly endemic to this region, including high rates of liver fluke infection caused by Opisthorchis viverrini (especially in northern Thailand and Lao PDR) and Clonorchis sinensis (China and North Korea). More than 20 million people are infected with liver flukes in these areas, which have been identified as carcinogens causing bile duct cancer (32, 33). About 1 million cases of an Asian form of schistosomiasis (Schistosoma japonicum) with an important water buffalo animal reservoir occur primarily along the tributaries and drainage basins of the Yangtze River in China and in the Philippines and one focal area of Indonesia (34). The west and Tibetan highland regions of China include areas where echinococcosis presents a major threat to health (35). Data on enteric protozoan infections are largely non-existent, while for the bacterial NTDs, almost one-half of the global trachoma cases were found to occur in China, Indonesia, and Cambodia, as does about 10% of the leprosy (Mycobacterium leprae) cases (31). East Timor has not achieved its leprosy elimination target of one case per 10 000 (36). Melioidosis (Burkholderia pesudomallei) is another important bacterial infection associated with sepsis and high mortality in northern Thailand, Malaysia, and Singapore (31).
NTDs in South Asia
Hookworm and other soil-transmitted helminth infections are extremely common in the most populous South Asian countries of India, Bangladesh, and Nepal, with an overall prevalence equivalent to that found in Southeast Asia and China (37). In addition, about 50% of the global disease burden of lymphatic filariasis occurs in South Asia. There is also a huge socioeconomic burden resulting from lymphatic filariasis because of diminished ability to work in both rural and urban pursuits (38). By some estimates, India loses close to $1 billion annually from lymphatic filariasis (8). VL is endemic to India (especially Bihar State), Nepal, and Bangladesh, where it is an opportunistic infection of HIV/AIDS. By some estimates more than 4 million cases occur, with another 200 million people at risk for infection (39). Amebiasis is also widespread, with seroprevalence estimates ranging between 2% and 55% in the 1990s, although there is minimal surveillance conducted for this infection (40). Among the bacterial NTDs, India annually reports the greatest number of new cases of leprosy annually, and three states in India have not yet achieved elimination targets of less than one per 10 000 cases (36). Along with East Timor and Brazil, Nepal is one of three countries worldwide not to have achieved this elimination target (36). Leptospirosis (Leptospira spp.) is also an important infection in South Asia.
NTDs in Latin America and the Caribbean (LAC)
The NTD burden and geographic distribution of the NTDs in LAC have been reviewed previously (41). Most of the NTDs in the Americas were imported from West Africa during the 500 years of the Middle Passage of the Atlantic slave trade (42). Among the ‘bottom 100 million’, referring to the people who live on less than US$2 per day, the most common NTDs include the soil-transmitted helminth infections, with the greatest number of cases occurring in Brazil, Mexico, and Guatemala. Approximately 65% of LAC’s 50 million cases of hookworm infection and more than 80% of the 2–7 million cases of intestinal schistosomiasis (S. mansoni) occur in Brazil (43). Indeed, Brazil accounts for more than 50% of all of the NTDs in the Americas (41). Almost 1 million cases of lymphatic filariasis still occur in four countries in the LAC region, led by Haiti with 80% of the cases followed by Brazil, Dominican Republic, and Guyana (43). Onchocerciasis is near elimination in the Americas through the Onchocerciasis Elimination Programme for the Americas (OEPA), and cysticercosis, fascioloiasis, and echinococcosis are important zoonotic helminthiases in focal areas. Chagas disease is the most common NTD in Latin America following the helminthic NTDs. Approximately 8–9 million cases occur in the LAC region, including tens of thousands of new cases annually (41, 44). Most of these cases occur in areas of extreme poverty, especially in Bolivia, where the quality of dwellings is sufficiently poor to facilitate the ecological habitats of the assassin bug intermediate host vectors. The disease is responsible for millions of cases of cardiomyopathy and possibly hundreds of thousands of cases of megaesophagous and megacolon (44) making it one of the highest disease burden conditions in LAC (41). Both forms of leishmaniasis are common in Latin America, and it has been suggested that guerilla activities and drug trafficking in the region may contribute to the emergence of these sandfly transmitted conditions (45). An estimated 1 million cases of trachoma occur mostly in the Amazon region of Brazil and neighboring countries, while leprosy has still not been eliminated in the nation of Brazil (36). Leptospirosis is an important zoonotic bacterial infection from rats living in the favelas of Brazilian cities (46), and bartonellosis (Bartonella spp.) is an important vector-borne transmitted bacterial infection in the Andes region, which like leishmaniasis is transmitted by sandflies. In the US, Chagas disease has now emerged as an important NTD in the states bordering with Mexico (47). However, neglected infections of poverty in the US are not exclusively related to immigration, as large numbers of African Americans living in poverty are affected by a variety of neglected infections including toxocariasis and the protozoan infections trichomoniasis and toxoplasmosis (47).
History and rationale of NTD vaccines
The history of large-scale control of the NTDs began with Jamot and his colleagues (1, 48) working in West Africa during the first part of the 20th century. Using mobilized teams in a military-style campaign, the prevalence and incidence of human African trypanosomiasis was greatly reduced through widespread case detection and treatment of individuals with T. br. gambiense in their blood or spinal fluid (1, 48). Later in the middle part of the 20th century, the drug diethylcarbamazine citrate (DEC) was shown to be effective in clearing microfilariae from the blood in patients with lymphatic filariasis (1), leading to the practice of treating large populations simultaneously with DEC through a program of mass drug administration (MDA) to effect a reduction in NTD prevalence and in some cases actually eliminate the infection as a public health problem (49). During the last decade of the 20th century, the People’s Republic of China expanded MDA to become the first large country to eliminate lymphatic filariasis through this practice (1). Today, highly cost effective MDA programs are in place for the control or elimination of lymphatic filariasis, onchocerciasis, leprosy, trachoma, and other NTDs using either extremely low-cost generic drugs or drugs donated free-of-charge by several different multinational pharmaceutical companies (49). As a result, these diseases have been eliminated in several countries and there is optimism that increased drug coverage could extend the list of nations that have eliminated some of their NTDs as major public health problems (49). The observation of extensive geographic overlap among many of the NTDs (Fig. 1), along with high rates of co-endemicity, has also led to stepped-up global efforts and financing to simultaneously administer several drugs or even combine them into a low-cost and highly cost-effective package in order to control several NTDs in parallel (6, 11, 31, 49, 50). Through support from the United States Agency for International Development (USAID) and the British Department for International Development (DFID), national control and elimination programs for NTDs based primarily on MDA are now in place for at least 14 countries, mostly in sub-Saharan African (5, 51).
For many NTDs, however, MDA is either not possible or efficient for purposes of control or elimination (51). For these diseases, there is an urgent need for new control tools, including vaccines (3, 9, 51). The major NTDs requiring vaccines include some of the high prevalence helminth and protozoan infections, i.e. hookworm infection, schistosomiasis, and amebiasis, and other enteric protozoan infections because of high rates of drug failure and/or rapid post-treatment re-infection, which have so far thwarted effective control through MDA (52, 53). In addition, there is an equal need for vaccines to combat the zoonotic and vector-borne NTDs associated with severe morbidity such as leptospirosis, leishmaniasis, and Chagas disease, anti-cancer vaccines to prevent neoplasms that result from chronic neglected infections caused by liver flukes and schistosomes, and therapeutic vaccines for atypical intracellular bacterial infections, including Buruli ulcer and possibly leprosy (3, 51). Some NTDs offer opportunities for the development of transmission-blocking vaccines, including cysticercosis, echinococcosis, Asian schistosomiasis, and some forms of leishmaniasis. Through this strategy, NTDs would be controlled indirectly by decreasing or removing the source of human infections via the pathogen’s animal reservoirs. Indeed, this strategy is showing great promise with new, effective recombinant vaccines against cysticercosis and echinococcosis beginning to be implemented (54, 55).
Because the NTDs almost exclusively affect the world’s poorest people, there is no traditional commercial market for new vaccines. As a result, R&D efforts for antipoverty vaccines have greatly lagged behind more traditional vaccines for childhood infections and other diseases. In addition, there are formidable scientific hurdles, which have thwarted NTD vaccine development, including complex genomes (especially for the eukaryotic pathogens), the absence of in vitro systems to maintain the NTD pathogens in the laboratory, suitable animal models of disease, and adequate correlates of protection.
The first generation of NTD vaccines developed in the 20th century was comprised of whole organisms, which were either attenuated (typically with radiation) or killed with heat or formalin (reviewed in 3). For instance, it was shown during the 1960s that living helminth larvae could be attenuated by X-ray or γ-irradiation; such vaccines were developed, with the hookworm and Dictyocaulus viviparous vaccine marketed as veterinary products (56, 57). In addition, whole cell vaccines (both killed and living vaccines) derived from eggs were also developed for Chlamydia infections, but in some cases these vaccines actually worsened the course of the disease, while heat-killed and formalinized whole cell vaccine from leptospiral cultures were developed in Japan (58). Leishmanization, which is the practice of injecting living Leishmania parasites from active lesions into human hosts, was developed in ancient times even before vaccination and subsequently used during the Iran–Iraq war during the 1980s (3, 59). For the most part, these vaccines were expensive to produce and, when living organisms were required, expensive to maintain in their laboratory. However, in the last decade, the availability of genomes and proteomes for NTD pathogens, access to new adjuvants, and partial financial support from the Bill & Melinda Gates Foundation and other sources, both public and private, has made it possible to expand R&D efforts for antipoverty vaccines. These initiatives are leading to new vaccines now entering clinical testing.
Technical challenges for NTD vaccines
The challenges of NTD vaccine development are not limited to the discovery of antigens, adjuvants or delivery methods, but also to product and clinical development of these vaccines (Table 3). Product development is the technological foundation that underlies the manufacture of new vaccines and is central for it to successfully reach the people for whom the vaccine is intended. Clinical development is the testing in humans from phase 1 to 4 of the safety, immunogenicity and efficacy of a vaccine. Herein, we discuss several of the technical challenges that are unique to the discovery and product and clinical development of NTD vaccines.
Table 3.
Technical challenges to develop neglected tropical disease (NTD) vaccines
| Antigen discovery |
| Complicated genetic structures of NTD pathogens and |
| Absence of genome databases or bioinformatic algorithms for selecting candidate antigens of promise. |
| Process development |
| Necessity to scale up production of NTDs vaccine at adequate yields and at low cost. |
| Failure of many bacterial expression systems to produce properly folded recombinant proteins and the requirement for eukaryotic or other less common expression vectors. |
| Preclinical development |
| Difficulty in maintaining cycle stages of NTD pathogens in vitro. |
| Paucity of laboratory animal models permissive to the NTD pathogens or that can accurately reproduce human disease or protective immunity. |
| Clinical development |
| Clinical trials in resource-poor settings. |
| Highly modulated immune response from infection with many NTDs, especially helmith NTDS, present some dangers for vaccination. |
Technical challenge 1: antigen discovery
Despite the recent availability genomic and bioinformatic data from completed genome projects for a number of NTD pathogens including schistosomes, filariae, and most of the protozoan and bacterial pathogens, efforts to develop vaccines against these organisms has been slow. Whereas so-called reverse vaccinology approaches based on the availability of pathogen genomes have led to recent successes in developing vaccines against a serogroup B meningococcus and a Group B streptococcus for instance (60), it has been difficult to apply similar successful paradigms to NTD pathogens (3). Outlined below are several other major challenges that confront the successful development of antipoverty vaccines.
While in silico approaches have helped to launch discovery programs for new vaccines targeting some viral and bacterial pathogens, the far more complicated genomes of eukaryotic parasites require the evaluation of considerably more gene targets. In some cases, innovative approaches using signal traps and other technologies have been used to specifically identify secreted and surface exposed eukaryotic proteins (61), but so far no universal approach to mining eukaryotic genomes and antigen selection has emerged.
Technical challenge 2: process development
Effective recombinant vaccine antigens which protect against infections with taeniid cestode parasites, such as those causing cysticercosis and echinococcosis, have been successfully produced using ‘simple’ bacterial (Escherichia coli) expression (62). However, for many eukaryotic antigens, similar expression systems do not produce recombinant proteins that fold properly and resemble native proteins. This observation has been made for a number of eukaryotic parasite proteins (63), including helminth antigens. To date, however, high throughput reverse vaccinology approaches have required bacterial expression systems (60), so that there remains an urgent need to adapt this approach for eukaryotic expression. Moreover, there is an additional constraint that most of the NTD vaccines must be made at extremely low cost. To ensure that antigens are expressed at lowest cost and maximal yield, either great care must be taken to ensure that parasite proteins can be expressed in prokaryotic systems in a manner which conform to the native antigen or high throughput expression systems must be developed using low cost yeast expression systems, such as Pichia pastoris or Saccharomyces cervisiae. Recently, a tobacco-based expression system has also emerged as a viable alternative (64), but it is unlikely this approach would be amenable to high throughput approaches.
Technical challenge 3: preclinical development
The life cycles of many viral and bacterial pathogens are relatively straightforward to maintain in vitro, and permissive animal models are available for the target pathogens, making the testing of vaccines for efficacy, immunogenicity, and potency straightforward. However, many of the NTDs are eukaryotic pathogens that are difficult to maintain in vitro or as laboratory strains; in some cases, only a single stage of the life cycle can be consistently maintained in vitro. A concomitant limitation is the paucity of laboratory animal models permissive to these pathogens. For example, in efforts to access material for the development of a vaccine against the food-borne trematode O. viverrini, the intermediate host is a cyprinoid fish, which must be harvested from local water sources and the encysted metacercarial stage of the pathogen removed and transported to laboratories in the US (32, 33). In other cases, uncommon small animals (e.g. jirds for Onchocerca volvulus) are the only permissive animal models, with limitations on immunological reagents and housing. Finally, some NTD pathogens require large and expensive animals models, many of which are considered ‘sensitive’ species (e.g. canines for hookworm or non-human primates for Schistosoma spp). In many cases, there is considerable scientific debate as to whether the animal models reproduce the natural history of the NTDs as they occur in the human host. Therefore, careful consideration must be given to determine how preclinical testing in laboratory animals can be used on the critical path for NTD vaccine development.
These limitations are most apparent in the potency testing stage of vaccine pre-clinical development (65). Potency testing is used to ensure the quality and consistency of vaccine manufacture, and usually performed for the ‘release’ of the drug product (vaccine formulation after cGMP manufacture) and then continually (usually in 6 month or yearly intervals) to ensure the ongoing stability of the vaccine formulation. The International Conference on Harmonization (ICH) defines potency testing as: ‘The measure of biological activity using a suitably quantitative biological assay (also called a potency assay or bioassay), based on the attribute of the product which is linked to the relevant biological properties,’ [ICH, Section Q6B (66)].
Traditionally, the term ‘potency’ has been reserved for bioassays that involve the lethal challenge of an animal immunized with a specific dose of the vaccine and then challenged with the target pathogen (reviewed in 67). If the vaccine formulation is potent, the animal will elicit an immune response that parallels protection in the human host (67). This model is used for a number of well-established vaccines, e.g. pertussis, tetanus, diphtheria, rabies, leptospira, and clostridial vaccines (reviewed in 67). In many cases, the potency of the vaccine is quantified as the Protective Dose 50 (PD50): the specific dose of the vaccine formulation that protects 50% of the animals in a dose group against the lethal challenge from the target pathogen (67, 68). As outlined discussed extensively in Jariwala et al (65), the ‘immunization and lethal challenge’ model for potency requires the following: (i) a lethal dose of the pathogen (ii) lethality by the target pathogen that can be induced by a similar mechanism as that induced lethality in the human host (not just toxicity), and (iii) a correlate of protection using the vaccine in humans. Many of the NTD pathogens fail to meet these requirements for the following reasons: (i) a pathogenesis that is often chronic and not lethal, (ii) clinical outcomes take years or even decades to manifest, (iii) vaccine endpoints that are nearly impossible to measure in laboratory animal models, or (iv) no naturally acquired immunity in humans against the NTD pathogend (e.g. hookworms). As such, traditional potency testing is seldom an option for NTD vaccine development. As pointed out by Arciniega (69), potency testing need not be the only tool to ensure the consistent quality in the manufacturing process of a vaccine. Many regulatory bodies now accept that a potency assay for an NTD vaccine may not need to directly measure the protective immune mechanism of a vaccine formulation and instead could measure some aspect of consistent manufacture, e.g. a consistent level of antibody in an animal model in response to a defined dose of the drug product. Table 4 is an example of how a potency test was developed for a recombinant NTD vaccine (65).
Table 4.
