Abstract
Diet, nutritional status, and certain dietary supplements are postulated to influence the development and progression of prostate cancer. Angiogenesis and inflammation are central to tumor growth and progression, but the effect of diet on these processes remains uncertain. We explored changes in 50 plasma cytokines and angiogenic factors (CAFs) in 145 men with prostate cancer enrolled in a pre-operative, randomized controlled phase-II trial with four arms: control (usual diet); low-fat (LF) diet; flaxseed-supplemented (FS) diet; and flaxseed-supplemented, low-fat diet. The mean duration of dietary intervention was 30–31 days. Among the individual arms, the largest number of significant changes (baseline vs pre-operative follow-up) was observed in the LF arm, with 19 CAFs decreasing and one increasing (p<.05). Compared to the control arm, 6 CAFs—including pro-angiogenic factors (stromal-cell derived-1α and myeloid factors (granulocyte-colony-stimulating factor, macrophage colony-stimulating factor — all decreased in the LF arm compared to controls; 3 and 4 CAFs changed in the FS and FS+LF arms, respectively. Weight loss occurred in the LF arms and significantly correlated with VEGF decreases (P <0.001). The CAFs that changed in the LF arm are all known to be regulated by nuclear factor-kappa B (NF-κB), and a pathway analysis identified NF-κB as the most likely regulatory network associated with these changes in the LF arm, but not in the FS-containing arms. These results suggest that a low-fat diet without flaxseed may reduce levels of specific inflammatory cytokines and angiogenic factors and suggests that the NF-κB pathway may be a mediator of these changes.
Keywords: angiogenesis-inducing agents, cytokines, prostatic neoplasms, diet, biomarkers
Introduction
Aside from non-melanoma skin cancer, prostate cancer is the most common cancer among men in the United States, with an estimated 217,730 new diagnoses in 2010 (1). Gathering evidence suggests that a plant-based, low-fat diet inhibits prostate cancer development and progression (2–4). Furthermore, obesity has been associated with an increased risk of high-grade, but a decreased risk of low-grade, prostate cancer (5).
A limited number of randomized clinical trials have suggested that low-fat diets and certain dietary supplements may inhibit prostate cancer development and progression and improve clinical outcomes in prostate cancer patients (6). In a small proof-of-concept randomized clinical trial, dietary fat restriction altered serum fatty acid levels, and decreased growth of LNCaP cancer cells treated with the collected patients’ sera (7). Specific mechanisms linking a high-fat diet with prostate cancer remain unclear; although previous studies have implicated NF-κB, a transcription factor whose activation has been linked to cancer. In a mouse model of prostate cancer, nuclear factor-kappa B (NF-κB) was found to be up-regulated in a moderate fat/carbohydrate group and these mice had shorter survival than mice on a high-fat/carbohydrate-free diet or a low-fat/high-carbohydrate diet (8).
NF-kb appears to be a key regulator energy balance and inflammation. Obesity and a diet high in fat have been found to induce an inflammatory response mediated by NF-κB (9, 10). Studies in mouse models of obesity show that a high-fat diet can increase NF-κB activation, which in turn leads to a sustained elevation of inhibitory κB (IκB) kinase ε (IKKε) levels in liver, adipocytes, and adipose-tissue macrophages, resulting in chronic low-grade inflammation and also increased levels of circulating pro-inflammatory cytokines (11, 12). Consistent with these observations, clinical studies have shown that a high-fat meal can lead to increased NF-κB activation from peripheral blood mononuclear cells and that obese individuals express higher levels of NF-κB (versus leaner individuals) (13). This is accompanied by higher circulating levels of proinflammatory cytokines and angiogenic factors (CAFs) such as tumor necrosis factor α (TNFα), interleukin 6 (IL-6), macrophage migration inhibitory factor (MIF), and matrix metallopeptidase 9 (MMP-9) (12, 13). Most recently, increased activation of NF-kB, along with elevated levels of proinflammatory TNF-α, IL-1β and COX-2, was detected in the mammary gland and visceral fat in both dietary (high fat diet) and genetic obesity mouse models (14). This study suggests that NF-κB is an important mediator of an inflammatory response following increased saturated fatty acid exposure and may contribute to breast cancer development through an inflammatory mediated induction of aromatase, which is involved in the biosynthesis of estrogens (14, 15). Taken together, these studies provide evidence of a linkage between obesity and an NF-κB-dependent inflammatory response that may ultimately contribute to the tumorigenic process.
Cytokines associated with obesity or secreted by adipose tissue may play a significant role in a sustained systemic state of inflammation which has been shown to increase the risk for the development of cancer (18–22). Additionally, tumor necrosis factor-alpha (TNF-α), which has been shown to contribute to insulin resistance in obesity and obesity-linked type-2 diabetes, induces several NF-kB-activating transcription factors and genes as well as other genes involved in cell growth, proliferation and inflammation (23, 24). Taken together, these findings potentially link obesity to upregulation of NF-kB-regulated pro-inflammatory CAFs that lead to a variety of adverse medical conditions, including increased risk for prostate cancer.
Other dietary changes including a diet high in lignans, such as traditional plant-based diets, have also been postulated to lower the incidence of prostate cancer (25). The most-abundant source of dietary lignans is flaxseed. Once consumed, the plant lignans are converted by the intestinal microflora to the two major mammalian estrogenic enterolignans: enterodiol and enterolactone (26). Both of these enterolignans are able to reduce cell viability of prostate cancer cells (27–29). Furthermore, flaxseed is a rich source of omega-3 fatty acids, which are also thought to inhibit neoplasia and to do so potentially through several mechanisms including modulation of the synthesis of prostaglandins and thromboxanes.
Previously, we conducted a randomized controlled phase II trial (RCT) of flaxseed supplementation (30 g of ground flaxseed/day) and/or a low-fat diet (fat intake < 20% of total energy intake) in prostate cancer patients (30). While non-significant trends toward lower Ki-67 proliferation rates were found with the low-fat diet, significantly lower proliferation rates were observed in both flaxseed arms. However, this effect was not accompanied by reductions in serum prostate-specific antigen (PSA), testosterone, sex hormone-binding globulin (SHBG), insulin-like growth factor (IGF)-1, or IGF binding protein-3 (IGFBP-3), which were hypothesized to be the responsible mechanisms of action.
Given the emerging associations between dietary factors, inflammation and prostate cancer progression, and the lack of understanding regarding the potential mechanisms by which these factors may be linked, in this study we have explored changes in circulating inflammatory cytokines and angiogenic factors occurring in men with prostate cancer following low-fat or flaxseed-supplemented diets.
Materials and Methods
Patients and study design
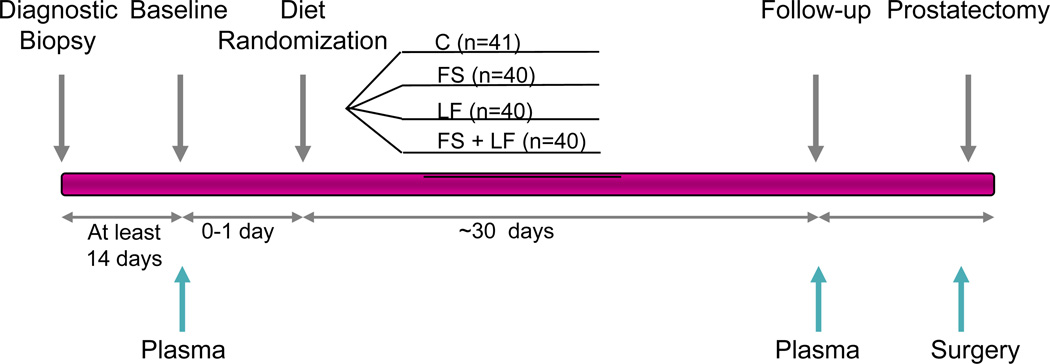
The methods and outcomes of this phase II RCT have been reported previously (30, 31). Briefly 161 men with clinically-confirmed prostate cancer who were scheduled for radical prostatectomy were randomized to one of the following conditions: usual diet (control); flaxseed (FS)-supplemented diet (30g/d); low-fat (LF) diet (fat intake less than 20% of total energy); or flaxseed-supplemented (30g/d), low-fat diet (fat intake less than 20% total energy) (FS+LF) (Figure 1). All participants adhered to the dietary regimen for at least 21 days, and on average for 30 days, prior to their scheduled surgery. The primary endpoint was tumor proliferation as determined by Ki-67 staining, and major secondary endpoints were the rate of apoptosis and expressions of a number of biomarkers associated with both prostate-cancer growth and dietary status. The study protocol was approved by the Institutional Review Board of Duke University Medical Center and sites associated with the University of Michigan Community Clinical Oncology Program. Written informed consent was obtained on all study participants. As noted in the main outcomes paper, proliferation rate significantly decreased in the flaxseed arms; however significant weight loss, as well as a decrease in serum cholesterol was noted in men assigned to the low-fat diet condition.
Fig. 1.
Clinical Trial Design Scheme of randomized, controlled trial of low-fat and/or flaxseed supplemented diets in men with prostate cancer (30). Prostate cancer patients were randomly assigned to control (C), flaxseed-supplemented diet (FS), low-fat diet (LF), or flaxseed-supplemented, low-fat diet (FS+LF). The blue arrows indicate the collection of plasma samples, collected at the prestudy evaluation and at follow-up (within 3 days of surgery) and prostate tissue (collected at least 14 days before baseline and during the prostatectomy).
Immunohistochemistry
The nuclear localization of p65 and the expression of VEGF and COX-2, were evaluated using an immunohistochemical method described previously (32). In brief, tumor samples were fixed with paraformaldehyde and embedded in paraffin. After being washed in PBS, the slides were blocked with protein block solution (DakoCytomation) for 20 min and incubated overnight with rabbit polyclonal anti-human p65 (Abcam, Cambridge, MA) and mouse monoclonal anti-human VEGF (Santa Cruz Biotechnology, Santa Cruz, CA) and COX-2 (Cayman Chemical Company, Ann Arbor, MI) (1:400, 1:100, and 1:200, respectively). Slides were washed and incubated with biotinylated link universal antiserum, followed by horseradish peroxidase-streptavidin conjugate (LSAB + kit). The slides were rinsed, and color was developed using 3, 3-diaminobenzidine hydrochloride as a chromogen. Finally, sections were rinsed in distilled water, counterstained with Mayer's hematoxylin, and mounted with DPX mounting medium for evaluation. Expression levels of p65, VEGF and COX-2 were determined by counting at least 500 tumor cells in 10 representative high-power fields and the percentage of p65 (nuclear), and the expression of VEGF and COX-2 were calculated in all cases. The percent positive cells were then represented graphically for each marker.
Plasma sample collection and analysis
Blood samples were collected in Vacutainer tubes (Becton Dickinson, San Jose, CA) containing EDTA anticoagulant and centrifuged at 1,500 × g for 10 minutes at 4°C within 30 minutes of collection. The plasma was placed in prelabelled cryovials and stored at −80°C until analysis. A total of 50 CAFs were measured using multiplexed bead suspension arrays (MBA) as previously described (33, 34) Samples were thawed overnight at 4°C prior to analysis, centrifuged at 1,500 × g to remove debris, and then aliquoted and analyzed in duplicate by MBA. Baseline and follow-up samples from a given patient were assessed simultaneously on the same plate to minimize the potential impact of interassay variability.
Statistical methods
All statistical analyses were performed using R (http://www.r-project.org) and SAS, Statistical Analysis System version 9.1 (SAS Institute, Cary, NC). Pre-post samples existed on 145 study participants consisting of 37 controls, 40 FS, 33 LF, and 35 FS+LF. Participants who were taking steroids during the study period (n=5) were removed from the analysis to reduce potential confounding effects that such agents or the conditions for which such agents are prescribed might have on inflammatory markers. The percent change from baseline was defined as the percentile of the median at follow-up divided by the median at baseline; significance was defined by p<0.05 by paired t-tests for each arm. For the primary analysis of CAF changes within arms, an adjustment for multiple comparisons (Bonferroni correction) was performed (Table 1). Two sample t-tests were used to identify significant changes in CAFs between the control arm and each of the modified diet conditions using change scores (the difference between baseline and follow-up). To produce heatmaps, the CAF change data was normalized by computing the z-score (subtracting by the average and dividing by the standard error). Common observations across 6 markers were used to generate the heat-map. Pathway analysis was conducted on the markers observed to change significantly in the modified diet arms; since this was an exploratory analysis, an alpha level of < 0.10 was used. The dataset containing the gene identifiers along with the corresponding mean differences was loaded into the Ingenuity Pathway Analysis program (Ingenuity Systems, Redwood City, CA). Given that significant weight loss was observed in the low-fat arms, and body weight status has been associated with prostate cancer progression, linear models were used to explore significant associations between changes in marker scores and changes in Body Mass Index (BMI). The correlation between changes in CAFs and BMI were calculated using Pearson correlation estimates and were graphed using scatter plots.
Table 1. Changes in cytokines and angiogenic factors (CAFs) during time on study within treatment arms.
Shown are CAFs with changes within an arm (follow-up vs baseline) with a p value less than 0.05 after adjusting for multiple testing (Bonferroni correction).
| Control | Low-Fat (LF) | Flaxseed Supplemented (FS) |
FS + LF |
|---|---|---|---|
| b-NGF ICAM-1* IFN-α2 IL-1a IL-2RA IL-3 IL-12 (p40) MCP-3 SCF SCGF-β TRAIL |
b-NGF Eotaxin GRO-α HGF ICAM-1* IFN-α2* IL-1a* IL-2RA* IL-3 IL-12 (p40) IL-16 IL-18 MCP-3 M-CSF* PDGF-BB SCF* SCGF-β SDF-1α* TNF-β* TRAIL |
ICAM-1* IL-1a IP-10 LIF MCP-3 PDGF-BB SCGF-β TRAIL TNF-α |
Eotaxin ICAM-1* IFN-α2 IL-1a IL-4 IL-16 IL-18 MCP-3 SCGF-β TNF-β* |
All are p<0.05 except as denoted by * p<0.001
Abbreviations:β-NGF beta-nerve growth factor, CAF cytokines and angiogenic factors, GRO-α growth-regulated protein-alpha, HGF hepatocyte growth factor, ICAM-1 intercellular adhesion molecule-1, IFN interferon, IL interleukin, IL-1RA interleukin-1 receptor antagonist, IL-2RA interleukin-2 receptor alpha, LIF leukemia inhibitory factor, MCP macrophage chemoattractant protein, M-CSF macrophage colony-stimulating factor, PDGF platelet-derived growth factor, SCF stem cell factor, SCGF-β stem cell growth factor-beta, SDF-1α stromal cell-derived factor-1alpha, TNF tumor necrosis factor, TRAIL TNF-related apoptosis-inducing ligand, VCAM-1 vascular cell adhesion molecule-1
Results
Patients
Of the 161 patients enrolled in this trial, a majority were over the age of 65 (74%) and were overweight (46%) or obese (35%). Most of the patients had earlier-stage disease with biopsy Gleason sums of 6 or less (66%). The average duration on the diet was 30–31 days. Baseline and follow-up (pre-surgery) plasma samples were available on 145 patients. An overview of the clinical trial design and the collection times for plasma and prostatic tissue is shown in Figure 1.
CAF changes within individual arms
We initially assessed changes in CAFs (preoperative follow-up vs. baseline) in each arm individually, and then compared CAF changes in the three intervention arms with those in the control arm. For the individual arms, CAFs that demonstrated statistically significant change (p<0.05) or a borderline trend towards change (p<0.1) between baseline and follow-up in each arm are shown in the Supplemental Table 1. The largest number of significant changes occurred in the LF arm (N=20), with all but one (PDGF-BB) decreasing, whereas 11, 9, and 10 CAFs changed significantly within the control, FS, and LF+FS arms, respectively. These changes remained significant when adjusted for multiple comparisons (Bonferroni correction), shown in Table 1. Many of these decreases were modest in magnitude, with ≥10% change noted for 9 CAFs in the LF arm vs two CAFs in the control arm and four in other two arms. CAFs decreasing in the LF arm included pro-angiogenic factors such as hepatocyte growth factor (HGF) and stromal-cell derived-1α SDF-1α) and inflammatory cytokines, including several involved in myeloid recruitment and proliferation such as macrophage-CSF (M-CSF), growth-regulated oncogene α (GRO-α), and eotaxin. There was also an observed decrease in the pro-inflammatory cytokine IL-10.
Comparison of changes in CAFs between the arms
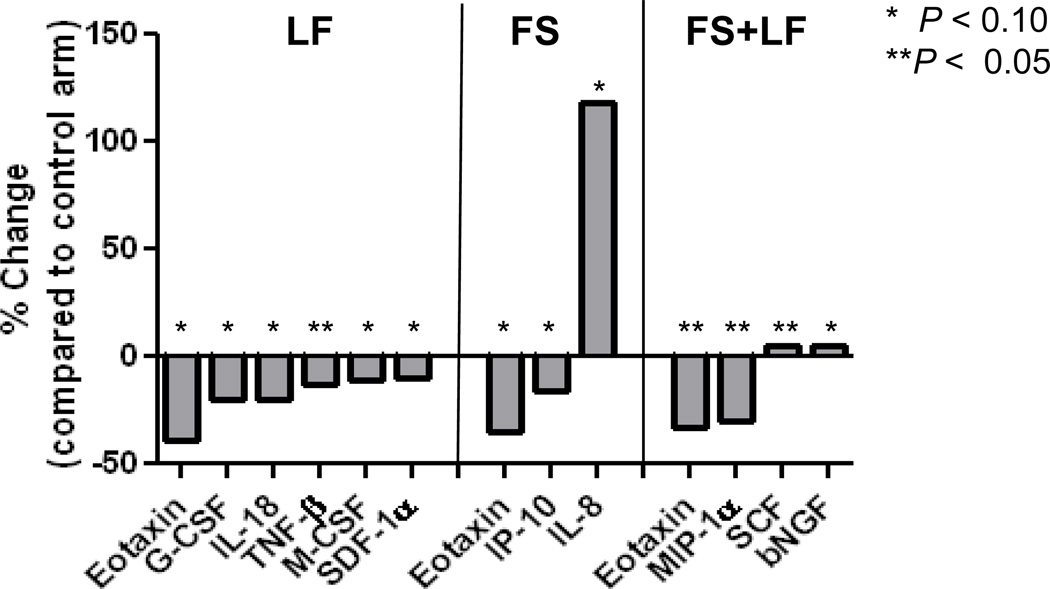
We then compared CAF changes for each analyte in the intervention arms with the changes for the same analyte in the control arm (i.e. change for analyte in control minus the change for the analyte in the intervention arm). As compared to the control, men in the LF arm experienced significantly greater decreases in 6 CAFs (Figure 2). Decreases in the mean difference between baseline and follow-up compared to the control were observed in the LF arm for the pro-angiogenic factors SDF-1α; the hematopoietic growth factor G-CSF (granulocyte colony-colony stimulating factor) as well as cytokines with roles in cell differentiation and recruitment of myeloid cells including eotaxin, macrophage-CSF (M-CSF), respectively. Decreases were also detected in the immunosuppressor TNF-β, which has been proposed to provide tumors with escape from immune surveillance (35).
Fig. 2.
Distinctive CAF changes following low-fat and flaxseed supplemented diet. The percent change in mean differences between the control arm and the treatment arm are shown. CAF mean differences were derived for each participant by subtracting the baseline from the follow-up for that biomarker.
By contrast, in the FS arm, only three CAFs changed significantly compared to the control arm, an increase in IL-8 and a decrease in eotaxin and IP-10 were observed (Figure 2).
When the combination arm, FS+LF, was compared to the control arm, the decrease in eotaxin continued to be observed, along with MIP1-α. However, two cytokines showed a significant increase, the hematopoietic growth factor SCF and the neurotrophic growth factor b-NGF (Figure 2).
Coordinate changes in CAFs in patients following a low-fat diet and their association with the NF-κB pathway
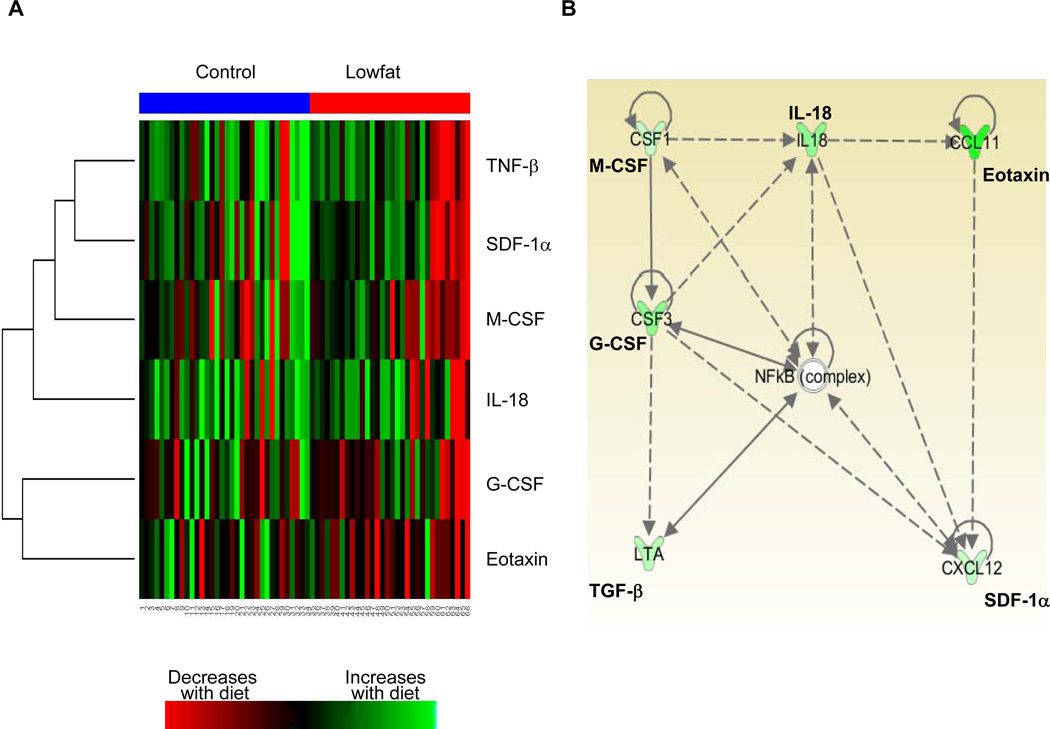
To gain a better understanding of the relationship between changes in the different CAFs, we performed hierarchical clustering analysis of the 6 CAFs previously identified to have significant changes between the control and the LF arm (Fig 3A). Each row represents the mean difference of each CAF from the control with each column representing a patient in either the control group (blue color) or the low-fat group (red color). Red denotes decreases in CAF concentration at follow-up compared to control and green indicates increased concentration at follow-up. Changes in many of these cytokines appeared to be coordinated with each other within a given patient, with a majority of these factors decreasing in the LF arm compared to the control arm. This suggests that the coordinate changes in these cytokines may potentially be regulated by common mechanism(s). To identify potential regulators of these 6 CAFs, we conducted an Ingenuity Pathway Analysis which revealed a network of significant relationships between these factors (Fig 3B). This analysis identified the NF-κB complex as a central node. NF-κB was not identified as a central node for the FS-containing arms (data not shown).
Fig. 3.
Unsupervised clustering identified coordinate changes in 6 CAFs in the LF arm. A. Relative mean differences of 6 CAFs are shown (red indicates high levels, green indicates low levels). CAF levels from patients in the control arm are grouped to the left under the blue bar and patients in the LF arm are under the red bar. B. Functional interacting network among the identified CAFs that changed in the LF arm compared to control. Pathway analysis was performed on 6 CAFs that significantly changed in the LF arm compared to the control arm (P<0.1) and the identified network is depicted. The genes that relate to our CAF dataset and falling in this network are green (down regulated) with the intensity of the color indicating the degree change.
Correlation between weight loss and decreases in serum cholesterol and CAF changes
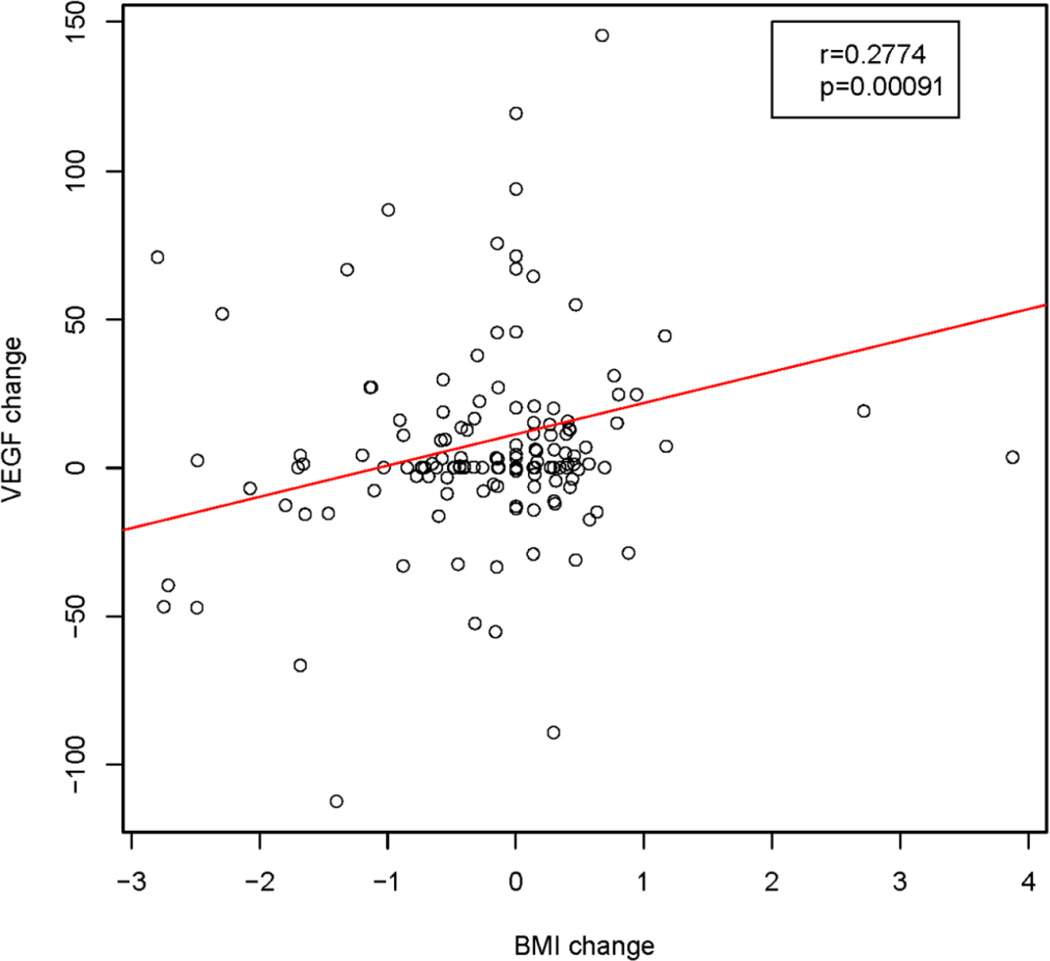
Given that the larger decreases in CAFs may have been mediated by weight loss, and also may be associated with serum lipids, we explored these associations (30), and detected a significantly positive association between the change in BMI and the change in VEGF (p=0.0009) as seen in Figure 4 and significant negative associations (P<0.05) with six other factors including the TNF-related apoptosis inducing ligand, TRAIL and IL-2RA (Supplemental Table 2). These three CAFs were among those for which the CAF changes were significantly associated with changes in cholesterol levels (Supplemental Table 3), suggesting a potential impact of the low fat diet. We also assessed the association between CAF and PSA and observed that changes in several members of the interleukin family (IL-1b, −4, −7, −9) as well as interferon (IFN)-γ were positively correlated with PSA changes (p<0.05 for all; Supplemental Table 4).
Fig. 4.
Correlation between CAFs and body mass index. Correlation scatterplot of changes in concentrations of VEGF correlating with changes in body mass index (BMI). The correlation was tested by Pearson’s correlation.
Tumor expression of angiogenic and inflammatory markers
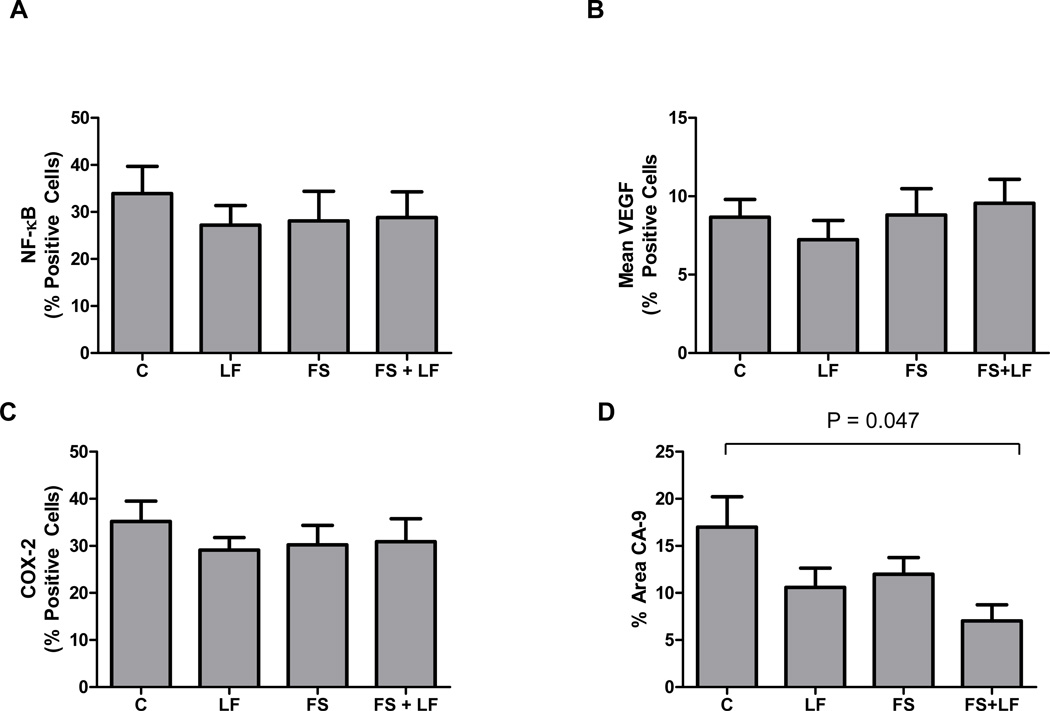
We analyzed protein levels of VEGF and markers for potential angiogenic factors and cytokine regulatory pathways including NF-κB, COX-2, and the hypoxia-inducible marker CA-9, in a limited subset of post-surgical prostate cancer specimens (N=40) by immunohistochemistry. VEGF was not reduced in the FS arm, but the other markers showed a non-significant trend (Fig. 5). Because sufficient pre-study biopsy specimens were not available, it was not possible to investigate tumor changes for individual patients during the study.
Fig. 5.
Changes in expression of tumor correlative markers in prostate cancer after diet modulation. The ability of LF diet, FS diet or the combination diet to affect changes in the prostate was determined by immunohistochemical staining for (A) VEGF, (B) CA-9, (C) NF-κB and (D) COX-2. Data are graphed as percent positive cells, *p<0.01, (t-test).
Discussion
This secondary analysis of 50 CAFs was undertaken to explore potential mechanisms by which the low fat diet or flaxseed supplementation may influence prostate cancer development and progression. The results suggest that while flaxseed supplementation influenced a few CAFs, the low-fat diet appeared to exert a greater impact and decreased levels of a number of circulating proinflammatory CAFs. In the LF arm, significant changes were observed for 6 CAFs, the majority of which were pro-inflammatory factors, involved in the myeloid (e.g., G-CSF, M-CSF, and eotaxin) or pro-angiogenic factors (e.g., SDF-1α). In comparison, in the combination arm (FS+LF) we only detected significant decreases in eotaxin and MIP-1α and increases in SCF and b-NGF. It is not known whether these changes are primarily due to the low fat diet itself, weight loss, or some other factor (s). The fact that we observed such a strong correlation between changes in BMI and changes in VEGF suggests that weight loss or low fat diet may impact inflammatory and angiogenic responses.
Moreover, the decreases in the six CAFs in the LF arm appeared to occur in a coordinated manner, suggesting that the expression of some or all of these CAFs may be governed by a common regulatory mechanism. Pathway analysis identified the NF-κB pathway as a signaling node that interacted with a majority of the CAFs that decreased in the LF arm. Each of these factors has previously been reported to be regulated by NF-κB (10, 36). In addition, the immunohistochemical analysis of a subset of prostatectomy tumor specimens revealed a trend toward lower levels of NF-κB and COX-2 expressions. For VEGF, lower expression was observed for the LF arm as compared to controls. Meanwhile, a slight increase in VEGF expression was observed for the FS and FS+LF arms as compared to the control. These findings are consistent with the decreased levels of proangiogenic factors detected in the plasma of these patients. Caution however is necessary in interpreting these findings since this analysis was highly exploratory and only was performed on a limited number of specimens. Thus, further study is needed.
NF-κB, a central mediator of the inflammatory response, angiogenesis, and invasion is constitutively activated in many cancers (35, 37–39). Several recent studies have indicated that the NF-κB pathway plays a critical role in regulating energy balance and provides a link between diet and inflammation. A high-fat diet has been demonstrated to increased NF-κB activation and further, knockout of the NF-κB target, IKKε, prevented diet-induced obesity and chronic inflammation in mouse models (11, 14, 40). Observations from a another study found that human subjects experience increases in NF-κB activation in peripheral blood mononuclear cells within hours of eating a high-fat meal (41). In light of these previous studies findings and the current study’s findings that the majority of CAFs that decreased in patients in the LF arm were known to be NF-κB-regulated, our data suggest that a LF diet induced downregulation of the NF-κB pathway may have contributed to changes in circulating proinflammatory and angiogenic CAFs. However, not all known NF-κB-regulated factors (e.g., TNF-α and IL-6) decreased between baseline and preoperative follow-up (just prior to prostatectomy) in the LF arm, indicating that other pathways known to impact inflammatory and angiogenic pathways, such as the COX-2 and hypoxia-inducible factor-1α (HIF-1α) pathways, may have contributed to the changes we observed (42).
The results of this study raise several questions when compared to the previously published analysis of prostate cancer specimens, in which tumors from men on the flaxseed arm had a lower rate of proliferation based on Ki-67 staining than those in the LF or control groups (30). The first issue is whether Ki-67 or the CAFs in this study are appropriate surrogate markers for clinical outcome in prostate cancer. It is worth noting that in the prior study, only post-operative Ki-67, and not changes in Ki-67, was assessed; therefore the impact of the dietary intervention on a particular patient could not be evaluated. Tumor proliferation, inflammation, and angiogenesis have all been linked to cancer progression in a variety of different settings, but given the design and size of the current study, it is not possible to determine which, if any, of the markers is associated with prostate cancer progression or clinical outcome in the pre-operative setting for prostate cancer. It is plausible that separate mechanisms govern the putative effects of flaxseed on tumor cell proliferation and of a low-fat diet on inflammatory and angiogenic CAFs. Further studies will be needed to identify these mechanisms and determine their impact on prostate cancer.
Another issue that remains unresolved is why the many changes in cytokines observed in the LF arm are not mirrored in the FS+LF arm. It is worth noting that while both the LF and FS+LF groups experienced a significant decrease in BMI (30), the LF group had a slightly higher loss which may have impacted the cytokine changes. Furthermore, it is conceivable that the addition of flaxseed may have altered the pattern of CAF changes, or that at least some of the changes in the LF arm were due to chance alone. The observations that 19/20 of the CAFs in the LF arm changed in the same direction (decreased), however, and that many of these changes occurred in a coordinated manner as seen in Figure 3, argue against these changes being solely due to chance.
To our knowledge, the current study is the first to report changes in plasma levels of CAFs resulting from dietary changes in prostate cancer patients. Prior studies have suggested that dietary factors influence prostate cancer development and progression. In addition, given that previous studies have shown higher rates of prostate cancer with high-fat diets, as well as associations between obesity and more aggressive disease, the mechanisms identified in this exploratory analysis involving the low-fat diet may merit further investigation (43, 44). Indeed, mounting evidence suggests that obesity is associated with chronic inflammation, which is characterized by an influx of circulating inflammation-related markers (45). Adipose tissue itself is highly vascularized and is capable of promoting angiogenesis through the production and release of angiogenic factors, such as VEGF (46–48). Preclinical studies have demonstrated that mice on a low-fat diet experience a reduction in prostate cancer growth and have lower serum insulin and IGF-1 levels, compared with mice fed a Western diet (49). Adding serum from men on a low-fat, high-fiber diet and an exercise intervention to androgen-independent LNCaP cells also has been found to decrease growth, increase apoptosis, and reduce NF-κB activation (47, 50). Taken together, these studies suggest that diet-induced changes in inflammatory and/or angiogenic factors may influence the growth of prostate cancer.
In the current study we also observed decreases in the proinflammatory cytokine TNF-β lymphotoxin) in the low-fat arm. TNF-β can bind to the TGF-βR1, the same receptor as TNF-α and TGF-R2 and is thus an important activator of NF-κB (51, 52). In a murine obesity model, elevated levels of TNF-α contributed to the development of hepatocellular carcinoma, suggesting obesity mediated increased TNF-1α contributes to the tumorigenic process (19). TNF-α was also found to be elevated in parallel with activated NF-kB in the mammary gland in a diet-induced murine obesity model (14). This is in line with our study, in which patients following a low-fat diet in the LF arm exhibited decreases in TNF-β that could potentially mediate decreased NF-κB activity and contribute to the changes in CAFs.
Additionally, significant decreases in eotaxin emerged in all three treatment arms. Eotaxin has pro-angiogenic effects through chemotaxis of microvascular endothelial cells and plays a role as a chemoattractant for eosinophils, basophils and Th2 lymphocytes (53). Eotaxin expression is induced by proinflammatory cytokines such as TNF-α, IFN-α and glucocorticoids (22). A previous study demonstrated that a link between obesity and eotaxin serum levels in obese (high-fat diet-induced) mouse models as well as in obese human serum and omental fat (54). Similar to the results in the current study, serum eotaxin levels were reduced in patients who experienced diet-induced weight loss. An NF-κB binding site is present within the eotaxin promoter (along with STAT-6, IFN-α response and glucocorticoid response element) (53). Suggesting that decreased NF-κB activity following a low-fat diet is a plausible mechanism for eotaxin changes observed in this study.
The current study had several potential limitations. Given the modest sample size, our findings might not be transferable to other studies. There was no adjustment for multiple comparisons given the exploratory nature of this analysis. A number of the CAFs we assessed can be affected by factors other than diet, such as stress, and may be greatly changed in a subset of individuals but would not reflect the effect of diet modulation on the cohort as a whole. The presence of a control diet arm, however, should reduce some of these confounding factors. Further, the biologic changes mediated by these changes in CAFs may not have been accurately demonstrated because of the short mean time period (30–31 days) of adhering to the diet on this study. Finally, it is not possible to determine whether changes in CAF were the result of changes in the tumor, the host, or a combination of the two and whether greater changes may have occurred in the tumor microenvironment than was observed in mixed venous blood. The findings from this study would therefore need to be validated in a larger study and a longer on-study duration time, and would ideally include paired tumor biopsies that would enable one to distinguish tumor vs host effects.
In summary, the current study was the first to investigate of the effects of LF and FS diets on circulating CAFs in prostate-cancer patients. While relatively few changes in CAFs were observed with flaxseed supplementation it does not negate the possibility that other mechanisms may be at play. In contrast, the low-fat diet appeared to spur a coordinated reduction among a set of inflammatory cytokines, particularly among those involved in myeloid-cell recruitment and proliferation, and several angiogenic factors. The changes with a low-fat diet occurred in factors regulated by NF-κB. Combined with emerging evidence that the NF-κB pathway plays a critical role in coupling energy balance with inflammatory and angiogenesis, our findings suggest that NF-κB is a potential mediator of the changes in circulating CAFs associated with a low-fat diet. Although these changes might be expected to reduce inflammation and tumor angiogenesis, the magnitude of these changes (detectable in mixed venous blood) were often modest and their impact on prostate cancer or other cancer types remains unknown and warrants further investigation. Therefore, the present study is hypothesis-generating, and additional studies are needed to investigate the potential links between diet, the NF-κB pathway, inflammation, angiogenesis and cancer biology.
Supplementary Material
Acknowledgments
Grant Support
This study was supported by National Institutes of Health grants CA85740, CA07464830, M01-RR-30, and CA16672, the Cancer Center Support Grant (CCSG) of The University of Texas M. D. Anderson Cancer Center. KAD was partially supported by the SPORE in Prostate Cancer (CA90270) at M.D. Anderson Cancer Center. JVH is a Damon Runyon-Lilly Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI 24-04) and the American Society for Clinical Oncology Career Development Award.
Footnotes
Disclosure of Potential Conflicts of Interest
References
- 1.Cancer Facts and Figures. American Cancer Society. 2010 [Google Scholar]
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, et al. Human prostate cancer risk factors. Cancer. 2004;101:2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 4.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. quiz 313-4. [DOI] [PubMed] [Google Scholar]
- 5.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 6.Van Patten CL, de Boer JG, Tomlinson Guns ES. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence. J Urol. 2008;180:2314–2321. doi: 10.1016/j.juro.2008.08.078. discussion 721-2. [DOI] [PubMed] [Google Scholar]
- 7.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 183:345–350. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavropoulos JC, Buschemeyer WC, 3rd, Tewari AK, Rokhfeld D, Pollak M, