To the Editor:
Chronic rhinosinusitis (CRS) significantly affects the quality of life of elderly people.1 However, the inflammatory mechanisms of CRS in the elderly have not been well studied. CRS is commonly divided into 2 subtypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).2 The inflammatory response in patients with CRSsNP has been shown to have a tendency toward TH1 polarization, whereas CRSwNP inflammatory responses are characterized by eosinophilia with a TH2 skewing.3 Recently, there has been increasing evidence that CRS and some allergic diseases might be linked to deficiencies in the barrier function of the skin or airway mucosal epithelium.4 The objective of the present study was to evaluate whether there are age-related differences in clinical presentation and/or immunologic markers in patients with CRS.
In the first part of the study, a retrospective chart review of 252 patients with CRS who visited the allergy and otolaryngology clinics at Northwestern University between January 2007 and May 2009 was performed to investigate age-related differences in general clinical characteristics. All subjects met the criteria for CRS as defined by nationally recognized consensus statements.2 The presence of CRS or NP was confirmed by office endoscopy and sinus computed tomography (CT) scans. The Lund-MacKay sinus CT scoring system was used as an objective measure of the severity of the disease.5 In the second part of the study, we obtained nasal lavage samples from 58 patients with CRSwNP, 54 patients with CRSsNP, and 50 healthy controls who consented for nasal lavage, and investigated immunologic markers. Details of the subjects’ characteristics are given in Table E1 (in this article’s Online Repository at www.jacionline.org). Subject numbers for ELISA values vary because of lack of sample availability for some subjects. Any patients who had taken oral or nasal steroids within a month prior to sample collection, had prior sinus surgeries, aspirin-exacerbated respiratory disease, or immunodeficiency were excluded from this study. This study was approved by the Institutional Review Board of Northwestern University. All statistical analyses were performed with Graph-Pad Prism version 5 (GraphPad Software, La Jolla, Calif), and ELISA data were analyzed by using the Mann-Whitney U test. A P value of less than .05 was considered statistically significant.
When the patients with CRS were divided into nonelderly (16–59 years) and elderly (60–77 years) groups, NP and asthma tended to be more prevalent in elderly patients with CRS compared with nonelderly patients, although this trend was not statistically significant (Table I). CT scores of the elderly group were significantly higher than those of the nonelderly group.
TABLE I.
Demographic and clinical characteristics of 252 patients with chronic rhinosinusitis
| Nonelderly (n = 230) |
Elderly (n = 22) |
P value | |
|---|---|---|---|
| Male/female | 124/106 | 13/9 | NS |
| Age (y), median (range) | 41 (16–59) | 67 (60–77) | |
| Nasal polyps, n (%) | 91 (39.6) | 15 (68.2) | NS |
| Asthma, n (%) | 65 (28.3) | 10 (45.5) | NS |
| Number of skin test positive/performed (%) |
74/93 (79.6) | 5/6 (83.3) | NS |
| CT score,* mean ± SD | 10.9 ± 5.4 | 13.7 ± 6.3 | <.05 |
CT, Computed tomography; NS, not significant.
Lund-Mackay CT Scoring System: Each sinus region is given a numeric score: 0, no opacification; 1, partial opacification; and 2, total opacification. The frontal, maxillary, anterior ethmoid, posterior ethmoid, and sphenoid sinuses are graded separately, and the ostiomeatal complex is also scored, for a total possible score of 24.
To evaluate the underlying immunologic mechanism of these clinical differences, we measured by using ELISA kits the levels of eosinophil cationic protein (ECP) (MBL International, Woburn, Mass), human neutrophil elastase (HNE) (Cell Sciences, Canton, Mass), S100A7 (psoriasin), S100A8/9 (calprotectin) (MBL International), IgA (Bethyl Laboratories, Montgomery, Tex), B-cell–activating factor of the TNF family (BAFF) (R&D Systems, Minneapolis, Minn), and IL-6 (BD Sciences, Franklin Lakes, NJ) in nasal lavage samples from healthy controls and from patients with CRS.
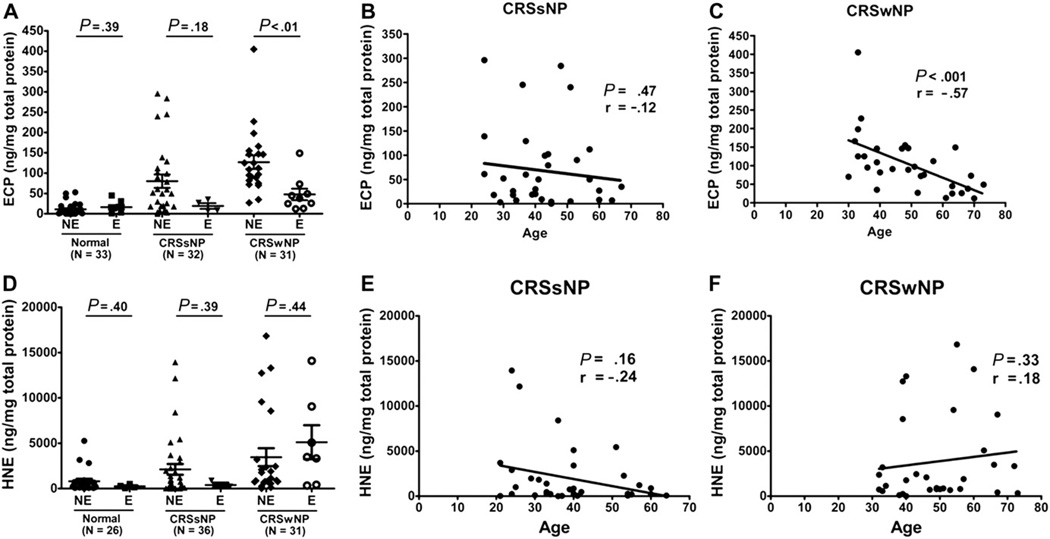
ECP is an important marker of eosinophilic inflammation in CRS, especially CRSwNP. In patients with CRSwNP, ECP levels in nonelderly patients were significantly higher compared with those in elderly patients (Fig 1, A). When we performed a linear regression analysis, there was a significant negative correlation between ECP levels and age in patients with CRSwNP (r = −.57; P < .001) but not in patients with CRSsNP (Fig 1, B and C). We also performed eosinophil counts in NP tissue (data not shown). Interestingly, there was no significant correlation between age and eosinophil count in NP, suggesting that infiltrating eosinophils in NP from elderly patients may produce less ECP.
FIG 1.
Age-related distribution of the ECP and HNE protein levels in nasal lavage fluids from normal subjects and from patients with CRS as determined by ELISA. A, In the CRSwNP subtype, ECP levels in elderly patients were significantly lower compared with those in nonelderly patients. B and C, There was a significant negative correlation between ECP levels and age in patients with CRSwNP, but not in patients with CRSsNP. D–F, The HNE levels from normal subjects and from patients with CRS were not different by age, and there was no correlation between HNE levels with aging. E, Elderly; NE, nonelderly.
We next analyzed a marker of neutrophilic inflammation, HNE, in nasal lavage samples. HNE levels in elderly subjects were not significantly different from those in nonelderly subjects (Fig 1, D), and there was no correlation between HNE levels and age in either subtype of CRS (Fig 1, E and F). There was no significant age-related difference in ECP and HNE levels in normal subjects (see Fig E1 in this article’s Online Repository at www.jacionline.org).
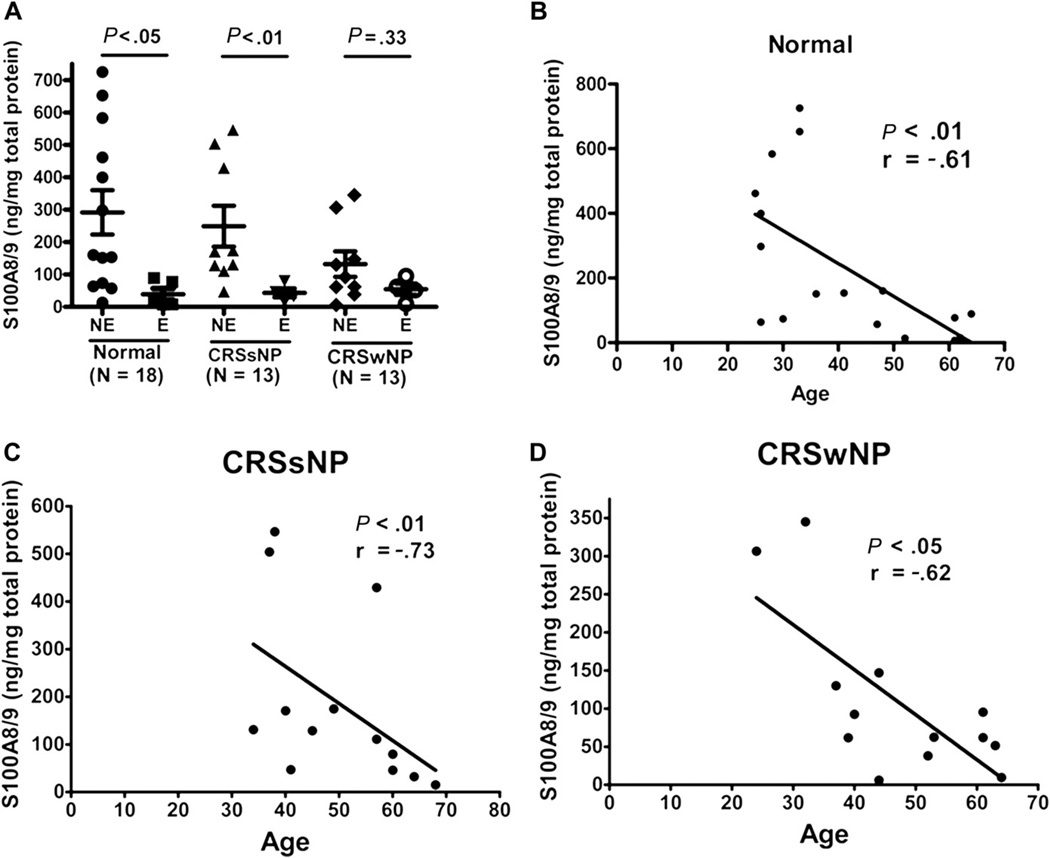
S100A7 and S100A8/9, members of the epidermal differentiation complex, are known to be important in epithelial barrier function. We previously reported that the expression of S100 proteins is reduced in the epithelium and nasal lavage fluid from patients with CRS.6 Levels of S100A8/9, but not S100A7, were significantly lower in elderly patients than in nonelderly patients with CRSsNP (Fig 2, A) and inversely correlated with increasing age in both subtypes of CRS (Fig 2, C and D). This finding of diminished S100A8/9 levels in elderly subjects was also observed in normal subjects (Fig 2, A and B), suggesting that the decline in S100A8/9 levels may be a part of the aging process. There were no significant age-related differences in the expression of other inflammatory markers such as IL-6, IgA, and BAFF (data not shown).
FIG 2.
Age-related distribution of the human S100A8/A9 protein levels in nasal lavage fluids from normal subjects and from patients with CRS as determined by ELISA. A, In normal controls and patients with CRSsNP, S100A8/9 levels in elderly subjects were significantly lower compared with those in nonelderly subjects. B–D, Levels of S100A8/A9 were significantly diminished with increasing age in normal controls and patients with both subtypes of CRS. E, Elderly; NE, nonelderly.
Our findings suggest that the impairment of epithelial barrier function persists and may worsen with age while eosinophilic inflammation appears to wane with age in the natural history of CRS. Severe disease is known to be associated with increased eosinophilic inflammation and high ECP level in patients with CRS.7 However, our results showed that ECP levels of elderly patients with CRSwNP were decreased compared with those of nonelderly patients despite a higher CT score and more NP formation in elderly patients. There are various age-related changes that occur in the human immune system.8 Immunosenescence affects various cell types in the bone marrow and the thymus, along with various components of the innate immune system. Aging is also associated with a decreased function of epithelial barriers of the skin and lungs. In this study, levels of S100A8/A9, but not S100A7, were diminished in association with the aging process. S100A7 has antimicrobial activity against Escherichia coli. On the other hand, S100A8/A9 has antimicrobial activity against Staphylococcus aureus, Klebsiella species, and fungi.9 One of the clinical implications of this difference could relate to potential alteration of the microbiome or pathogens in elderly patients with CRS.
In summary, this study demonstrates that despite the higher CT score and more NP formation observed in elderly patients, they had lower ECP levels than did nonelderly patients with CRS. In addition, S100A8/9 level was significantly decreased in CRS with the aging process. These results suggest that epithelial barrier dysfunction may continue to play an important role in the pathogenesis of CRS while eosinophilic inflammation may subside with age.
Supplementary Material
Acknowledgments
S.H.C. has received grant support from the AAAAI-Geriatrics Junior Faculty Development Award and Earnest Bazley Fund, and R.P.S. and R.C.K. have received grant support from the National Institutes of Health (grant no. 2 R01 HL078860).
Footnotes
Disclosure of potential conflict of interest: R. C. Kern and R. P. Schleimer received research support from the National Institutes of Health. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2004;193:3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:S155–S212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Tieu DD, Peters AT, Carter RG, Suh L, Conley DB, Chandra R, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–675. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lund VJ, Kennedy DW. Quantification for staging sinusitis: the staging and therapy group. Ann Otol Rhinol Laryngol. 1995;167:S17–S21. [PubMed] [Google Scholar]
- 6.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B-cell activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–1392. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Lorenzo G, Drago A, Esposito Pellitteri M, Candore G, Colombo A, Gervasi F, et al. Measurement of inflammatory mediators of mast cells and eosinophils in native nasal lavage fluid in nasal polyposis. Int Arch Allergy Immunol. 2001;125:164–175. doi: 10.1159/000053811. [DOI] [PubMed] [Google Scholar]
- 8.Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010;126:690–699. doi: 10.1016/j.jaci.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross KF, Herzberg MC. Calprotectin expression by gingival epithelial cells. Infect Immun. 2001;69:3248–3254. doi: 10.1128/IAI.69.5.3248-3254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.