A case study in a technical challenge for an neglected tropical disease (NTD) vaccines: developing a potency test for an recombinant protein NTD vaccine
The Na-GST-1 Hookworm vaccine consists of recombinant Na-GST-1, produced in Pichia pastoris, adsorbed to Alhydrogel® (aluminum hydroxide gel) and is intended for the prevention of moderate and heavy hookworm infections caused by Necator americanus, the leading cause of human hookworm infection (see full description below). The primary ‘biological activity’ of the Na-GST-1 hookworm vaccine is a reduction intestinal blood loss by limiting the number of adult hook worms resident in the host lumen. To measure the ‘essential biological activity’ of the Na-GST-1 vaccine, a protection against hookworm-induced iron deficiency anemia (IDA) would need to be achieved, which is an endpoint not easily attained in current animal models of hookworm infection (65). The most appropriate models to study hookworm infection are canines and hamsters. Canines can be experimentally infected with Ancylostoma caninum, which very closely resembles human Necator hookworm infection; and the hamster Mesocricetus auratus can be infected with Ancylostoma ceylanicum and N. americanus (70). While these animal models are extremely valuable for antigen discovery, they are of limited use in a bioassay for potency of a recombinant hookworm vaccine for the following reasons (65):
|
| For the Na-GST-1 vaccine, we developed a potency test that would be appropriate for the stage of vaccine’s development (preclinical) as well as for its indications for use (see below). While our available data indicate that Na-GST-1 will probably require neutralizing antibodies to be protective and that these antibodies will have to have the correct conformation in order to elicit this protective response, the correlation of these responses with clinical protection has yet to be established and, therefore, could not be used to claim that the potency test was measuring an ‘attribute essential for effect’(65). As described for the development of recombinant malaria vaccines (68), the potency assay for Na-GST-1 Hookworm Vaccine does not measure the biological acitivity related to vaccine efficacy. As stated in Jariwala et al (65), it measures an IgG response in mice immunized with a predetermined dose of the drug product as an indicator of consistent manufacture and stability over time” (65). The Na-GST-1 potency assay is one assay among many quality assurance measures and does predict or reflect clinical efficacy (65). |
Technical challenge 4a: clinical trials in resource-poor settings
Clinical testing of NTD vaccines is affected by the economic and geographical characteristics of NTDs, which often occur among the bottom billion (2), i.e. those individuals typically resident in the low and middle income countries in the tropics where NTDs are endemic. These resource poor settings pose numerous challenges (Table 5) for the clinical development of NTD vaccines, including little or no infrastructure for early vaccine clinical development and few trained research personnel (75). This is most critical during the early stages of clinical development: phase 1 or first-in-human testing. In acute shortage are the clinical laboratories necessary for the accurate and certified clinical chemistry evaluations; in many case, the clinical trial infrastructure (e.g. clinics, research pharmacies, certified clinical laboratories, personnel trained in Good Clinical Practices, etc.) are implemented by the sponsor.
Table 5.
Barriers to performing clinical trials where neglected tropical diseases are endemic
|
Another important barrier is that the nature of the patient population enrolled into phase 1 through 3 testing. Many of the bottom billion fall into the category of a ‘vulnerable populations’ from the perspective of ethical committees due to their socioeconomic and educational conditions, which often include illiteracy. The obligation of researchers to ensure that potential volunteers understand the risks and benefits of clinical trial participation is especially challenging with such populations (76, 77). Traditionally, ‘informing’ potential research subjects and obtaining their voluntary permission to participate has been accomplished by means of reading and signing an informed consent document. By signing the informed consent, it is assumed that the clinical trial volunteer has freely exercised his or her will in deciding to participate and that this was decision was formed an independent evaluation of the proposed research; that is, the participant made a truly informed decision about participating in the proposed research. However, research indicates that despite the use of thorough informed consent documents, the comprehension of the proposed research and an the understanding of the potential risks and benefits of participating in a clinical trial are less than ideal among population resident in resource poor settings (76, 77). Hence, much effort often goes into educating and informing these populations not only of the nature of the current clinical trials but the basic distinction between medical ‘research’ and medical ‘care’ (78–80). At times, even the basic components of the disease itself must be explained to participants in order for them to decide on the risk and benefits proposed by participating in a clinical trial.
Most problematic is that many of populations in which NTD vaccines will undergo early clinical testing are often underserved by the local medical infrastructure and are unfamiliar with the distinction between standard of care medical practice and clinical research. The latter poses problems of enrolling truly informed and consenting participants into clinical trials. The daunting complexity of the technical and scientific information presented during the informed consent process can prove especially challenging to volunteers with limited education (78–80). Often even the most simply written informed consent document contains extensive and complex information that may not satisfactorily convey an understanding of the study procedures to be undertaken or of the potential risks and benefits of participation to individuals in such settings. In an effort to adequately inform volunteers, investigators conducting early phase clinical research on NTD vaccines in resource-poor areas have increased the amount of information in informed consent documents as well as developed several strategies involved in community preparation (81).
Technical challenge 4b: the immune response to NTD infection and the ‘IgE trap’
As noted above, many of the NTDs are endemic to the same geographic area (co-endemic): a single individual can often have several of NTD infections at once. This is most apparent in helminth infection, where it is common for individuals (especially children) to have several of these infections simultaneously. Many of the NTD pathogens, especially the helminths, are associated with a systemic downmodulation of the immune response, with measurable attenuation of responses to bystander antigens and routine vaccine vaccination (82–84); for example, it is well accepted that T-helper 2 (Th2) responses are elicited during natural helminth infections, e.g. schistosomiasis, onchocerciasis, and filariasis (82–84). As part of this Th2 response, individuals develop elevated levels of total and parasite-specific immunoglobulin E (IgE), as well as increased levels of interleukin-4 (IL-4), IL-5 and IL-13, with concomitant increases in eosinophils and mast cells (83). The Th2 response during helminth infection is induced against a background of potent, parasite-induced immunoregulation, referred to as a ‘modified’ Th2 response. This modified Th2 response can consist of alternatively activated macrophages, Foxp3+ CD4 regulatory T (Treg) cells, and CD4+ Tr1-IL-10-producing T cells (82, 83). The effect of this response is to create an immune environment so extensively downregulated that it should protect the host not only from the strong inflammatory effects of helminth infections but also against the effects of other IgE-mediated disorders such as atopy, asthma, and anaphylaxis (85). Reduced allergic responses have been shown in studies of infection of mice with various helminth infections (reviewed in 86). Moreover, epidemiological evidence suggests that hookworm infection is associated with reduced skin reactivity to common allergens and a lowered risk of extrinsic asthma (87).
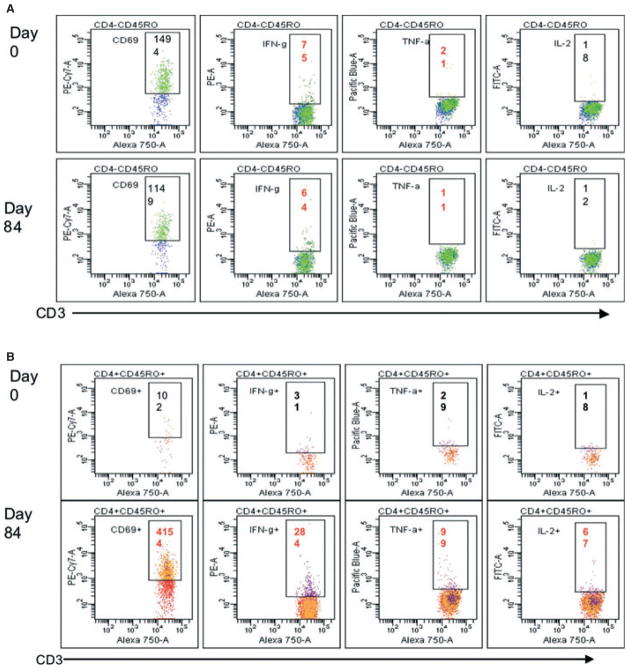
However, this response can also pose other problems for helminth vaccines. Recombinant N. americanus Ancylostoma Secreted Protein-2 (Na-ASP-2) is a 21.3 kDa protein secreted by infective hookworm larvae upon entry into the human host (88–90). Immune responses to administered Na-ASP-2 showed significant protection in laboratory animal models (91). In a Phase 1 study conducted in hookworm-naive adults living in the US, Na-ASP-2 adjuvanted with Alhydrogel was well-tolerated and immunogenic (92). However, in a parallel Phase 1 trial of this vaccine in adults living in a hookworm endemic area of Brazil, vaccination with a single dose of Na-ASP-2 (10 μg) resulted in generalized urticarial reactions in several volunteers. Subsequent analysis showed that the urticarial reactions were associated with elevated levels of IgE antibodies specific for Na-ASP-2, present before receiving immunization from their previous hookworm infection. A survey of adults and children from the same hookworm-endemic area revealed that a significant proportion had elevated levels of IgE to Na-ASP-2. Hence, vaccinating with Na-ASP-2 posed risks for the population in general. To date, the only feasible alternatives has been to either re-engineer the Na-ASP-2 antigen to remove or mutate epitopes recognized by IgE or identify new vaccine antigens that are protective but not recognized by IgE antibodies induced by natural infection (discussed below). Currently, screening for pre-existing levels of antigen-specific IgE is used as a critical step in our selection of potential vaccine antigens.
Vaccines for soil-transmitted helminths
Hookworm vaccines
The soil-transmitted helminth (STH) infections are among the most common afflictions of humankind, especially the three most common STH infections, i.e. ascariasis, trichuriasis, and hookworm (37). They are also among the most significant NTDs in terms of disease burden with some estimates indicating that the three major STH infections result in 39.1 million DALYs lost annually (93), a value roughly equivalent to malaria or tuberculosis (94). The current approach to the control of these major STH infections in developing countries is the annual or twice-yearly administration of a single dose of either albendazole (400 mg) or mebendazole (500 mg) (37). This strategy is sometimes referred to as ‘deworming’ and is currently practiced extensively in low- and middle-income countries especially in schools to reduce the worm burdens of children (49), with resultant improvements in child growth and cognition (37). In a recent meta-analysis, it was determined that single dose albendazole or mebendazole is most effective for producing cures or reducing the worm burdens of the STH infection ascariasis but much less so for trichuriasis and hookworm infection (95). Of particular concern are the findings that single dose mebendazole produces only 15% cure rates for hookworm infection (95), and the efficacy of mebendazole can diminish with frequent and periodic use (96), leading to suggestion that anthelminthic drug resistance may be developing against hookworm, particularly N. americanus. Moreover, high rates of post-treatment re-infection are common for hookworm as they are other STH infections (97). Therefore, while anthelminthic chemotherapy approaches remain the mainstay of control for ascariasis and trichuriasis, for hookworm new controls tools are considered necessary such as a vaccine (56, 98).
Recent developments in hookworm vaccines
The prospects for developing a vaccine against human hookworm infection, particularly for N. americanus infection, which is responsible for almost 90% of the human hookworm cases worldwide has been reviewed (56, 98, 99) and is briefly summarized here. As mentioned above (Technical challenges for NTD vaccines), the initial lead candidate antigen of the HHVI was a 21 kDa recombinant protein known as Ancylostoma secreted protein 2 (ASP-2) (56, 88, 91). During phase 1 testing in a hookworm endemic area of Brazil, pre-existing levels of Na-ASP-2-specific IgE among adults resulted in generalized urticaria response after a single vaccination (unpublished observation). Based on the outcome of this phase 1 study, the HHVI is no longer pursing larval antigens (such as ASP-2) as candidates for vaccine development (100).
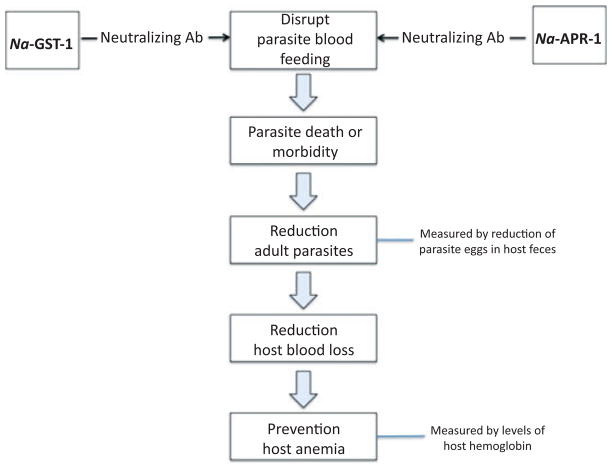
The HHVI is now focused on candidate antigens from the adult hookworm, especially antigens involved in parasite blood feeding (reviewed in 100) (Fig. 2). Hookworms ingest blood, and approximately 25–30 adult hookworms can cause the blood loss of approximately 1 ml daily, which contains an amount of iron roughly equivalent to a child’s daily iron intake (101). Much of the pathology associated with human hookworm infection is associated with the blood loss from feeding adults, which can lead to iron deficiency and anemia and protein malnutrition (14). Interfering with hookworm blood ingestion through vaccination represents a viable and alternative strategy to larval vaccination (56).
Fig. 2.
Revised strategy for a human hookworm vaccine: a bivalent recombinant protein vaccine targeting blood feeding of Necator americnaus.
Two lead antigens have emerged as promising candidates for a human hookworm vaccine based on this strategy (100). One of these is a 45 kDa aspartic protease, known as Na-APR-1 (102–104). Na-APR-1 is a hemoglobin-digesting protease found in the hookworm alimentary canal (105). The enzyme is critical for parasite hemoglobin digestion. The immunization of canines with recombinant Ac-APR-1 induced antibody and cellular responses that resulted in significantly reduced worm burdens and fecal egg counts in vaccinated dogs compared with control dogs after challenge with infective larvae of Ancylostoma caninum. More importantly, vaccinated dogs were protected against blood loss and did not develop anemia compared with control canines. In addition, the IgG from vaccinated canines decreased the catalytic activity of the recombinant enzyme in vitro, and the antibody bound in situ to the intestines of worms recovered from vaccinated dogs, implying that the vaccine interferes with the parasite’s ability to digest blood (102). Because it is not practical to immunize humans with an enzymatically active protease, Na-APR-1 cloned from N. americanus was inactivated by site directed mutagenesis (two aspartic acid residues to alanines). When expressed as a recombinant protein, the mutagenized gene elicited neutralizing antibodies and host protection (103). Na-APR-1 is currently undergoing process development.
A second adult-stage hookworm antigen, Na-GST-1, is also involved in parasite blood feeding. This 24 kDa glutathione S-transferase (GST) from N. americanus (or A. caninum) reduced host worm burdens immunized in hamsters (106–108). The mechanism of action of vaccines containing Na-GST-1 also appears to be antibody mediated. It was shown that hookworm GST-1 molecules belong to a unique Nu class of enzymes, which are involved in heme binding (107, 109). From the X-ray crystal structure of Na-GST-1 (109), it has been hypothesized that the molecule forms homodimers large enough to accommodate heme, hematin, and related molecules. Hence, Na-GST-1 may function to detoxify heme (107–109). Na-GST-1 expressed in the yeast P. pastoris has completed both process development and cGMP manufacture and is expected to undergo a regulatory submission and possibly Phase 1 clinical testing soon. Ultimately, Na-GST-1 and Na-APR-1 would be used together a bivalent vaccine (100).
Much of the product and clinical development of the human hookworm vaccine will be conducted in Brazil (100). With 32 million cases, Brazil has the largest number of cases of hookworm in the western hemisphere. Moreover, it has a sophisticated biotechnology infrastructure through both its Oswaldo Cruz Foundation (FIOCRUZ) and Instituto Butantan, which create ideal partners for the HHVI. The HHVI will work with both the US FDA and ANVISA, the national regulatory authority in Brazil to advance regulatory filings in both countries and downstream consider product licensure in that country.
Vaccines for blood flukes
Schistosomiasis vaccines
Schistosomiasis is caused by blood flukes of the genus Schistosoma and is arguably the most important human helminth infection in terms of global mortality. Recently, King et al. (13) increased their assessment of the public health impact of schistosomiasis by including not only gross organ pathology in the calculation of DALYs, but also the anemia, pain, diarrhea, exercise intolerance, and under-nutrition that results from chronic infection. However, recent progress in the control of schistosomiasis has led some to suggest that it may be ‘consigned to history’ by 2015 – the target stated in the MDGs (110). Since the 1990s, the major approach to schistosomiasis control has been periodic treatment with praziquantel (PZQ), with the most recent version of schistosomiasis control consisting of the integration of PZQ into control programs for other neglected tropical diseases (11, 111). However, the sustainability of PZQ treatment for the long-term control of schistosomiasis remains a concern. Indeed, the justification for developing vaccines against schistosomiasis have not changed for over a generation, i.e. high disease burden, high rates of post-treatment reinfection, the inability of mass chemotherapy to interrupt transmission and control morbidity. Most remarkable is the exclusive reliance on praziquantel for control, even in the face of significant concerns about drug resistance and an absence of new drugs in the development pipeline (112, 113).
A central assumption in schistosomiasis control programs is that the repeated use of PZQ leads to a regression of the end organ pathology related to infection. Indeed, treatment with PZQ has been shown to reverse both liver and urinary tract pathology due to S. mansoni and S. haematobium, respectively (reviewed in 114). However, there is no evidence that PZQ acts directly on the liver and urinary tract to reduce granulomata and fibrosis – in fact, this is unlikely since PZQ has no direct effect on the schistosome eggs that are the cause of the pathology (114). The benefit of PZQ in this regard is probably due to a temporary reduction in the number of egg-laying adult worms in the host, thereby slowing the progression to an advanced disease state and even allowing for regression of existing lesions. However, post-therapy reversal of both peri-portal liver fibrosis and urinary tract pathology is variable and temporary, with lesions usually recurring from 12 to 18 months after treatment, at least in the case of S. haematobium (115, 116). Therefore, use of PZQ to prevent and treat organ pathology would require sustained chemotherapy efforts, applied systematically and periodically on a mass scale for an indefinite period of time, which does not appear to be a sustainable proposition.
While the development and spread of PZQ resistance is uncertain, the possibility of resistance reinforces the need for alternatives to single drug treatment. The reduced efficacy of PZQ treatment has already been reported in both Egypt and Senegal (117–119), and PZQ-resistant schistosomes can be selected for in the laboratory (reviewed in 120). It is not an unreasonable supposition, given the experience with Plasmodium falciparum and gastrointestinal nematodes of livestock, that the selection of drug-resistant schistosomes is inevitable. For these reasons, the window of opportunity provided by PZQ should be considered transitory, and the time afforded should be used to develop a vaccine, which can be used once PZQ is no longer effective, or even before then, to prevent or limit resistance. Additionally, an effective drug discovery program should be strongly encouraged to sufficiently arm the chemotherapeutic arsenal against schistosomiasis (121).
Human immune response
As with other helminth infections, there is very little evidence to conclude that protective immunity develops in response to chronic schistosome infection or can be induced by repeated treatment with PZQ. The best evidence for the acquisition of immunity to schistosome infection comes from studies of populations living in endemic areas, where declining levels of infection are seen with increasing age. This age – intensity relationship has been observed for all three of the major schistosomes (S. mansoni, S. haematobium, and S. japonicum) and is commonly referred to as the ‘convex age infection curve’, in which the mean intensity of infection (usually measured as fecal egg counts) rises throughout childhood, peaks in late adolescence, and then declines rapidly in adults (122). Several hypotheses have been proposed to explain this curve, including the slow acquisition of immunity triggered by antigens released by dying worms in the host (123), hormonal and physiological changes of adolescence that alter the ability of schistosomes to penetrate the skin, or behavioral changes that result in reduced environmental exposure (124). Evidence that the curve is due to acquired immunity comes from the observation of a ‘peak shift’ in which maximum infection intensity occurs at younger ages in areas of higher transmission (125), presumably because more intense exposure to infection results in earlier acquisition of immunity, similar to what is observed with P. falciparum malaria.
Over a decade ago, groups of individuals were identified as ‘Putatively Resistant’ (PR) by remaining egg-negative despite constant exposure to S. mansoni transmission (126, 127). More specifically, PR individuals were defined as (1) negative over 5 years for S. mansoni infection based on fecal egg counts; (2) never treated with anthelmintic drugs; (3) continually exposed to infection; and (4) maintaining a vigorous cellular and humoral immune response to crude schistosome antigen preparations (126–128). A role for immunity in protecting these individuals is inferred from their vigorous, but very different immune response to the crude S. mansoni antigen extracts [i.e. schistosomula tegument extract (STEG) and soluble adult worm antigen preparation (SWAP)] than individuals who are chronically infected.
Proof of concept
The ‘gold standard’ against which Schistosoma spp vaccines are judged is the attenuation of invasive cercariae with ionizing radiation (gamma, X-rays, or UV) (reviewed in 129). Based upon the development of successful viral and bacterial vaccines in the early 20th century, this attenuation strategy was developed, optimized and standardized in laboratory models during the late 1970s (130–132). The attenuation of infective cercariae has traditionally been achieved using a gamma source of radiation (131), although X-rays have also been used (133). In this model, protection is measured by enumerating the adult worms recovered by perfusion of the portal vasculature from vaccinated mice compared with control (unvaccinated or vaccinated with adjuvant) mice (129). A single exposure to 500 optimally radiation-attenuated cercariae can achieve protection of 60–70% (129). While nearly all studies of the radiation attenuated cercariae vaccine have been performed in C57Bl/6 mice (which are considered to be a high responder strain), protective immunity in other strains has been achieved, with levels depending upon various genetic factors, including host MHC (129). The radiation-attenuated vaccine has also been used in many different host species and against S. mansoni, S. haematobium, Schistosoma bovis, and S. japonicum (134–137). The radiation-attenuated vaccine for S. mansoni has been shown to protect in a variety of host species such as rats (138) and non-human primates, including baboons and chimpanzees (139, 140). Radiation-attenuated larvae of S. haematobium induce protection in baboons (141). Over the past 25 years, a substantial inventory of data has accrued which reveals many features of the radiation-attenuated larvae vaccine that are critical to our understanding of how to induce protective immunity and are well reviewed in Hewitson et al. (129).
Context for the development of schistosomiasis vaccines
The radiation attenuated vaccine model raised hopes for the development of molecular vaccines against schistosomes. However, no single antigen has consistently induced these same levels of protection, particularly in recombinant form. Nearly 15 years ago, the WHO initiated an independent trial of the six most promising vaccine candidates of S. mansoni origin. This was a reflection of the advances made in molecular biology during the 1980s that enabled the selection and purification of recombinant schistosome molecules, which could be tested in laboratory animal models (mice). As reported by Bergquist and Colley (142), these trials failed to identify a candidate antigen protective above the 40% threshold set by the WHO. Moreover, studies of the human immune response to these candidates also failed to identify one with outstanding potential (143, 144).
Ongoing development of schistosomiasis vaccines
Only one schistosome antigen has entered into clinical trials. The Institut Pasteur together with the French Institut National de la Santé et de la Recherche Médicale have taken a recombinant 28 kDa GST cloned from S. haematobium through both phase 1 and 2 clinical testing in Europe and West Africa (Senegal and Niger). Sh28-GST (Bilhvax) is a recombinant protein formulated with an aluminum hydroxide adjuvant (145, 146). Bilhvax appears to be immunogenic and well-tolerated in healthy adults from non-endemic (France) and S. haematobium endemic areas in African (reviewed in 145, 146). A number of other antigens have shown promise in preclinical studies (reviewed in 57, 112). Of note is a 14 kDa fatty acid binding protein known as Sm14 (147), which in experimental animals (mice and rabbits) elicits protection against S. mansoni as well as Fasciola hepatica, a trematode fluke responsible for human and veterinary fascioliasis (148). Recombinant Sm14 is being developed as an anthelminthic vaccine for use against both fascioliasis of livestock and human schistosomiasis due to S. mansoni. Previous problems with dimerization have been solved. Sm 14 now appears to be a viable and stable vaccine candidate for clinical testing (149). Sm-p80 is another S. mansoni antigen at an advanced stage of pre-clinical development. This antigen encodes the large subunit of a calcium-dependent neutral protease (150–152), and has been tested as DNA vaccine ina DNA prime and protein-boost schedule as well as with a more conventional recombinant protein schedule. In all cases, Smp80 has shown excellent protection in a variety of animal models, including a non-human primates (150–152).
Recent developments in schistosomiasis vaccines
Over the past few years several major advances in schistosome molecular biology have occurred: the transcriptome (153), the genome (154, 155), and much of the tegument proteome of S. mansoni (156–159) have either been completed or mostly characterized. This upsurge in molecular information (particular the marriage of nucleotide and protein sequence data to rapidly link proteins to mRNAs) is now bearing fruit in terms of a whole new suite of promising vaccine antigens. These proteomic and transcriptomic analyses have also reminded us that the most important target of the schistosome is the tegument. Indeed, there is some consensus that previous failures to develop an efficacious schistosome vaccine were due to the complex immunoevasive strategies employed by the parasite to avoid elimination from its intravascular environment (160), with much of this immune evasion attributed to the dynamic nature of the tegument. Mammalian stage schistosomes have a host-interactive outer surface tegument consisting of a single, contiguous, double-bilayer (heptalaminate) membrane that covers the entire worm. The tegument is thought to be involved in several key physiologic processes: parasite nutrition, osmoregulation, and the evasion of host immunity (reviewed in 161). For many microbial pathogens, the host-exposed capsular surface is the target of the most protective vaccines and includes successful examples of metazoan parasite vaccines, such as the cattle tick Boophilus microplus (162, 163), the gastrointestinal nematode Haemonchus contortus (164), and several species of cestode parasites (62). Based on this knowledge, the schistosome tegument is now the target of intensive development of a vaccine (167).
Substantial recent proteomic analyses have been utilized to identify the proteins present in the tegument and exposed to the host (157–159). Despite the abundance of proteins found within this structure (157), few tegument proteins are found in the outer tegument of live worms, where they are likely to be exposed to the host immune system (158). To identify proteins that contain membrane-targeting signals and are putatively expressed in the outer tegument, we used signal sequence trapping to identify two S. mansoni cDNAs of particular interest – Sm-tsp-1 and Sm-tsp-2 (168). These mRNAs encoded novel tetraspanins, i.e. four-transmembrane domain proteins homologous to surface receptors on B and T cells. Tetraspanins have two extracellular (EC) domains – the small loop (EC-1) and the large loop (EC-2). In recent descriptions of the S. mansoni adult worm tegument (157, 159), TSP-2 was one of relatively few integral membrane proteins to be consistently found in the tegument, and not in underlying tissues. Sm-TSP-2 is thought to play a critical role in tegument development and maturation (169). The ultrastructural morphology of adult worms and schistosomula treated in vitro with Sm-tsp-2 double-stranded RNA (dsRNA) displays a distinctly vacuolated and thinner tegument compared with controls, suggestive of impaired closure of tegumentary invaginations (169). A marked and significant reduction (83%) of adult parasites were recovered from mice injected with schistosomulae pre-treated with Sm-tsp-2 dsRNA than control mice injected with untreated schistosomulae (169). These data suggest that tetraspanins are important role in maintaining the integrity of the tegument, including its structure and development. We have identified and are evaluating other tetraspanins in experimental animal models such as Sm-tsp-2, which is the most highly upregulated mRNA in maturing schistosomulae (61). Finally, addition, there is some precedent for the evaluation tetraspanins as vaccine candidates: Sj23 is a tegument tetraspanin used in DNA vaccine for water buffaloes, an important reservoir for S. japonicum in China (170).
Because the TSPs are putatively exposed to the host immune system, we screened the sera of individuals who are putatively resistant to S. mansoni infection from Brazil for antibodies against recombinant versions of these proteins. These putatively resistant individuals had elevated levels of the cytophilic antibodies IgG1 and IgG3 compared with age, sex, and water contact matched individuals chronically infected with S. mansoni from the same endemic area (61). Previous studies in Brazil (144) and Egypt (143) assessed the immune responses of resistant and susceptible individuals to a panel of S. mansoni vaccine antigens, mostly those tested by the WHO, with no single antigen uniquely recognized by putatively resistant individuals. However, unlike Sm-TSP-2, none of these proteins tested by WHO were apical membrane proteins exposed to the host in the outer tegument membrane (158). Of note in our studies in Brazil was the absence of IgE to Sm-TSP-2 in both putatively resistant and chronically infected individuals, enabling us to avoid one of the more recently identified technical challenges for helminth vaccines – the IgE trap (see above).
The second EC domain fragment of a schistosome tetraspanin known as Sm-TSP-2 has been selected by the HHVI for development as a human vaccine antigen. When the 9 kDa EC domain was expressed in either P. pastoris or E. coli and formulated with either Freund’s complete adjuvant (61), aluminum hydroxide, or aluminum hydroxide together with CpGs, it provided high levels of protection in mice vaccinated with the antigen followed by challenge with S. mansoni cercariae. The Sm-TSP-2 recombinant schistosomiasis vaccine would be intended primarily for school-aged children living in the S. mansoni endemic regions of sub-Saharan Africa and Brazil. This population was selected because they are considered at greatest risk for acquiring the largest number of schistosomes and because they suffer the greatest morbidity compared to any other age-group. The vaccine would be administered as an injectable product and ideally would prevent the reacquisition of schistosomes in the blood stream following initial treatment with PZQ (vaccine-linked chemotherapy) (reviewed in 112). The ‘proof of concept’ for the efficacy of the vaccine would be obtained in a phase 2b study that follows safety studies (phase 1) and would be based on reductions in schistosome egg counts in school-aged children compared with age-matched controls.
The absence of a commercial market for a schistosomiasis vaccine linked with PZQ chemotherapy requires that the vaccine be developed through a PD-PPP mechanism (Global access of NTD vaccines, below). It also requires that a schistosomiasis vaccine be produced at extremely low cost; our economic studies indicate that helminth vaccines require costing below US$1–2 per dose. Such economic requirements likely prevent expensive vaccine biotechnologies, including mammalian cell culture, insect expression vectors, and prime-boost strategies using adenovirus vectors or DNA vaccines. Therefore, we are focusing expressing this protein in extremely low-cost bacteria and yeast expression vectors.
Veterinary (transmission-blocking) vaccines
Cysticercosis and echinococcosis vaccines
Cysticercosis and echinococcosis (hydatid disease) are caused by infection with larval stages of the taeniid tapeworm parasites Taenia solium and Echinococcus granulosus, respectively. These are zoonotic diseases and livestock animals are involved in their transmission. Vaccination of humans would provide the most direct means to prevent cysticercosis and echinococcosis; however, an alternative option would be to utilize vaccines in the normal animal hosts of the parasites, indirectly achieving a reduction in human incidence by decreasing or removing the source of infective material for humans. The latter strategy would be considerably less expensive to develop and implement.
Two different mammalian hosts are involved in the life cycle of taeniid cestode parasites, in a prey-predator cycle. The adult tapeworm lives in the small intestine of a carnivore (definitive host) while the larval stages encyst in the body tissues of an omnivore or herbivore (intermediate host). The life cycle is completed when tissues infected with the larval stages are eaten by a suitable definitive host species. For T. solium, humans act as the obligate definitive host and pigs act as the animal intermediate host. Dogs act as definitive hosts for E. granulosus, and while numerous herbivorous species may be intermediate hosts, sheep and goats are most commonly associated with transmission of the parasite leading to infections in humans. Humans may act as intermediate hosts for both T. solium (cysticercosis) and E. granulosus (echinococcosis/hydatid disease), and it is these infections of the body organs with the parasites’ metacestode stages that causes substantial human morbidity and mortality globally.
Potentially both the definitive and intermediate hosts of these species could be targeted for development of transmission blocking vaccines. Notwithstanding some recent encouraging data (171, 172), there is little convincing evidence in favor of the existence of immunologically mediated resistance to infection with taeniid cestodes in their definitive hosts (173). This contrasts with the situation in the parasites’ intermediate hosts where unequivocal evidence exists for immunologically mediated resistance to infection. This fact has favored the successful development of transmission blocking vaccines and the following discussion focuses on vaccination against infection in the parasites’ intermediate hosts.
Acquired immunity
Taeniid cestodes are unusual eukaryotic parasites because acquired immunity can be readily demonstrated. Indeed, the first convincing proof that it was possible to achieve immunity against a metazoan parasite with obtained for infection with Taenia taeniaeformis, a natural taeniid cestode parasite of rodents, when it was discovered that infected animals were immune to a subsequent re-exposure to the parasite (174, reviewed in 175). Subsequently it was shown that acquired immunity could be demonstrated for many, if not all, species of taeniid cestode in their intermediate hosts (reviewed in 176).
Correlates of protection
Early investigations into acquired immunity to Taenia and Echinococcus species found that immunity could be transferred with colostrum from an infected dam or to a naive recipient with passively transferred serum or purified IgG from an infected donor (174, 177–179). The protective efficacy of specific antibody against T. taeniaeformis in both in rats (180) and mice (181) was found to be abrogated entirely by cobra venom factor, implicating complement in the mechanism by which host protective immunity was manifest. Passive protection was found to be effective only if the antibodies were transferred within the first few days of an infection (180–182), indicating that the susceptible phase in the parasite’s development was the invasive or early developing parasite and that mature parasites were relatively insusceptible to host immune attack. While all of this information is not available for taeniid species other than T. taeniaeformis, the available evidence suggests that these general features are common to many or all taeniid cestode infections in their intermediate hosts (173, 175, 179).
Impact on development of a vaccine
Shortly after Miller (174) established that immunity to re-infection with T. taeniaeformis occurred in rats, immunization studies showed that immunity could also be stimulated by immunization with parasite extracts (183). Subsequently, it has been found that protection could be afforded against other taeniid species by immunization of their hosts with non-living parasite extracts (176). Rajasekariah and colleagues (184) discovered that the richest source of host protective antigens was the infective form of the parasite known as the oncosphere.
Proof of concept: animal models
Research towards the development of transmission blocking vaccines for cysticercosis and echinococcosis affecting humans took a major step forward with the successful development of a recombinant vaccine against cysticercosis in sheep caused by Taenia ovis (165, 185). This was the first highly successful recombinant vaccine against any eukaryotic parasite and has been recognized as a milestone in the history of parasitology (186). Not only did the T. ovis vaccine development program provide a blueprint for how an effective vaccine could be developed, it also provided cDNA probes, which could be used as tools for identification of potential antigen-encoding genes in other taeniid species.
Proof of concept: in vitro models
Antibody is the principal, if not the only, specific host protective immune mechanism which protects the intermediate hosts of taeniid cestodes against a challenge infection with eggs. This is the case both for immunity stimulated by prior infection as well as immunity stimulated by vaccination with oncosphere antigens. The presence of protective antibody in serum can be demonstrated through their capacity to kill oncospheres or early developing parasites in in vitro culture. This phenomenon was first demonstrated for the parasite Taenia saginata by Silverman (187) and been utilized for investigations into protective antibodies against several taeniid species (188–190).
Successful development of effective vaccines against cysticercosis and echinococcosis
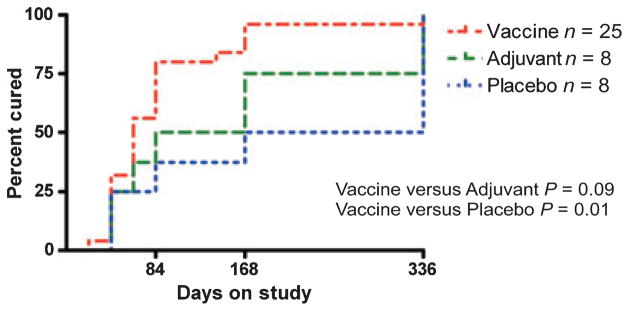
Following the development of the recombinant vaccine against T. ovis in 1989 (165), the knowledge and tools developed with that parasite were utilized to assist with the production of effective recombinant vaccines against infection with several other taeniid cestode species (reviewed in 62). Vaccine trials in Australia, New Zealand, Argentina, and China confirmed the efficacy of the EG95 recombinant antigen against E. granulosus infection in sheep and other host species (54, 166, 191). Vaccine trials in pigs against cysticercosis caused by T. solium confirmed the effectiveness of recombinant oncosphere antigens to protect against this species also. Independent vaccine trials carried out in pigs with the TSOL18 antigen in Mexico, Peru, Honduras, and Cameroon have all achieved 99–100% protection against an experimental challenge infection with T. solium (62, 192, 193). The effectiveness of these vaccines in experimental challenge trials in the parasites’ natural host species is highlighted in Table 6.
Table 6.
Effectiveness of transmission blocking vaccines against Taenia solium cysticercosis (TSOL18) and Echinococcus granulosus echinococcosis (hydatid disease; EG95) in challenge trials against experimental infections in pigs and sheep, respectively
| Parasite/Group
|
Number of T. solium cysticerci or E. granulosus hydatid cysts in individual animals
|
Protection* %
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pigs, T. solium† | |||||||||||||
| GST/MBP controls | 69 | 136 | 186 | 1021 | 1146 | 1711 | 1785 | 2143 | 2810 | 5336 | – | ||
| TSOL18 vaccinated | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 99.9 | ||||
| Sheep/E. granulosus‡ | |||||||||||||
| GST controls | 165 | 40 | 30 | 15 | 10 | 9 | 8 | 7 | 3 | 2 | – | ||
| EG95 vaccinated | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 99.6 |
Calculated on the total number of cysticerci or hydatid cysts detected at necropsy expressed as a percentage reduction in the mean number of cysts in vaccinated pigs compared with the mean number in control animals.
Pigs were vaccinated with 200 μg protein plus 1 mg Quil A on two occasions twice 4 weeks apart. Pigs were challenged with T. solium eggs 1–3 weeks after the last immunization and the animals necropsied approximately 12 weeks later.
Sheep were vaccinated with 50 μg protein plus 1 mg Quil A twice, 1 month apart, and challenged with E. granulosus eggs from parasites obtained from a naturally infected dog. Levels of protection were assessed 12–14 months after experimental infection. Data from (165).
Field trials of the EG95 vaccine against echinococcosis are currently underway in the Patagonian region of Argentina. Recently, results were published of the first field trial of the TSOL18 vaccine, which was carried out in far north Cameroon. The vaccine completely eliminated the transmission of T. solium by the pigs involved in the trial (194). This represents an extraordinary level of success for an anti-parasite vaccine and augers well for the implementation of programs to eradicate T. solium (195, 196).
Challenges for further development and implementation
The most significant challenge facing the TSOL18 and EG95 vaccines against cysticercosis and echinococcosis relate to the lack of incentive for livestock owners to vaccinate their animals against the parasites causing these diseases. There is relatively little direct economic impact due to T. solium or E. granulosus infections in livestock. The overwhelming importance of the parasites lies in their effects on human morbidity and mortality. There are not compelling economic reasons why livestock owners would choose to spend time or money to prevent these infections being transmitted. For this reason, control of cysticercosis and echinococcosis is likely to depend on disease control investments made by governments and philanthropic agencies.
Delivery systems
At this time, the TSOL18 and EG95 vaccines both require at least two immunizations to stimulate a high level of immunity. The necessity to treat each animal individually on more than one occasion is not ideal in the often poor and remote environments in which these vaccines are needed most. Alteration to the vaccine delivery technology such that only a single exposure of the animals was required to stimulate long lasting immunity would represent a major improvement. Currently available technologies suggest this could possibly be achieved through the adoption of live recombinant vaccine delivery technologies.
To date the TSOL18 and EG95 vaccines have been utilized as stand-alone vaccines. An alternative approach to the vaccines’ delivery could provide incentives for livestock owners to use the vaccines. The development of products that combined the cestode antigens with vaccines against economically important pathogens of livestock would be likely to enhance the acceptability and application of these vaccines. For example, combination of TSOL18 with one of the existing commercial vaccines against classical swine fever or a future vaccine against African swine fever. For echinococcosis, the very commonly used clostridial vaccines provide obvious candidates for development of combination vaccines with EG95 for use in sheep, goats and cattle.
Formulation
Formulation of vaccines for application in livestock animals does not pose the same level of restrictions as are imposed in the development of vaccines for use in humans. The adjuvant that has been utilized successfully in experimental trials of the TSOL18 and EG95 vaccines to date, Quil A, and its less pure parent saponin, are already licensed for use in a variety of veterinary vaccines.
Progress on clinical development and commercial-scale production
The EG95 hydatid vaccine has undergone substantial clinical development as well as the establishment of Good Manufacturing Practice production protocols. The vaccine was licensed for application in China in June 2007 and is currently being assessed for registration in Argentina. Field trials are underway in Argentina in the Tehuelche communities of Chubut province and the Mapuche communities of Rio Negro province. Clinical trials are underway in Turkey with a clostridial/EG95 combination vaccine for sheep. Clinical development and scale up of the TSOL18 cysticercosis vaccine for pigs are in their infancy; substantial progress is anticipated in these areas over the next few years.
Enteric protozoa vaccines
Giardia lamblia (9.5 cases/100 000 USA population) is the most common parasite identified in stool samples of individuals in the US, present in about 4% of stool specimens submitted to clinical laboratories for O&P testing. It is also the most common cause of diarrhea in returning travelers (197). There are two major genotypes of G. lamblia that may differ in their propensity to cause diarrhea (198, 199). Water and food-borne transmission are the most frequent mechanisms of spread, with person-to-person spread important in day care settings and among sexually active homosexual males. Giardiasis is highly infectious, ingestion of as few as 10–25 cysts produces disease in human volunteers. Giardiasis occurs in all parts of the world and is a common cause of water-borne outbreaks of diarrhea in the United States. Giardia exists in the small intestine, where EC trophozoites remain in intimate contact with the intestinal epithelium. Vaccination against giardiasis is potentially complicated by the fact that the parasite can undergo antigenic variation. Cyst wall proteins are candidate antigens for a giardia vaccine (200, 201). Recently disruption of antigenic variation was demonstrated to be an approach to vaccination (202).
Cryptosporidium spp. infection (1.4 cases/100 000 USA population) results from ingestion of fecally contaminated water or food containing the infectious oocyst form. Cryptosporidium parvum and Cryptosporidium hominis are the predominant causes of cryptosporidiosis in humans (203). In one study of diarrheal disease in Dhaka Bangladesh cryptosporidiosis was responsible for 2.8% of patients with diarrhea severe enough to warrant a hospital visit (198). Sporozoites are released from the oocyst in the small intestine and attach to the epithelial cell surface. Upon invasion of the epithelial cells the parasite undergoes both the sexual and asexual stages of the life cycle. Infection with Cryptosporidium spp. in a normal host leads to days to several weeks of non-bloody diarrhea. In patients with AIDS and diminished CD4+ T cells, infection may be persistent and require therapy. Individuals with diminished mannose-binding lectin levels (204) and with certain HLA haplotypes (205) are also more susceptible. The most effective therapy for cryptosporidiosis in AIDS is antiretroviral treatment (ART) of HIV, which has led to a 90% decrease in the incidence of cryptosporidiosis in the US. In patients with severe AIDS who are not responding to or are intolerant of ART, neither paromomycin nor nitazoxanide are effective treatment (203, 206). Vaccination against C. parvum is focused on immunodominant antigens expressed on the surface of sporozoites (207–210). Immune sera against these antigens are able to partially block sporozoite invasion. Additional sporozite antigens, to which invasion-neutralizing antibody responses are directed, included CpMuc4 and CpMuc5 which are mucin-like glycoproteins (209). Because the entire parasite life cycle takes place in the intestinal epithelium, a mucosal immune response is considered critical, and recent approaches have used salmonella as a vaccine delivery vehicle (207).
E. histolytica infection is foremost a problem for children in the developing world (211). The WHO estimates that approximately 50 million people worldwide suffer from invasive amebic infection each year, resulting in 40 to 100 thousand deaths annually (212). Carefully conducted serologic studies in Mexico, where amebiasis is endemic, demonstrated antibody to E. histolytica in 8.4 % of the population (213). In the urban slum of Fortaleza, Brazil, 25% of the people tested carried antibody to E. histolytica; the prevalence of anti-amebic antibodies in children aged 6 to 14 years was 40% (214). Our prospective study of preschool children in a slum of Dhaka Bangladesh demonstrated new E. histolytica infection in 39% of children over a 1-year period of observation, with 10% of the children having an E. histolytica infection associated with diarrhea and 3% with dysentery (215). Amebiasis is also the second most common cause of diarrhea in returning travelers (197). Amebiasis is also a Category B biodefense agent. There are several unfortunate properties of E. histolytica that could be exploited for misuse in an act of bioterror or war. The organism has a low infectious dose (<10 cysts) and is resistant to chlorination. Infection can be acquired from fecal contamination of municipal water supplies, as most recently seen in Tblissi in the Republic of Georgia (216).
Vaccination against amebiasis in our laboratory is focusing on the parasite Gal/GalNAc lectin. It is an attractive vaccine antigen, as it has essential roles in parasite adherence, killing and phagocytosis, and is antigenically conserved between strains. Trophozoites adhere to the colonic mucus and epithelial cells through interaction of a galactose and N-acetyl-D-galactosamine (Gal/GalNAc) specific lectin with host Gal/GalNAc-containing glycoconjugates (217). The trophozoite kills host epithelial and immune cells at points of invasion in a process that requires the activity of the Gal/GalNAc lectin. Finally, E. histolytica ingests the corpse of the dead cell, in part via the lectin (218). A critical recent advance by our team of investigators has been the discovery of acquired immunity in children to intestinal amebiasis (219) (Fig. 3). Immunity is associated with a mucosal IgA response against the carbohydrate recognition domain (CRD) of the Gal/GalNAc lectin: children with this response had 86% fewer new infections over 1 year of prospective observation, with an overall median duration of protection of approximately 600 days (220). Immunizations with native Gal/GalNAc lectin, native Igl, and with recombinant proteins containing parts of the cysteine-rich EC portion of Hgl, have been protective in several different investigators labs with the gerbil model of amebic liver abscess, and in our lab for the murine model of amebic colitis (reviewed in 222). LecA absorbed in alum is an effective vaccine in the murine model of amebic colitis. The LecA fragment contains all of the neutralizing antibody epitopes of the complete Gal/GalNAc lectin. Vaccination with the alum-absorbed LecA fragment of the parasite Gal/GalNAc lectin provided 68% protection from amebic colitis in the mouse model (221). Work is underway to develop LecA absorbed in alum as a vaccine product for the protection of children in the developing world, as well as international travelers.
Fig. 3. Immunoglobulin (Ig) A and immunity to amebiasis.
Children with fecal IgA antibodies against the Gal/GalNAc lectin carbohydrate recognition domain (CRD) [IgA anti-CRD (+); n = 81] had a lower incidence of new intestinal Entamoeba histolytica infection compared with children lacking this response [IgA anti-CRD (−); n = 149]. The two groups are statistically significantly different (P ≤ 0.04) at every time point (from Haque et al. 219).
Vaccines for leishmaniasis
Leishmaniasis causes human suffering on a global scale, threatening approximately 350 million people in endemic areas, with an estimated 12 million current cases and 2 million additional cases annually (222). Several species of protozoan parasites of the genus Leishmania are transmitted by the bite of infected sand flies and cause human infections ranging from disfiguring cutaneous lesions to potentially fatal VL. Available chemotherapeutics are largely effective, though often toxic, and drug-resistance is an issue (223). Even if ideal drugs were available, elimination of leishmaniasis can only be achieved through vaccination as humans are the main reservoir for many Leishmania spp., and elimination of insect vectors is not an alternative. For these reasons, we at the Infectious Diseases Research Institute (IDRI) have been focused on vaccine development.
Leishmania parasites reside mainly within macrophages, and therefore vaccines that stimulate cellular immune responses are required for control of intracellular replication. Appropriate CD4+ T-cell responses correlate with protection against leishmaniasis in humans and in animal models (224). The discovery of the Th1/Th2 separation of CD4 response based on cytokine production was aided largely by studies using resistant and susceptible inbred mouse strains (225–227). Using crude or defined antigens with appropriate adjuvants, protection against visceral and cutaneous disease has been achieved in mice, hamsters, dogs, and non-human primates (59, 228–235). Protection studies, particularly in mice, have corroborated the Th1-dependence of effective immunity against Leishmania. Thus, understanding how to induce protective immune responses against Leishmania has broad relevance to the development of T-cell vaccines and vaccines against intracellular organisms.
Partial clinical efficacy has been obtained using first generation vaccines, primarily for cutaneous leishmaniasis (CL) (236–238), though results have been inconsistent. These studies involved the use of crude preparations that cannot be standardized or be optimally formulated to induce desired immune responses while avoiding undesirable immune responses. Defined antigens delivered as plasmid DNA, vectored DNA, or as recombinant protein have advantages in this regard, and have proven to be effective in animal models. Of these platform technologies, only recombinant proteins have advanced to licensure in human vaccines, while both protein- and DNA-based vaccines have advanced as veterinary products. While recombinant proteins provide a versatile, scalable, and cost-effective approach for vaccine development, they generally induce only weak T-cell responses. However, this can be overcome with the inclusion of adjuvants. We have optimized adjuvants for vaccine targets requiring potent CD4+ T-cell responses, including leishmaniasis. For our human vaccine development we are emphasizing recombinant protein/adjuvant, while our canine vaccine program includes evaluation of nucleic acid as well as protein vaccine constructs.
The development of a safe, effective, and practical vaccine against leishmaniasis involves: (i) identification of effective antigens and (ii) delivering antigens in formulations that induce effective T-cell responses. Although partial efficacy has been demonstrated with crude first generation vaccines, attempts to turn such preparations into sustainable products have been unsuccessful. To date, there has been no licensure of effective T-cell vaccines, although several are in development (e.g. tuberculosis, malaria, HIV, leishmaniasis), as well as immune-therapeutics for cancer. Our approach has been to identify protein antigens, create polyprotein fusions, optimize the proteins for maximum immunogenicity, and develop antigen delivery platforms, including adjuvant formulations that promote appropriate T-cell responses and protection in animal models. Criteria for antigen selection have also included conservation among Leishmania species, potentially allowing the development of a vaccine effective against both VL and CL.
Turning antigens into effective immunogens requires understanding of the nature of the desired immune response and selection of delivery platforms capable of inducing such a response. Our first defined vaccine against leishmaniasis consisted of a combination of four recombinant antigens formulated with GM-CSF (used before the availability of other adjuvants). This vaccine was successfully used to treat drug refractory mucosal leishmaniasis (ML) caused by Leishmania braziliensis (239, 240) and was the first example of a defined vaccine being successfully used for immunochemotherapy for this disease, providing proof-of-concept for this approach.
A major breakthrough in the development of vaccine candidates against leishmaniasis, as well as other diseases requiring potent and directed T-cell responses, occurred with the identification of adjuvants capable of inducing Th1 responses. The discovery that properly formulated Toll-like receptor (TLR) agonists can stimulate Th1 immune responses has profoundly impacted vaccine development against intracellular pathogens such as Leishmania. In particular, the extensive experience with monophosphoryl lipid A (MPL), a TLR4 agonist obtained from the cell wall of Salmonella, and MPL’s approval in vaccines for hepatitis B and human papilloma virus have demonstrated the safety and efficacy of engaging TLR4. MPL is the only TLR agonist in approved vaccines and thus has an extensive history of safety and efficacy. We have used MPL in preclinical models of leishmaniasis and have demonstrated efficacy in several species. The next generation vaccine candidate consisted of a poly-protein, designed and produced to be more cost effective than came with the use of recombinant fusion protein Leish-111f (L111f) together with MPL formulated in an oil-in-water emulsion (MPL-SE) (59, 241). This vaccine antigen (Fig. 4) was shown to protect mice, hamsters, and rhesus macaques, when formulated with an effective adjuvant or as DNA (230, 241–244), and has subsequently been used in multiple clinical trials. L111f was shown to be safe and immunogenic as well as to have therapeutic efficacy in humans (240, 245, 246) and in dogs (233, 244). The antigens were chosen based on their ability to protect mice or, in the case of LeIF, to act as an adjuvant through the stimulation of IL-12, as well as on their conservation among Leishmania species (247).
Fig. 4. Leish-111f (L111f): a tandemly linked protein of three subunits.
Each component of L111f, the first recombinant Leishmania vaccine candidate to enter clinical trials, was cloned from a Leishmania major expression library. LeIF, (ribosomal initiation factor identified by serological screening with human sera from healthy infected individuals); LmSTI1 (temperature inducible protein, identified by screening with sera from BALB/c mice infected with L. major); thiol-specific antioxidant (TSA, identified through screening with sera from BALB/c mice immunized with protective Leishmania antigens.
The primary patient (and reservoir) populations for leishmaniasis are humans and dogs. Dogs are a natural host for VL and represent both a disease protection model as well as a target for epidemiological intervention of disease transmission. In endemic regions of the Mediterranean and Latin America, dogs are the most important reservoir of Leishmania infantum. Humans are the VL reservoir in the Indian subcontinent and parts of Africa. Relatively little is known with regard to the generation of protective T-cell responses in dogs, although we (233, 244) and others (234, 235) have demonstrated partial efficacy in both prophylactic and therapeutic vaccine approaches in canine leishmaniasis. Thus, canine VL studies have provided important proof of concept for the use of vaccines to effectively treat fatal VL. Clinical trials to evaluate this concept in human VL will begin next year.
We have performed several clinical trials using recombinant Leishmania antigens formulated in granulocyte macrophage colony stimulating factor (GM-CSF) or monophosphoryl lipid A-squalene (MPL-SE). Several interesting observations have emerged from our clinical studies. Individuals with active CL or ML have strong anti-Leishmania immune responses, including high antibody levels and significant T-cell responses. Thus, treating these individuals with vaccine may seem counterintuitive. However, we have found that infected individuals responded poorly to vaccine antigens prior to immunization, but responded to the vaccine antigens with both specific antibody (Fig. 5) and T cells (Fig. 6A) following immunization. Thus, infected individuals can be immunized with properly formulated antigens, which often times are not well recognized by the infected individual, resulting in the generation of vaccine antigen-specific T cells which seems to correlate with disease resolution (Fig. 6B).
Fig. 5. Induction of specific antibody following immunization of active cutaneous leishmaniasis patients.
Immunoglobulin G antibody titers (per-protocol population). Black bar represents median vaccine versus adjuvant or placebo, day 84 P < 0.0001; day 168 P = 0.0019.
Fig. 6. (A). Induction of specific cellular immune responses following immunization of active cutaneous leishmaniasis patients using Fluorescence Activated Cell Sorting (FACS).
FACS analysis of un-vaccinated mucosal leishmaniasis (ML) patient subject’s cells collected before (day 0) and after (day 84) three immunizations with Leish-111f (L111f) in MPL-SE which does not cure (chemotherapy only). Cells were analyzed following in vitro stimulation with vaccine antigen. (B). FACS analysis of ML patient subject’s cells collected before (day 0) and after (day 84) three immunizations with L111f in MPL-SE in combination with standard antimony therapy (immunochemotherapy). Cells were analyzed following in vitro stimulation with vaccine antigen.
In a recent study in CL patients in Brazil, a significantly faster cure rate was observed in patients who received vaccine in addition to chemotherapy, as opposed to chemotherapy alone (Fig. 7). Cumulatively, our clinical results have demonstrated safety and partial efficacy of therapeutic vaccination and point to the possibility of using this approach in patients who fail chemotherapy, as well as potentially devising protocols involving reduced doses of drug in combination with vaccination. Further innovation has been in the area of adjuvant development. Because of the effectiveness of MPL-based adjuvants in animal models of leishmaniasis, as well as in humans, we have focused on improving MPL. Although we have a license to MPL from GSK, there are several reasons for emphasizing the development of synthetic molecules based on MPL. For one thing, continued cost effective access to the molecule cannot be guaranteed. In addition, we developed structures with increased potency over MPL, allowing the use of comparatively smaller doses. Using information from the crystal structure of the human TLR4, we selected one molecule for further development, based on the ability of this molecule to fit in the human MD2 structure. We have developed formulations of this novel synthetic TLR4 agonist glucopyranosyl lipid A (GLA), which is more potent than MPL in in vitro studies with human cells, and have shown that GLA is an effective adjuvant in models of CL and VL. Furthermore, GLA can be synthesized in large amounts (we currently have nearly 1 million human doses in inventory) and is independent from control by pharmaceutical companies. In addition to being an effective adjuvant molecule, GLA can synergize with ligands of other TLRs.
Fig. 7. Beneficial effects of vaccination of cutaneous leishmaniasis patients receiving antimony chemotherapy; decreased time to cure.
All patients received antimony therapy plus three injections of saline (placebo), MPL-SE, 25 ug/dose (adjuvant), or vaccine (Leish-111f, 5–25 ug/dose in MPL-SE).
It is evident that solid protection can be achieved in experimental models using recombinant proteins properly formulated in safe and effective adjuvants. The fact that certain protective antigens are highly shared between Leishmania species, that protection can be achieved with an adjuvant approved in vaccines in over 100 countries, that VL vaccine development can be pursued in both dogs and humans, and that vaccine products can be pursued for both prophylactic and therapeutic applications are all advantages for targeting Leishmania for vaccine development. IDRI has completed or has ongoing clinical trials in several countries, including USA, Brazil, Peru, Colombia, Venezuela, India, and Sudan. It is hoped and expected that information from these trials will lead to one or more safe and effective vaccines for human and canine leishmaniasis.
Vector saliva: a neglected component of neglected diseases
Some 50% of the neglected tropical diseases listed by the WHO are vector-borne (http://www.who.int/neglected_diseases). These include leishmaniasis transmitted by phlebotomine sand flies, American trypanosomiasis (Chagas disease) by triatomine bugs, African trypanosomiasis (human sleeping sickness) by tsetse flies, dengue by Aedes mosquitoes, lymphatic filariasis by mosquitoes, onchocerciasis (river blindness) by black flies, and Buruli ulcer by aquatic insects. Over the past two decades, there has been a steady and mounting evidence of the immunogenicity and immunomodulatory properties of the salivary proteins of these diverse vectors and their significant influence over vector-transmitted diseases (248–252). This is not surprising, since salivary molecules of disease vectors are composed of a myriad of potent and pharmacologically active molecules evolved to assist with blood meal acquisition (248, 253).
Exacerbation of disease by vector saliva
Several reports have demonstrated that saliva of certain vectors enhances disease by promoting survival of the pathogen they transmit. Saliva of the sand flies Lutzomyia longipalpis (254) and Phlebotomus papatsi (255), vectors of visceral and cutaneous leishmaniasis, respectively, enhanced infection with Leishmania major in mice. More recently, hyaluronidase, an enzyme present in both Phlebotomus and Lutzomyia species, was proposed a one of the factors responsible for disease exacerbation by sand fly saliva (256). As for triatomines, saliva of Rhodnius prolixus attracted inflammatory cells to the bite site and blocked nitric oxide production by Trypanosoma cruzi-exposed macrophages resulting in disease enhancement in mice (257). Similarly, Caljon et al. (258, 259) proposed that saliva of the tsetse fly, the vector of African trypanosomiasis, diminishes the host inflammatory response at the site of infection biasing host immunity towards a Th2 response and putatively enhancing Trypanosoma brucei infection in mice (259). Additionally, saliva of the mosquito Aedes aegypti, the primary vector of dengue virus, also promotes a Th2 immune response in the host upregulating anti-inflammatory cytokines and downregulating the pro-inflammatory cytokine interferon-γ (IFN-γ) (260, 261). Subsequently, Boppana et al. (262) identified a salivary protein from Ae. aegypti (SAAG-4) that modulated CD4+ Th immune responses inducing Th2 responsiveness and IL-4 production while reducing expression of the antiviral Th1 cytokine IFN-γ. This modulation of the host immune response by mosquito saliva was put forward as a possible mechanism for potentiation of viral infections (251, 261). Saliva of the black fly is also suspected to contribute to efficient transmission of O. volvulus, the etiological agent of human onchocerciasis. Recently, saliva of black fly Simulium vittatum was shown to contain an immunosuppressive protein that inhibits proliferation of both CD4+ and CD8+ T cells and induces their apoptosis (263). Understanding the mechanism of exacerbation and revealing the identity of the salivary molecules responsible for it will permit the reversal/neutralization of their effect and a consequent reduction of disease burden. Maxadilan is a good example of such an approach. A potent vasodilator and immunomodulator in Lu. longipalpis saliva, maxadilan was shown to exacerbate infection with L. major, and immunization against it neutralized its disease enhancing effect resulting in protection (264). The emergence of powerful tools such as high throughput genomics, bioinformatics, functional genomics tools, and sensitive multiplex technologies should accelerate the discovery of the identity, putative function, and immunomodulatory properties of other salivary molecules from these vectors (265–267).
Adaptive immunity to saliva and disease
The disease exacerbative properties of saliva, often resulting from the bioactive property of one or more of its molecules, should not be confounded with antigenic molecules in saliva that induce an adaptive immune response in the host. This acquired immunity can be either protective or exacerbative depending of the nature and dominance of the salivary components of a vector species. Exposure to uninfected bites of the sand fly Phlebotomus papatasi induces a strong delayed-type hypersensitivity response and IFN-γ production at the bite site that confers protection in mice challenged by L. major-infected flies (274); in contrast, acquired immunity to Lutzomyia intermedia saliva results in disease exacerbation not protection (268). Moreover, P. papatasi saliva, despite its overall protective property, was shown to contain molecules that alone induce a protective (PpSP15) or exacerbative (PpSP44) immune response in the host (269, 270). It is likely that Lu. intermedia saliva also contains molecules with similar profiles despite the overall exacerbative effect of total saliva. Interestingly, the protective nature of salivary molecules was also reported for aquatic insects, where pre-exposure to members of the family Naucoridae that transmit M. ulcerans protected mice from Buruli ulcers (271), demonstrating the broadness of this phenomenon. This emphasizes the importance of vector saliva as an untapped source of antigens for vaccines against vector-borne neglected diseases. Unfortunately, for the majority of neglected diseases, the adaptive immune response generated by vector saliva or its components remains unknown.
Correlates of protection
Pathogens of vector-borne neglected diseases are diverse including viruses, bacteria, and protozoan and eukaryotic parasites. It is unclear for many how immunity to vector saliva can impact transmitted pathogens, often attributing it to a similarity of antigens between the two. Though this is not impossible, for most vector-borne diseases, the reason immunity to vector saliva has such an impact on pathogens revolves around the fact that pathogens are co-deposited into the host skin together with saliva. The immune response generated against salivary molecules will therefore impact the pathogens residing in the vicinity. Knowing the correlates of protection from neglected diseases and generating them using a salivary protein is the rationale for development of vector-based vaccines.
As an example, it is established that immunity to leishmaniasis is a cell-mediated response characterized by the presence of a CD4+ Th1 effector response; conversely, a Th2 response signals susceptibility to infection (272, 273). In most experimental models studied, immunity to sand fly saliva in general (274–276) and to certain sand fly salivary proteins specifically (269, 270, 277) reproduced the correlates of protection from Leishmania, driving a strong adaptive immune response dominated by IFN-γ-producing CD4+ Th1 cells; this saliva-induced immune response resulted in robust protection against both CL and VL. Of note, protection by vector saliva against visceral disease (277) underlines its importance in initiating a faster and stronger Leishmania-specific immune response and therefore of having a long-lasting effect on host immunity. We hypothesize that inherent properties of protective sand fly salivary proteins govern early events involving antigen processing, cell recruitment, and cytokine induction to produce a distinctly rapid and vigorous immune response that in the presence of Leishmania drives an accelerated and improved parasite-specific Th1 response.
Animal models of vector salivary vaccines: sand flies as an example
Having a defined antigen brings the process of vaccine development a step closer towards an effective vaccine. The use of modern transcriptomic analysis combined with functional genomics produced a number of potential sand fly salivary vaccines (270, 277–279). In rodent models of infection, vaccination with a DNA plasmid encoding a salivary protein induced a strong immune response resulting in protection from both CL and VL in the absence of adjuvant. The salivary protein PpSP15 from P. papatasi conferred powerful protection against L. major infection (270). Furthermore, this protection was observed in B-cell-deficient C57BL/6 mice establishing the cell-mediated basis of this protection (269). In a hamster model of VL, vaccination with LJM19, a salivary protein from Lu. longipalpis, protected animals against the fatal outcome of infection by L. infantum chagasi (277). The protective effect was associated with an early saliva-specific Th1 cellular immune response in the skin and correlated with a later Leishmania-specific Th1 protective immune response in the liver and spleen (277). Promisingly, the Th1 anti-saliva immunity observed in rodents was also observed in dogs, a key reservoir of leishmaniasis (279).
Immunization of dogs with the salivary proteins LJM17 and LJL143 from Lu. Longipalpis using a combination of DNA plasmid, recombinant protein, and canarypox virus resulted in a strong and long lasting Th1 cellular and humoral immunity. Importantly, salivary gland-stimulated peripheral blood mononuclear cells from vaccinated dogs killed L. infantum within macrophages upon the addition of autologous T cells (279). Importantly, in a similar assay L. infantum chagasi was killed by sand fly saliva-stimulated peripheral blood mononu-clear cells from human volunteers exposed to uninfected sand fly bites (276) suggesting that protective anti-saliva immune responses may be relevant in humans.
Challenges for vector salivary vaccine development: sand flies as an example
The concept of vector saliva-based vaccines is relatively recent. Thus far, defined salivary vaccine candidates for vectors of neglected diseases have only been identified for sand flies. At this point a major challenge is to move these potential candidates towards large-scale production and clinical trials. In general, properties of salivary molecules should make them amenable for large-scale production: they are secreted proteins increasing the probability of soluble recombinant protein expression, the majority shares no sequence homology to proteins in humans or other mammals, and they are immunogenic. Another important consideration is the heavy financial burden of such an undertaking. Thus, the selection process of a vaccine candidate must be stringent. Emphasis should be placed on the use of materials from animals targeted by the vaccine (instead of mouse models) to establish a reliable prediction of the type, quality, and magnitude of the immunity induced by the salivary vaccine candidates. The combination of recent technological advances in genomics and immunology are opening the path for this transition. The rapid evolution of high throughput transcriptomics linked with high throughput DNA plasmid construction, nucleofection technology and multiparameter flow cytometry now allow the rapid screening of complete repertoires of salivary molecules and the identification of inducers of the desired immune response. These salivary proteins can then be moved forward for development.
Impact on vaccine development
Salivary molecules of vectors of neglected diseases offer several advantages as vaccines or components of vaccines against the pathogens they transmit: they are mostly foreign molecules that have no homologs in humans; they are mostly well-tolerated as demonstrated by repeatedly exposed humans in endemic areas; and most importantly, they provide a unique opportunity to fight neglected diseases on two fronts, driving a better pathogen-specific immune response while maintaining an independent saliva-specific immune response that contributes to and ameliorates protection. Another tantalizing aspect of vaccinating with a salivary molecule is that subjects will be naturally boosted by bites of the targeted vector, which will promote the maintenance of memory cells and prolong the efficacy of a salivary vaccine.
Despite the promising properties of a salivary vaccine, they also offer some challenges. Ideally, a vector salivary vaccine should be conserved within and between different vector species/populations. Mejia et al. (253) reflected on the possibility of a ‘pan arthropod vaccine’. This would indeed be the perfect vaccine; however, it is difficult to envision a universal antigen considering the diversity of vectors and the divergent evolution of their salivary proteins. More likely, we foresee situations where development of a vector salivary vaccine is warranted, for instance, having a primary vector largely responsible for pathogen transmission within a sizeable geographical region or targeting vectors in areas of high mortality and morbidity.
For most of the neglected vector-borne diseases mentioned here, potentiation and enhancement of infection by vector saliva has been reported. Additionally, as shown for leishmaniasis, distinct salivary proteins induce a potent immune response in the host with profound effects on disease outcome. It is therefore clear that vector saliva represents a valuable yet neglected component in vaccine development for neglected vector-borne diseases. Investing in research on vector salivary vaccine candidates offers much to gain and nothing to lose.
Overcoming economic challenges: PD-PPP and the pharmaceutical industry
In addition to the technological hurdles, the economic challenges have until very recently discouraged the multinational pharmaceutical companies from embarking on NTD vaccine R&D. Instead, leading the way for the production of a new generation of vaccines to combat NTDs are the PD-PPPs (Table 7). The PD-PPPs are non-profit organizations that use industry practices or partner with industry for purposes of developing, manufacturing, and clinically testing vaccines (Table 6). Because of their non-profit status, they attract private and public donor support (3, 9). Among the first PD-PPPs was the International Vaccine Institute (IVI) (http://www.ivi.org). Established in the 1990s through initial support of the United Nations Development Program (UNDP) and the Korean government and located in Seoul, Korea, IVI is advancing the clinical testing of enteric and arboviral vaccines as well as taking leadership on developing new vaccines for dengue. Following scale-up funding of the Gates Foundation in this new century, additional support became available for malaria vaccines through a PATH-MVI (http://www.malariavaccine.org/) and tuberculosis vaccines through Sequella, later renamed Aeras Global TB Vaccine Foundation (http://www.aeras.org/home/home.php). Subsequently, funds became available to establish the following PD-PPPs: the IDRI (http://www.idri.org/) in Seattle to develop leishmaniasis vaccines and to expand the availability of adjuvants for other antipoverty vaccines; the Sabin Vaccine Institute (http://www.sabin.org) linked to George Washington University in Washington DC, for hookworm, schistosomiasis, and other helminth vaccines; Fraunhofer Center for Molecular Biology in Newark, Delaware for human African trypanosomiasis vaccines; and other organizations. However, recently both Novartis and Merck & Co. have made major commitments to developing new NTD vaccines, establishing spin-off enterprises in Siena, Italy and Delhi, India, respectively. There is great excitement that the major multinational pharmaceutical companies have cautiously begun a concerted effort to enter into the NTD space, with the possibility that additional companies will follow.
Table 7.
Major PD-PPPs for NTDs (antipoverty vaccines)
| Organization
|
Website
|
Location
|
Major diseases
|
Innovative technologies
|
Developing country partners
|
|---|---|---|---|---|---|
| Fraunhofer Center for Molecular Biology | http://www.fraunhofer-cmb.org | Newark, DE, USA | Human African Trypanosomiasis | Tobacco plant expression systems | Sub-Saharan Africa |
| Infectious Diseases Research Institute | http://www.idri.org | Seattle, WA, USA | Leishmaniasis, Leprosy, Trachoma | Recombinant protein vaccines, Platform adjuvants | Brazil, India, Peru |
| Institut Pasteur and INSERM | http://www.bilhvax.inserm.fr | Lille, France | Schistosomiasis | Recombinant protein vaccines | Niger, Senegal |
| International Vaccine Institute | http://www.ivi.org | Seoul, Korea | Cholera and other Enteric bacterial infections, Dengue | Whole cell vaccines, Chimeric and inactivated viruses, Recombinant protein vaccines | Bangladeshi, India, Sub-Saharan Africa, Vietnam |
| MSD Wellcome Trust Hilleman Laboratories | http://www.hillemanlaboratories.in | Delhi, India | TBD | TBD | India |
| Novartis Vaccines Institute for Global Health | http://www.novartis.com/research/corporate-research/nvgh.shtml | Siena, Italy | Enteric bacterial infections | Conjugate vaccine technologies | Sub-Saharan Africa |
| Sabin Vaccine Institute | http://www.sabin.org | Washington, DC, USA | Hookworm, Schistosomiasis, Onchocerciasis | Recombinant protein vaccines | Brazil, India, Malaysia |
PD-PPPs, product development-public private partnerships; NTD, neglected tropical diseases; TBD, to be determined.
Several PD-PPPs have developed strategic partnerships with vaccine manufacturers in middle income countries, most of which belong to an umbrella organization known as the Developing Country Vaccine Manufacturers Network. For example, IDRI and the Sabin Vaccine Institute work closely with the public sector Institute Butantan in Sao Paulo Brazil, and to FIOCRUZ, the Oswaldo Cruz Foundation. Additional opportunities are under exploration with both public and private vaccine manufacturers in China, India, and Indonesia, which have joined in a Developing Country Vaccine Manufacturers Network (http://www.dcvmn.com). It is anticipated that such middle income country vaccine manufacturers may be especially tolerant of the low-profit margins anticipated for NTD vaccines or in some cases zero profit margins, in the expectation that final products would be purchased by governments in the endemic countries or by the Global Alliance for Vaccines and Immunization (GAVI). Each of the PD-PPPs has written global access roadmaps to ensure their vaccines are produced and developed at affordable prices.
Additional partnerships may be anticipated. The observation that almost one-half of the NTDs occurs in the world’s Islamic countries, including Asian nations such as Indonesia and Bangladesh, as well as the African countries of Sudan, Niger, Mali, Chad, and others, adds an additional geopolitical dimension to the urgent need to control these conditions (280). Discussions are in progress by some of the PD-PPPs to partner with some of the wealthier countries in the Middle East as well as with Malaysia and Singapore to advance NTD vaccines to address the diseases of the most impoverished Islamic nations. The observation that polio vaccines were developed jointly by the US and the Soviet Union at the height of the Cold War has led to calls to embark on similar ‘vaccine diplomacy’ efforts in the Middle East and elsewhere (281).
Global access for NTD vaccines
While the technical hurdles to develop new NTD vaccines as outlined in the previous section are substantial, equally formidable are the barriers that prevent the global uptake and access to these new vaccines. Even for commercially successful products such as the yeast-derived recombinant hepatitis B vaccine, almost 30 years following proof of the vaccine’s efficacy were required before high levels of coverage began to be achieved in developing countries (282); similarly, coverage for the Haemophilus influenzae type B (Hib) vaccine is still extremely low almost 20 years since the Hib vaccine was licensed (282).
In the cases of the hepatitis B and Hib vaccines, a key barrier to their global update and access has been cost. Both vaccines required a high degree of technological sophistication to produce as well as years of labor-intensive and expensive R&D activities. The vaccine manufacturers, in turn, charged consumers living in the US, Europe, Japan, and elsewhere costs sufficient to ensure that they recouped their financial investment in R&D. Ultimately, only as the decades passed would the costs of these vaccines trickle down to a point where they might become affordable in low- and middle-income countries in Asia, Africa, and the Americas. Efforts to greatly shorten the time frame for trickle down and to ensure other financing and global access strategies are now actively under development through the activities of the Global Alliance for Vaccines and Immunization (GAVI) (http://www.gavial-liance.org), a 10-year-old global partnership established to accelerate the access and use of vaccines newly developed by the major pharmaceutical manufacturers.
The trickle down model outlined above will not apply to most of the NTD vaccines. Because the NTDs occur only among the world’s subsistence farmers and their families or the urban slum dwellers, for most NTD vaccines there is no significant US or European market. Further, in contrast to vaccines to combat malaria or tuberculosis, there is no real market for international travelers or the military. Indeed up until just a few years ago, the major vaccine manufacturers had largely avoided embarking on R&D efforts to develop NTD vaccines. This situation has started to change with the pharmaceutical giants Novartis and Merck & Co. recently establishing global health vaccine ventures, the Novartis Vaccines Institute for Global Health (Siena, Italy) (http://www.novartis.com/research/corporate-research/nvgh.shtml), and the MSD-Well-come Hilleman Laboratories (New Delhi, India) (http://www.hillemanlaboratories.in/), respectively. However, today most of the NTD vaccines are being developed through PDPs, i.e. non-profit organizations that use industry business practices (283). While many of the PDPs are committed to small molecule drug development, several NTD vaccine PDPs are now in place including Sabin Vaccine Development, the PDP of the Sabin Vaccine Institute in Washington DC, which is developing vaccines for hookworm, schistosomiasis, and liver fluke infection, the IDRI in Seattle, WA, for leishmaniasis and leprosy vaccines, and the IVI in Seoul, Korea, for dengue and enteric bacterial pathogen vaccines.
Among the elements of global access strategies for these organizations are those to ensure that their NTD vaccines are produced at the lowest possible costs and with a technology platform, which can be readily integrated into health systems found in low-income countries. Thus, in the area of vaccine design, low cost expression vectors and column resins are preferred, while for vaccine development the NTD vaccine PDPs frequently partner with developing country manufacturers and clinical trials sites located in innovative developing countries (IDCs), such as Brazil, India, and China (284). For instance, both Sabin Vaccine Development and IDRI develop, manufacture, and tests new NTD vaccines in close collaboration with Brazilian institutions such as the Instituto Butantan and FIOCRUZ (Oswaldo Cruz Foundation).
Another key aspect of global access is vaccine introduction, which includes efforts to integrate new NTD vaccines into existing health systems. Because many of the NTDs are important causes of morbidity among older children, adolescents, and adults, the vaccines for these conditions may not need to be administered to infants and as part of the Expanded Programme on Immunization (EPI), which was first launched in Ethiopia in 1980 in order to expand access to vaccines for diphtheria, pertussis, tetanus, polio, measles, and tuberculosis during infancy (http://www.who.int/countries/eth/areas/immunization/en/). For example, recombinant vaccines for hookworm, schistosomiasis, and possibly other NTDs will likely be administered to school-aged or preschool children either in schools or through child health days. Therefore, considerable advocacy and consensus building with the WHO and other international agencies will be required to ensure global access, including possible efforts to link vaccine campaigns for the NTDs with those being put forward for other school-based childhood vaccines including the human papillomavirus vaccine (285).
Another important aspect of global access will be identifying mechanisms to finance these new NTD vaccines, particularly since the people who need them most will not be able to afford them. Based on past successes with the revolving fund of the Pan American Health Organization, it may one day be possible to distribute some NTD vaccines free of charge in this part of the world (286), while governments such as Brazil with its large-scale manufacturing capabilities may be able to purchase and distribute vaccines nationally and for all of Latin America. For the poorest countries in Africa and Asia, however, there will need to be stepped-up efforts similar to those being advanced for other childhood vaccines currently procured through GAVI. Recently, an innovative sustainable immunization financing mechanism was created jointly between GAVI, the Sabin Vaccine Institute, and the health ministries of several disease-endemic countries (http://www.sabin.org/advocacy-eduation/sustainable-immunization-financing). Finally, patents and other intellectual property will need to be made available to vaccine manufacturers in IDCs, while in parallel knowledge is publicly disseminated through the peer-reviewed literature, including open access journals (http://www.plosntds.org).
Acknowledgments
J. M. B. and P. J. H. are funded by the Bill and Melinda Gates Foundation through the Sabin Vaccine Institute. Research funding (M. W. L) from the Wellcome Trust grant 075818 and the Australian National Health and Medical Research Council grants 350279, 400109, 628320.
References
- 1.Hotez P. Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and their Impact on Global Health and Development. Washington, DC: American Society for Microbiology Press; 2008. [Google Scholar]
- 2.Collier P. The Bottom Billion: Why the Poorest Countries Are Failing and What Can Be Done About It. Oxford: Oxford University Press; 2007. [Google Scholar]
- 3.Hotez PJ, Ferris MT. The antipoverty vaccines. Vaccine. 2006;24:5787–5799. doi: 10.1016/j.vaccine.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Hotez P. Combating parasitic diseases in innovative developing countries by reverse vaccinology. Parasite Immunol. 2006;28:242–243. [Google Scholar]
- 5.Hotez PJ. A plan to defeat neglected tropical diseases. Sci Am. 2010;302:90–94. doi: 10.1038/scientificamerican0110-90. [DOI] [PubMed] [Google Scholar]
- 6.Hotez PJ, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 7.Musgrove P, Hotez PJ. Turning neglected tropical diseases into forgotten maladies. Health Aff (Millwood) 2009;28:1691–1706. doi: 10.1377/hlthaff.28.6.1691. [DOI] [PubMed] [Google Scholar]
- 8.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 9.Hotez PJ, Brown AS. Neglected tropical disease vaccines. Biologicals. 2009;37:160–164. doi: 10.1016/j.biologicals.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 14.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 15.Sakti H, et al. Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Trop Med Int Health. 1999;43:322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 16.Jukes MC, et al. Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 17.Miguel E, Kremer M. Worms: identifying impacts on education and health in the presence oof treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- 18.Bleakley H. Disease and development: evidence from hookworm eradication in the American South. Q J Economics. 2007;122:73–117. doi: 10.1162/qjec.121.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotez PJ. Empowering women and improving female reproductive health through control of neglected tropical diseases. PLoS Negl Trop Dis. 2009;3:e559. doi: 10.1371/journal.pntd.0000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooker S, Hotez PJ, Bundy DA. Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl Trop Dis. 2008;2:e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjetland EF, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 22.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 23.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Dracunculiasis eradication. Wkly Epidemiol Rec. 2008;83:159–167. [PubMed] [Google Scholar]
- 26.Eckert J, Gemmell MA, Meslin F-X, Pawlowski ZS. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: WHO/-FAO; 2001. [Google Scholar]
- 27.Murrell KDE. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniasis/Cysticercosis. Paris: OIE/WHO/FAO; 2005. [Google Scholar]
- 28.WHO. Human African trypanosomiasis (sleeping sickness) Wkly Epidemiol Rec. 1999;74:245–246. [PubMed] [Google Scholar]
- 29.Reithinger R, Brooker S, Kolaczinski JH. Visceral leishmaniasis in eastern Africa –current status. Trans R Soc Trop Med Hyg. 2007;101:1169–1170. doi: 10.1016/j.trstmh.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stauffer W, Abd-Alla M, Ravdin JI. Prevalence and incidence of Entamoeba histolytica infection in South Africa and Egypt. Arch Med Res. 2006;37:266–269. doi: 10.1016/j.arcmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Hotez PJ, Ehrenberg JP. Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv Parasitol. 2010;72:31–53. doi: 10.1016/S0065-308X(10)72002-9. [DOI] [PubMed] [Google Scholar]
- 32.Sripa B, et al. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sripa B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang LD, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 35.Craig PS, Liu D, Ding Z. Hydatid disease in China. Parasitol Today. 1991;7:46–50. doi: 10.1016/0169-4758(91)90188-t. [DOI] [PubMed] [Google Scholar]
- 36.Gautam VP. Treatment of leprosy in India. J Postgrad Med. 2009;55:220–224. doi: 10.4103/0022-3859.57410. [DOI] [PubMed] [Google Scholar]
- 37.Bethony J, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 38.Perera M, Whitehead M, Molyneux D, Weerasooriya M, Gunatilleke G. Neglected patients with a neglected disease? A qualitative study of lymphatic filariasis. PLoS Negl Trop Dis. 2007;1:e128. doi: 10.1371/journal.pntd.0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi A, et al. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45:105–111. [PubMed] [Google Scholar]
- 40.Bansal D, Malla N, Mahajan RC. Drug resistance in amoebiasis. Indian J Med Res. 2006;123:115–118. [PubMed] [Google Scholar]
- 41.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotez P. Neglected diseases amid wealth in the United States and Europe. Health Aff (Millwood) 2009;28:1720–1725. doi: 10.1377/hlthaff.28.6.1720. [DOI] [PubMed] [Google Scholar]
- 43.Hotez PJ. The giant anteater in the room: Brazil’s neglected tropical diseases problem. PLoS Negl Trop Dis. 2008;2:e177. doi: 10.1371/journal.pntd.0000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franco-Paredes C, et al. Chagas disease: an impediment in achieving the Millennium Development Goals in Latin America. BMC Int Health Hum Rights. 2007;7:7. doi: 10.1186/1472-698X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyrer C, Villar JC, Suwanvanichkij V, Singh S, Baral SD, Mills EJ. Neglected diseases, civil conflicts, and the right to health. Lancet. 2007;370:619–627. doi: 10.1016/S0140-6736(07)61301-4. [DOI] [PubMed] [Google Scholar]
- 46.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotez PJ. Neglected diseases and poverty in “The Other America”: the greatest health disparity in the United States? PLoS Negl Trop Dis. 2007;1:e149. doi: 10.1371/journal.pntd.0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jannin J, Louis FJ, Lucas P, Simarro PP. [Control of human African trypanosomiasis: back to square one] Med Trop (Mars) 2001;61:437–440. [PubMed] [Google Scholar]
- 49.Hotez PJ. Mass drug administration and integrated control for the world’s high-prevalence neglected tropical diseases. Clin Pharmacol Ther. 2009;85:659–664. doi: 10.1038/clpt.2009.16. [DOI] [PubMed] [Google Scholar]
- 50.Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions”: how a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hotez PJ, Pecoul B. “Manifesto” for advancing the control and elimination of neglected tropical diseases. PLoS Negl Trop Dis. 2010;4:e718. doi: 10.1371/journal.pntd.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotez PJ, Bethony JM, Oliveira SC, Brindley PJ, Loukas A. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Rev Vaccines. 2008;7:745–752. doi: 10.1586/14760584.7.6.745. [DOI] [PubMed] [Google Scholar]
- 53.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heath DD, Jensen O, Lightowlers MW. Progress in control of hydatidosis using vaccination – a review of formulation and delivery of the vaccine and recommendations for practical use in control programmes. Acta Trop. 2003;85:133–143. doi: 10.1016/s0001-706x(02)00219-x. [DOI] [PubMed] [Google Scholar]
- 55.Lightowlers MW. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol. 2010;40:1183–1192. doi: 10.1016/j.ijpara.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6:733–741. doi: 10.1016/S1473-3099(06)70630-2. [DOI] [PubMed] [Google Scholar]
- 57.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koizumi N, Watanabe H. Leptospirosis vaccines: past, present, and future. J Postgrad Med. 2005;51:210–214. [PubMed] [Google Scholar]
- 59.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Mora M, Veggi D, Santini L, Pizza M, Rappuoli R. Reverse vaccinology. Drug Discov Today. 2003;8:459–464. doi: 10.1016/s1359-6446(03)02689-8. [DOI] [PubMed] [Google Scholar]
- 61.Tran MH, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 62.Lightowlers MW. Cestode vaccines: origins, current status and future prospects. Parasitology. 2006;133(Suppl):S27–S42. doi: 10.1017/S003118200600179X. [DOI] [PubMed] [Google Scholar]
- 63.Monsalve RI, Lu G, King TP. Expression of yellow jacket and wasp venom Ag5 allergens in bacteria and in yeast. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 1999;(93):181–188. [PubMed] [Google Scholar]
- 64.Mett V, Farrance CE, Green BJ, Yusibov V. Plants as biofactories. Biologicals. 2008;36:354–358. doi: 10.1016/j.biologicals.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Jariwala AR, et al. Potency testing for the experimental Na-GST-1 hookworm vaccine. Expert Rev Vaccines. 2010;9:1219–1230. doi: 10.1586/erv.10.107. [DOI] [PubMed] [Google Scholar]
- 66.International Conference on Harmonization. Q6B specifications test procedures and acceptance criteria for biotechnological/biological products. 1999. [Google Scholar]
- 67.Hendriksen CF. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Rev Vaccines. 2009;8:313–322. doi: 10.1586/14760584.8.3.313. [DOI] [PubMed] [Google Scholar]
- 68.Giersing BK, Dubovsky F, Saul A, Denamur F, Minor P, Meade B. Potency assay design for adjuvanted recombinant proteins as malaria vaccines. Vaccine. 2006;24:4264–4270. doi: 10.1016/j.vaccine.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Arciniega J. What’s in a name? The meaning of the term “potency” as applied to immunobiologics for human use and its implications for alternatives and validation. Dev Animal Vet Sci. 2000;31:979–986. [Google Scholar]
- 70.Fujiwara RT, Geiger SM, Bethony J, Mendez S. Comparative immunology of human and animal models of hookworm infection. Parasite Immunol. 2006;28:285–293. doi: 10.1111/j.1365-3024.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 71.Miller TA. Vaccination against the canine hookworm diseases. Adv Parasitol. 1971;9:153–183. doi: 10.1016/s0065-308x(08)60161-x. [DOI] [PubMed] [Google Scholar]
- 72.Miller TA. The diagnosis of hookworm disease. Mod Vet Pract. 1974;55:706–709. [PubMed] [Google Scholar]
- 73.Miller TA. Industrial development and field use of the canine hookworm vaccine. Adv Parasitol. 1978;16:333–342. doi: 10.1016/s0065-308x(08)60577-1. [DOI] [PubMed] [Google Scholar]
- 74.Xue J, et al. Necator americanus: optimization of the golden hamster model for testing anthelmintic drugs. Exp Parasitol. 2005;111:219–223. doi: 10.1016/j.exppara.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Cutts FT, Enwere G, Zaman SM, Yallop FG. Operational challenges in large clinical trials: examples and lessons learned from the Gambia pneumococcal vaccine trial. PLoS Clin Trials. 2006;1:e16. doi: 10.1371/journal.pctr.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krosin MT, Klitzman R, Levin B, Cheng J, Ranney ML. Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clin Trials. 2006;3:306–313. doi: 10.1191/1740774506cn150oa. [DOI] [PubMed] [Google Scholar]
- 77.Sudore RL, Landefeld CS, Williams BA, Barnes DE, Lindquist K, Schillinger D. Use of a modified informed consent process among vulnerable patients: a descriptive study. J Gen Intern Med. 2006;21:867–873. doi: 10.1111/j.1525-1497.2006.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, et al. Differences in perception of dysentery and enteric fever and willingness to receive vaccines among rural residents in China. Vaccine. 2006;24:561–571. doi: 10.1016/j.vaccine.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 79.Pack R, et al. Willingness to be vaccinated against shigella and other forms of dysentery: a comparison of three regions in Asia. Vaccine. 2006;24:485–494. doi: 10.1016/j.vaccine.2005.07.094. [DOI] [PubMed] [Google Scholar]
- 80.Kaljee LM, Pack R, Pach A, Nyamete A, Stanton BF. Sociobehavioural research methods for the introduction of vaccines in the Diseases of the Most Impoverished Programme. J Health Popul Nutr. 2004;22:293–303. [PubMed] [Google Scholar]
- 81.Gazzinelli MF, et al. Health education through analogies: preparation of a community for clinical trials of a vaccine against hookworm in an endemic area of Brazil. PLoS Negl Trop Dis. 2010;4:e749. doi: 10.1371/journal.pntd.0000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maizels RM. Infections and allergy – helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 83.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites – masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 84.Maizels RM, Holland MJ, Falcone FH, Zang XX, Yazdanbakhsh M. Vaccination against helminth parasites – the ultimate challenge for vaccinologists? Immunol Rev. 1999;171:125–147. doi: 10.1111/j.1600-065x.1999.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 85.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 86.Erb KJ. Helminths, allergic disorders and IgE-mediated immune responses: where do we stand? Eur J Immunol. 2007;37:1170–1173. doi: 10.1002/eji.200737314. [DOI] [PubMed] [Google Scholar]
- 87.Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174:514–523. doi: 10.1164/rccm.200603-331OC. [DOI] [PubMed] [Google Scholar]
- 88.Goud GN, et al. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine. 2005;23:4754–4764. doi: 10.1016/j.vaccine.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 89.Goud GN, et al. Cloning, yeast expression, isolation, and vaccine testing of recombinant Ancylostoma-secreted protein (ASP)-1 and ASP-2 from Ancylostoma ceylanicum. J Infect Dis. 2004;189:919–929. doi: 10.1086/381901. [DOI] [PubMed] [Google Scholar]
- 90.Hawdon JM, Narasimhan S, Hotez PJ. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol Biochem Parasitol. 1999;99:149–165. doi: 10.1016/s0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 91.Bethony J, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 2005;19:1743–1745. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- 92.Bethony JM, et al. Randomized, placebo-controlled, double-blind trial of the Na-ASP-2 hookworm vaccine in unexposed adults. Vaccine. 2008;26:2408–2417. doi: 10.1016/j.vaccine.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 93.Chan MS. The global burden of intestinal nematode infections – fifty years on. Parasitol Today. 1997;13:438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 94.Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Diemert D, Loukas A. New technologies for the control of human hookworm infection. Trends Parasitol. 2006;22:327–331. doi: 10.1016/j.pt.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 96.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 97.Albonico M, et al. Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans R Soc Trop Med Hyg. 1995;89:538–541. doi: 10.1016/0035-9203(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 98.Bethony JM, Loukas A, Hotez PJ, Knox DP. Vaccines against blood-feeding nematodes of humans and livestock. Parasitology. 2006;133(Suppl):S63–S79. doi: 10.1017/S0031182006001818. [DOI] [PubMed] [Google Scholar]
- 99.Diemert DJ, Bethony JM, Hotez PJ. Hookworm vaccines. Clin Infect Dis. 2008;46:282–288. doi: 10.1086/524070. [DOI] [PubMed] [Google Scholar]
- 100.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 101.Crompton DW. The public health importance of hookworm disease. Parasitology. 2000;121(Suppl):S39–S50. doi: 10.1017/s0031182000006454. [DOI] [PubMed] [Google Scholar]
- 102.Loukas A, et al. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Med. 2005;2:e295. doi: 10.1371/journal.pmed.0020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pearson MS, et al. An enzymatically inactivated hemoglobinase from Necator americanus induces neutralizing antibodies against multiple hookworm species and protects dogs against heterologous hookworm infection. FASEB J. 2009;23:3007–3019. doi: 10.1096/fj.09-131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pearson MS, et al. Neutralizing antibodies to the hookworm hemoglobinase Na-APR-1: implications for a multivalent vaccine against hookworm infection and schistosomiasis. J Infect Dis. 2010;201:1561–1569. doi: 10.1086/651953. [DOI] [PubMed] [Google Scholar]
- 105.Ranjit N, et al. Proteolytic degradation of hemoglobin in the intestine of the human hookworm Necator americanus. J Infect Dis. 2009;199:904–912. doi: 10.1086/597048. [DOI] [PubMed] [Google Scholar]
- 106.Xiao S, et al. The evaluation of recombinant hookworm antigens as vaccines in hamsters (Mesocricetus auratus) challenged with human hookworm, Necator americanus. Exp Parasitol. 2008;118:32–40. doi: 10.1016/j.exppara.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Zhan B, et al. Biochemical characterization and vaccine potential of a heme-binding glutathione transferase from the adult hookworm Ancylostoma caninum. Infect Immun. 2005;73:6903–6911. doi: 10.1128/IAI.73.10.6903-6911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhan B, et al. Molecular cloning, biochemical characterization, and partial protective immunity of the heme-binding glutathione S-transferases from the human hookworm Necator americanus. Infect Immun. 2010;78:1552–1563. doi: 10.1128/IAI.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asojo OA, et al. X-ray structures of Na-GST-1 and Na-GST-2 two glutathione S-transferase from the human hookworm Necator americanus. BMC Struct Biol. 2007;7:42. doi: 10.1186/1472-6807-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fenwick A. Waterborne infectious diseases – could they be consigned to history? Science. 2006;313:1077–1081. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- 111.Hotez P, Ottesen E, Fenwick A, Molyneux D. The neglected tropical diseases: the ancient afflictions of stigma and poverty and the prospects for their control and elimination. Adv Exp Med Biol. 2006;582:23–33. doi: 10.1007/0-387-33026-7_3. [DOI] [PubMed] [Google Scholar]
- 112.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–117. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Bethony JM, Diemert DJ, Oliveira SC, Loukas A. Can schistosomiasis really be consigned to history without a vaccine? Vaccine. 2008;26:3373–3376. doi: 10.1016/j.vaccine.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 114.Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86:161–183. doi: 10.1016/s0001-706x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 115.Campagne G, Garba A, Barkire H, Vera C, Sidiki A, Chippaux JP. [Continued ultrasonic follow-up of children infected with Schistosoma haematobium after treatment with praziquantel] Trop Med Int Health. 2001;6:24–30. doi: 10.1046/j.1365-3156.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 116.Hatz CF, et al. Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg. 1998;59:775–781. doi: 10.4269/ajtmh.1998.59.775. [DOI] [PubMed] [Google Scholar]
- 117.Botros S, Sayed H, Amer N, El-Ghannam M, Bennett JL, Day TA. Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. Int J Parasitol. 2005;35:787–791. doi: 10.1016/j.ijpara.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 118.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 119.Ismail M, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 120.Hagan P, Appleton CC, Coles GC, Kusel JR, Tchuem-Tchuente LA. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 121.Utzinger J, Xiao SH, Tanner M, Keiser J. Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs. 2007;8:105–116. [PubMed] [Google Scholar]
- 122.Fulford AJ, et al. Water contact observations in Kenyan communities endemic for schistosomiasis: methodology and patterns of behaviour. Parasitology. 1996;113:223–241. doi: 10.1017/s0031182000082007. [DOI] [PubMed] [Google Scholar]
- 123.Woolhouse ME, Hagan P. Seeking the ghost of worms past. Nat Med. 1999;5:1225–1227. doi: 10.1038/15169. [DOI] [PubMed] [Google Scholar]
- 124.Fulford AJ, Webster M, Ouma JH, Kimani G, Dunne DW. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol Today. 1998;14:23–26. doi: 10.1016/s0169-4758(97)01168-x. [DOI] [PubMed] [Google Scholar]
- 125.Woolhouse ME. Patterns in parasite epidemiology: the peak shift. Parasitol Today. 1998;14:428–434. doi: 10.1016/s0169-4758(98)01318-0. [DOI] [PubMed] [Google Scholar]
- 126.Caldas IR, et al. Susceptibility and resistance to Schistosoma mansoni reinfection: parallel cellular and isotypic immunologic assessment. Am J Trop Med Hyg. 2000;62:57–64. doi: 10.4269/ajtmh.2000.62.57. [DOI] [PubMed] [Google Scholar]
- 127.Correa-Oliveira R, Caldas IR, Gazzinelli G. Natural versus drug-induced resistance in Schistosoma mansoni infection. Parasitol Today. 2000;16:397–399. doi: 10.1016/s0169-4758(00)01740-3. [DOI] [PubMed] [Google Scholar]
- 128.Brito CF, Caldas IR, Coura Filho P, Correa-Oliveira R, Oliveira SC. CD4+ T cells of schistosomiasis naturally resistant individuals living in an endemic area produce interferon-gamma and tumour necrosis factor-alpha in response to the recombinant 14 kDa Schistosoma mansoni fatty acid-binding protein. Scand J Immunol. 2000;51:595–601. doi: 10.1046/j.1365-3083.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 129.Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol. 2005;27:271–280. doi: 10.1111/j.1365-3024.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 130.Bickle QD, James ER. Resistance against Schistosoma mansoni induced by immunization of mice with cryopreserved schistosomula. Trans R Soc Trop Med Hyg. 1978;72:677–678. doi: 10.1016/0035-9203(78)90043-3. [DOI] [PubMed] [Google Scholar]
- 131.Minard P, Dean DA, Jacobson RH, Vannier WE, Murrell KD. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978;27:76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- 132.Murrell KD, Clark S, Dean DA, Vannier WE. Influence of mouse strain on induction of resistance with irradiated Schistosoma mansoni cercariae. J Parasitol. 1979;65:829–831. [PubMed] [Google Scholar]
- 133.Hsu SY, Hsu HF, Burmeister LF. Schistosoma mansoni: vaccination of mice with highly x-irradiated cercariae. Exp Parasitol. 1981;52:91–104. doi: 10.1016/0014-4894(81)90065-5. [DOI] [PubMed] [Google Scholar]
- 134.Agnew AM, Murare HM, Doenhoff MJ. Specific cross-protection between Schistosoma bovis and S. haematobium induced by highly irradiated infections in mice. Parasite Immunol. 1989;11:341–349. doi: 10.1111/j.1365-3024.1989.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 135.Anderson S, Shires VL, Wilson RA, Mountford AP. In the absence of IL-12, the induction of Th1-mediated protective immunity by the attenuated schistosome vaccine is impaired, revealing an alternative pathway with Th2-type characteristics. Eur J Immunol. 1998;28:2827–2838. doi: 10.1002/(SICI)1521-4141(199809)28:09<2827::AID-IMMU2827>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 136.Dean DA, Mangold BL, Harrison RA, Ricciardone MD. Homologous and heterologous protective immunity to Egyptian strains of Schistosoma mansoni and S. haematobium induced by ultraviolet-irradiated cercariae. Parasite Immunol. 1996;18:403–410. doi: 10.1046/j.1365-3024.1996.d01-129.x. [DOI] [PubMed] [Google Scholar]
- 137.Ruppel A, Shi YE, Moloney NA. Schistosoma mansoni and S. japonicum: comparison of levels of ultraviolet irradiation for vaccination of mice with cercariae. Parasitology. 1990;101:23–26. doi: 10.1017/s0031182000079701. [DOI] [PubMed] [Google Scholar]
- 138.Ford MJ, Bickle QD, Taylor MG. Immunization of rats against Schistosoma mansoni using irradiated cercariae, lung schistosomula and liver-stage worms. Parasitology. 1984;89:327–344. doi: 10.1017/s0031182000001347. [DOI] [PubMed] [Google Scholar]
- 139.Soisson LA, Reid GD, Farah IO, Nyindo M, Strand M. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J Immunol. 1993;151:4782–4789. [PubMed] [Google Scholar]
- 140.Yole DS, Pemberton R, Reid GD, Wilson RA. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology. 1996;112:37–46. doi: 10.1017/s0031182000065057. [DOI] [PubMed] [Google Scholar]
- 141.Harrison RA, et al. Immunization of baboons with attenuated schistosomula of Schistosoma haematobium: levels of protection induced by immunization with larvae irradiated with 20 and 60 krad. Trans R Soc Trop Med Hyg. 1990;84:89–99. doi: 10.1016/0035-9203(90)90393-s. [DOI] [PubMed] [Google Scholar]
- 142.Bergquist NR, Colley DG. Schistosomiasis vaccine: research to development. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 143.Al-Sherbiny M, Osman A, Barakat R, El Morshedy H, Bergquist R, Olds R. In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop. 2003;88:117–130. doi: 10.1016/s0001-706x(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 144.Ribeiro de Jesus A, et al. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect Immun. 2000;68:2797–2803. doi: 10.1128/iai.68.5.2797-2803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Capron A, Capron M, Dombrowicz D, Riveau G. Vaccine strategies against schistosomiasis: from concepts to clinical trials. Int Arch Allergy Immunol. 2001;124:9–15. doi: 10.1159/000053656. [DOI] [PubMed] [Google Scholar]
- 146.Capron A, Riveau G, Capron M, Trottein F. Schistosomes: the road from host–parasite interactions to vaccines in clinical trials. Trends Parasitol. 2005;21:143–149. doi: 10.1016/j.pt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Moser D, Tendler M, Griffiths G, Klinkert MQ. A 14-kDa Schistosoma mansoni polypeptide is homologous to a gene family of fatty acid binding proteins. J Biol Chem. 1991;266:8447–8454. [PubMed] [Google Scholar]
- 148.Tendler M, Simpson AJ. The biotechnology-value chain: development of Sm14 as a schistosomiasis vaccine. Acta Trop. 2008;108:263–266. doi: 10.1016/j.actatropica.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 149.Ramos CR, et al. Stability improvement of the fatty acid binding protein Sm14 from S. mansoni by Cys replacement: structural and functional characterization of a vaccine candidate. Biochim Biophys Acta. 2009;1794:655–662. doi: 10.1016/j.bbapap.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 150.Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol. 2009;31:156–161. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ahmad G, et al. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a non-human primate model. Vaccine. 2009;27:2830–2837. doi: 10.1016/j.vaccine.2009.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ahmad G, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105:1767–1777. doi: 10.1007/s00436-009-1646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verjovski-Almeida S, et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–157. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- 154.Berriman M, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Knudsen GM, Medzihradszky KF, Lim KC, Hansell E, McKerrow JH. Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol Cell Proteomics. 2005;4:1862–1875. doi: 10.1074/mcp.M500097-MCP200. [DOI] [PubMed] [Google Scholar]
- 157.van Balkom BW, et al. Mass spectrometric analysis of the Schistosoma mansoni tegumental sub-proteome. J Proteome Res. 2005;4:958–966. doi: 10.1021/pr050036w. [DOI] [PubMed] [Google Scholar]
- 158.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 159.Braschi S, Curwen RS, Ashton PD, Verjovski-Almeida S, Wilson A. The tegument surface membranes of the human blood parasite Schistosoma mansoni: a proteomic analysis after differential extraction. Proteomics. 2006;6:1471–1482. doi: 10.1002/pmic.200500368. [DOI] [PubMed] [Google Scholar]
- 160.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 161.Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–263. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 162.Willadsen P, et al. Immunologic control of a parasitic arthropod identification of a protective antigen from Boophilus microplus. J Immunol. 1989;143:1346–1351. [PubMed] [Google Scholar]
- 163.Willadsen P. The molecular revolution in the development of vaccines against ectoparasites. Vet Parasitol. 2001;101:353–368. doi: 10.1016/s0304-4017(01)00560-x. [DOI] [PubMed] [Google Scholar]
- 164.Smith TS, Munn EA, Graham M, Tavernor AS, Greenwood CA. Purification and evaluation of the integral membrane protein H11 as a protective antigen against Haemonchus contortus. Int J Parasitol. 1993;23:271–280. doi: 10.1016/0020-7519(93)90150-w. [DOI] [PubMed] [Google Scholar]
- 165.Johnson KS, et al. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature. 1989;338:585–587. doi: 10.1038/338585a0. [DOI] [PubMed] [Google Scholar]
- 166.Lightowlers MW, et al. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol. 1996;18:457–462. doi: 10.1111/j.1365-3024.1996.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 167.Jazwinski SM, Chen JB, Sun J. A single gene change can extend yeast life span: the role of Ras in cellular senescence. Adv Exp Med Biol. 1993;330:45–53. doi: 10.1007/978-1-4615-2926-2_4. [DOI] [PubMed] [Google Scholar]
- 168.Smyth D, McManus DP, Smout MJ, Laha T, Zhang W, Loukas A. Isolation of cDNAs encoding secreted and transmembrane proteins from Schistosoma mansoni by a signal sequence trap method. Infect Immun. 2003;71:2548–2554. doi: 10.1128/IAI.71.5.2548-2554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tran MH, et al. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog. 2010;6:e1000840. doi: 10.1371/journal.ppat.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Da’dara AA, et al. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sciutto E, et al. Renewed hope for a vaccine against the intestinal adult Taenia solium. J Parasitol. 2007;93:824–831. doi: 10.1645/GE-1018R1.1. [DOI] [PubMed] [Google Scholar]
- 172.Zhang W, McManus DP. Vaccination of dogs against Echinococcus granulosus: a means to control hydatid disease? Trends Parasitol. 2008;24:419–424. doi: 10.1016/j.pt.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 173.Lightowlers MW, Mitchell GF, Rickard MD. Cestodes. In: Warren KS, Agabian N, editors. Immunology and Molecular Biology of Parasitic Infections. Cambridge: Blackwell Scientific; 1993. pp. 438–472. [Google Scholar]
- 174.Miller HM, Jr, Gardiner ML. Passive immunity to infection with a metazoan parasite, Cysticercus fasciolaris, in the albino rat. J Prev Med. 1932;6:479–496. [Google Scholar]
- 175.Lightowlers MW. Fact or hypothesis: concomitant immunity in taeniid cestode infections. Parasite Immunol. 2010;32:582–589. doi: 10.1111/j.1365-3024.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rickard MD, Williams JF. Hydatidosis/cysticercosis: immune mechanisms and immunization against infection. Adv Parasitol. 1982;21:229–296. doi: 10.1016/s0065-308x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- 177.Mitchell GF, Rajasekariah GR, Rickard MD. A mechanism to account for mouse strain variation in resistance to the larval cestode, Taenia taeniaeformis. Immunology. 1980;39:481–489. [PMC free article] [PubMed] [Google Scholar]
- 178.Musoke AJ, Williams JF. Immunoglobulins associated with passive transfer of resistance to Taenia taeniaeformis in the mouse. Immunology. 1975;28:97–101. [PMC free article] [PubMed] [Google Scholar]
- 179.Lightowlers MW, Gemmell MA, Harrison GBL, Heath DD, Rickard MD, Roberts MG. Control of tissue parasites. II. Cestodes. In: Yong WK, editor. Animal Parasite Control Utilizing Biotechnology. Boca Raton: CRC Press,Inc; 1992. pp. 171–198. [Google Scholar]
- 180.Musoke AJ, Williams JF. The immunological response of the rat to infection with Taenia taeniaeformis V. Sequence of appearance of protective immunoglobulins and the mechanism of action of 7Sgamma2a antibodies. Immunology. 1975;29:855–866. [PMC free article] [PubMed] [Google Scholar]
- 181.Mitchell GF, Goding JW, Rickard MD. Studies on immune responses to larval cestodes in mice. Increased susceptibility of certain mouse strains and hypothymic mice to Taenia taeniaeformis and analysis of passive transfer of resistance with serum. Aust J Exp Biol Med Sci. 1977;55:165–186. doi: 10.1038/icb.1977.13. [DOI] [PubMed] [Google Scholar]
- 182.Miller HM., Jr Specific immune serums as inhibitors of infections of a metazoan parasite (Cysticercus fasciolaris) Am J Hyg. 1934;19:270–277. [Google Scholar]
- 183.Miller HM. The production of artificial immunity in the albino rat to a metazoan parasite. J Prev Med. 1931;5:429–452. [Google Scholar]
- 184.Rajasekariah GR, Mitchell GF, Rickard MD. Taenia taeniaeformis in mice protective immunization with oncospheres and their products. Int J Parasitol. 1980;10:155–160. doi: 10.1016/0020-7519(80)90028-4. [DOI] [PubMed] [Google Scholar]
- 185.Harrison GB, et al. Identification and cDNA cloning of two novel low molecular weight host-protective antigens from Taenia ovis oncospheres. Int J Parasitol. 1996;26:195–204. doi: 10.1016/0020-7519(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 186.Cox FEG. Milestones in parasitology. Parasitol Today. 1993;9:347–348. [Google Scholar]
- 187.Silverman PH. A technique for studying the in vitro effect of serum on activated taeniid hexacanth embryos. Nature. 1955;176:598–599. doi: 10.1038/176598b0. [DOI] [PubMed] [Google Scholar]
- 188.Heath DD, Smyth JD. In vitro cultivation of Echinococcus granulosus, Taenia hydatigena, T. ovis, T. pisiformis and T. serialis from oncosphere to cystic larva. Parasitology. 1970;61:329–343. doi: 10.1017/s0031182000041184. [DOI] [PubMed] [Google Scholar]
- 189.Heath DD, Lawrence SB. Echinococcus granulosus cysts: early development in vitro in the presence of serum from infected sheep. Int J Parasitol. 1981;11:261–266. doi: 10.1016/0020-7519(81)90035-7. [DOI] [PubMed] [Google Scholar]
- 190.Kyngdon CT, et al. In vitro oncosphere-killing assays to determine immunity to the larvae of Taenia pisiformis, Taenia ovis, Taenia saginata, and Taenia solium. J Parasitol. 2006;92:273–281. doi: 10.1645/GE-619R.1. [DOI] [PubMed] [Google Scholar]
- 191.Lightowlers MW, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol. 1999;29:531–534. doi: 10.1016/s0020-7519(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 192.Gonzalez AE, et al. Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg. 2005;72:837–839. [PubMed] [Google Scholar]
- 193.Flisser A, et al. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect Immun. 2004;72:5292–5297. doi: 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Assana E, et al. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int J Parasitol. 2010;40:515–519. doi: 10.1016/j.ijpara.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lightowlers MW. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol. 2010;40:1183–1192. doi: 10.1016/j.ijpara.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 196.Schantz PM, Cruz M, Sarti E, Pawlowski Z. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ. 1993;27:397–403. [PubMed] [Google Scholar]
- 197.Freedman DO, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 198.Haque R, et al. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009;48:1191–1197. doi: 10.1086/597580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ng CT, Gilchrist CA, Lane A, Roy S, Haque R, Houpt ER. Multiplex real-time PCR assay using Scorpion probes and DNA capture for genotype-specific detection of Giardia lamblia on fecal samples. J Clin Microbiol. 2005;43:1256–1260. doi: 10.1128/JCM.43.3.1256-1260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davids BJ, et al. A new family of giardial cysteine-rich non-VSP protein genes and a novel cyst protein. PLoS One. 2006;1:e44. doi: 10.1371/journal.pone.0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee P, Abdul-Wahid A, Faubert GM. Comparison of the local immune response against Giardia lamblia cyst wall protein 2 induced by recombinant Lactococcus lactis and Streptococcus gordonii. Microbes Infect. 2009;11:20–28. doi: 10.1016/j.micinf.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 202.Rivero FD, Saura A, Prucca CG, Carranza PG, Torri A, Lujan HD. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat Med. 2010;16:551–557. doi: 10.1038/nm.2141. [DOI] [PubMed] [Google Scholar]
- 203.Chen XM, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. N Engl J Med. 2002;346:1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- 204.Carmolli M, et al. Deficient serum mannose-binding lectin levels and MBL2 polymorphisms increase the risk of single and recurrent Cryptosporidium infections in young children. J Infect Dis. 2009;200:1540–1547. doi: 10.1086/606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kirkpatrick BD, et al. Association between Cryptosporidium infection and human leukocyte antigen class I and class II alleles. J Infect Dis. 2008;197:474–478. doi: 10.1086/525284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.White AC., Jr Nitazoxanide: an important advance in anti-parasitic therapy. Am J Trop Med Hyg. 2003;68:382–383. [PubMed] [Google Scholar]
- 207.Benitez AJ, McNair N, Mead JR. Oral immunization with attenuated Salmonella enterica serovar Typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin Vaccine Immunol. 2009;16:1272–1278. doi: 10.1128/CVI.00089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jenkins MC. Present and future control of cryptosporidiosis in humans and animals. Expert Rev Vaccines. 2004;3:669–671. doi: 10.1586/14760584.3.6.669. [DOI] [PubMed] [Google Scholar]
- 209.O’Connor RM, et al. Polymorphic mucin antigens CpMuc4 and CpMuc5 are integral to Cryptosporidium parvum infection in vitro. Eukaryot Cell. 2009;8:461–469. doi: 10.1128/EC.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.O’Connor RM, Wanyiri JW, Cevallos AM, Priest JW, Ward HD. Cryptosporidium parvum glycoprotein gp40 localizes to the sporozoite surface by association with gp15. Mol Biochem Parasitol. 2007;156:80–83. doi: 10.1016/j.molbiopara.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Amebiasis. N Engl J Med. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 212.WHO. Amoebiasis. Wkly Epidemiol Rec. 1997;72:97–99. [PubMed] [Google Scholar]
- 213.Caballero-Salcedo A, et al. Seroepidemiology of amebiasis in Mexico. Am J Trop Med Hyg. 1994;50:412–419. doi: 10.4269/ajtmh.1994.50.412. [DOI] [PubMed] [Google Scholar]
- 214.Braga LL, et al. Seroepidemiology of Entamoeba histolytica in a slum in northeastern Brazil. Am J Trop Med Hyg. 1996;55:693–697. doi: 10.4269/ajtmh.1996.55.693. [DOI] [PubMed] [Google Scholar]
- 215.Haque R, et al. Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect Immun. 2006;74:904–909. doi: 10.1128/IAI.74.2.904-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Barwick RS, Mohammed HO, McDonough PL, White ME. Epidemiologic features of equine Leptospira interrogans of human significance. Prev Vet Med. 1998;36:153–165. doi: 10.1016/s0167-5877(98)00069-5. [DOI] [PubMed] [Google Scholar]
- 217.Petri WA, Jr, Haque R, Mann BJ. The bittersweet interface of parasite and host: lectin–carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu Rev Microbiol. 2002;56:39–64. doi: 10.1146/annurev.micro.56.012302.160959. [DOI] [PubMed] [Google Scholar]
- 218.Boettner DR, et al. Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family. PLoS Pathog. 2008;4:e8. doi: 10.1371/journal.ppat.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA., Jr Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183:1787–1793. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 220.Chaudhry OA, Petri WA., Jr Vaccine prospects for amebiasis. Expert Rev Vaccines. 2005;4:657–668. doi: 10.1586/14760584.4.5.657. [DOI] [PubMed] [Google Scholar]
- 221.Guo X, et al. Protection against intestinal amebiasis by a recombinant vaccine is transferable by T cells and mediated by gamma interferon. Infect Immun. 2009;77:3909–3918. doi: 10.1128/IAI.00487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Scientific Working Group on Leishmaniasis. Meeting report; 2–4 February 2004; Geneva, Switzerland. Available from http://appswhoint/tdr/publications/tdr-research-publications/swg-report-leishmaniasis/pdf/swg_leishpdf. [Google Scholar]
- 223.Murray HW. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob Agents Chemother. 2001;45:2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reed SG, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 225.Scott P, Pearce E, Cheever AW, Coffman RL, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 226.Sypek JP, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper Type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol. 2001;Chapter 19(Unit 19):2. doi: 10.1002/0471142735.im1902s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant poly-protein vaccine that protects against visceral leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Coler RN, et al. Immunization with a poly-protein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70:4215–4225. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Goto Y, et al. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27:2884–2890. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG. Protective immunization against visceral leishmaniasis using leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007;25:7450–7458. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Trigo J, et al. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f + MPL-SE. Vaccine. 2010;28:3333–3340. doi: 10.1016/j.vaccine.2010.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Borja-Cabrera GP, et al. Long lasting protection against canine kalaazar using the FML-QuilA saponin vaccine in an endemic area of Brazil (Sao Goncalo do Amarante, RN) Vaccine. 2002;20:3277–3284. doi: 10.1016/s0264-410x(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 235.Borja-Cabrera GP, et al. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine. 2004;22:2234–2243. doi: 10.1016/j.vaccine.2003.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Greenblatt CL. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- 237.Handman E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev. 2001;14:229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Palatnik-de-Sousa CB. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine. 2008;26:1709–1724. doi: 10.1016/j.vaccine.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 239.Badaro R, et al. Immunotherapy for drug-refractory mucosal leishmaniasis. J Infect Dis. 2006;194:1151–1159. doi: 10.1086/507708. [DOI] [PubMed] [Google Scholar]
- 240.Badaro R, et al. Successful use of a defined antigen/GM-CSF adjuvant vaccine to treat mucosal leishmaniasis refractory to antimony: a case report. Braz J Infect Dis. 2001;5:223–232. doi: 10.1590/s1413-86702001000400008. [DOI] [PubMed] [Google Scholar]
- 241.Reed SG, Coler RN, Campos-Neto A. Development of a leishmaniasis vaccine: the importance of MPL. Expert rev vaccines. 2003;2:239–252. doi: 10.1586/14760584.2.2.239. [DOI] [PubMed] [Google Scholar]
- 242.Campos-Neto A, et al. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a Recombinant Poly-protein Vaccine that protects against visceral Leishmaniasis by the elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–454. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Miret J, et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26:1585–1594. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nascimento E, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 246.Velez ID, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28:329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 247.Skeiky YA, et al. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce Interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 249.Titus RG, Bishop JV, Mejia JS. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006;28:131–141. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 250.Andrade BB, de Oliveira CI, Brodskyn CI, Barral A, Barral-Netto M. Role of sand fly saliva in human and experimental leishmaniasis: current insights. Scand J Immunol. 2007;66:122–127. doi: 10.1111/j.1365-3083.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 251.Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Oliveira F, Jochim RC, Valenzuela JG, Kamhawi S. Sand flies, Leishmania, and transcriptome-borne solutions. Parasitol Int. 2009;58:1–5. doi: 10.1016/j.parint.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mejia JS, Bishop JV, Titus RG. Is it possible to develop panarthropod vaccines? Trends Parasitol. 2006;22:367–370. doi: 10.1016/j.pt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 254.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 255.Belkaid Y, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Volfova V, Hostomska J, Cerny M, Votypka J, Volf P. Hyaluronidase of bloodsucking insects and its enhancing effect on leishmania infection in mice. PLoS Negl Trop Dis. 2008;2:e294. doi: 10.1371/journal.pntd.0000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mesquita RD, et al. Trypanosoma cruzi infection is enhanced by vector saliva through immunosuppressant mechanisms mediated by lysophosphatidylcholine. Infect Immun. 2008;76:5543–5552. doi: 10.1128/IAI.00683-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Caljon G, Van Den Abbeele J, Sternberg JM, Coosemans M, De Baetselier P, Magez S. Tsetse fly saliva biases the immune response to Th2 and induces anti-vector antibodies that are a useful tool for exposure assessment. Int J Parasitol. 2006;36:1025–1035. doi: 10.1016/j.ijpara.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 259.Caljon G, Van Den Abbeele J, Stijlemans B, Coosemans M, De Baetselier P, Magez S. Tsetse fly saliva accelerates the onset of Trypanosoma brucei infection in a mouse model associated with a reduced host inflammatory response. Infect Immun. 2006;74:6324–6330. doi: 10.1128/IAI.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 1999;21:35–44. doi: 10.1046/j.1365-3024.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 261.Schneider BS, Soong L, Zeidner NS, Higgs S. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to sindbis virus infection. Viral Immunol. 2004;17:565–573. doi: 10.1089/vim.2004.17.565. [DOI] [PubMed] [Google Scholar]
- 262.Boppana VD, Thangamani S, Adler AJ, Wikel SK. SAAG-4 is a novel mosquito salivary protein that programmes host CD4 T cells to express IL-4. Parasite Immunol. 2009;31:287–295. doi: 10.1111/j.1365-3024.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tsujimoto H, Gray EW, Champagne DE. Black fly salivary gland extract inhibits proliferation and induces apoptosis in murine splenocytes. Parasite Immunol. 2010;32:275–284. doi: 10.1111/j.1365-3024.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 264.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 265.Ribeiro JM, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6. doi: 10.1186/1471-2164-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Andersen JF, Pham VM, Meng Z, Champagne DE, Ribeiro JM. Insight into the sialome of the Black Fly, Simulium vittatum. J Proteome Res. 2009;8:1474–1488. doi: 10.1021/pr8008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Alves-Silva J, et al. An insight into the sialome of Glossina morsitans morsitans. BMC Genomics. 2010;11:213. doi: 10.1186/1471-2164-11-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.de Moura TR, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis. 2007;1:e84. doi: 10.1371/journal.pntd.0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Valenzuela JG, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Oliveira F, Lawyer PG, Kamhawi S, Valenzuela JG. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLoS Negl Trop Dis. 2008;2:e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Marsollier L, et al. Protection against Mycobacterium ulcerans lesion development by exposure to aquatic insect saliva. PLoS Med. 2007;4:e64. doi: 10.1371/journal.pmed.0040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 273.Okwor I, Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res. 2008;41:123–136. doi: 10.1007/s12026-008-8016-2. [DOI] [PubMed] [Google Scholar]
- 274.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 275.Thiakaki M, Rohousova I, Volfova V, Volf P, Chang KP, Soteriadou K. Sand fly specificity of saliva-mediated protective immunity in Leishmania amazonensis-BALB/c mouse model. Microbes Infect. 2005;7:760–766. doi: 10.1016/j.micinf.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 276.Vinhas V, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. Eur J Immunol. 2007;37:3111–3121. doi: 10.1002/eji.200737431. [DOI] [PubMed] [Google Scholar]
- 277.Gomes R, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci U S A. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Oliveira F, et al. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 279.Collin N, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hotez PJ. The neglected tropical diseases and their devastating health and economic impact on the member nations of the Organisation of the Islamic Conference. PLoS Negl Trop Dis. 2009;3:e539. doi: 10.1371/journal.pntd.0000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hotez PJ. Peace through vaccine diplomacy. Science. 2010;327:1301. doi: 10.1126/science.1189028. [DOI] [PubMed] [Google Scholar]
- 282.Mahoney RT, Maynard JE. The introduction of new vaccines into developing countries. Vaccine. 1999;17:646–652. doi: 10.1016/s0264-410x(98)00246-1. [DOI] [PubMed] [Google Scholar]
- 283.Moran M, Guzman J, Ropars AL, Illmer A. The role of product development partnerships in research and development for negelcted diseases. Int Health. 2010;2:114–122. doi: 10.1016/j.inhe.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 284.Morel CM, et al. Health innovation networks to help developing countries address neglected diseases. Science. 2005;309:401–404. doi: 10.1126/science.1115538. [DOI] [PubMed] [Google Scholar]
- 285.Watson M, Shaw D, Molchanoff L, McInnes C. Challenges, lessons learned and results following the implementation of a human papilloma virus school vaccination program in South Australia. Aust N Z J Public Health. 2009;33:365–370. doi: 10.1111/j.1753-6405.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 286.Andrus JK, de Quadros C, Matus CR, Luciani S, Hotez P. New vaccines for developing countries: will it be feast or famine? Am J Law Med. 2009;35:311–322. doi: 10.1177/009885880903500204. [DOI] [PubMed] [Google Scholar]
- 287.Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions”: how a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]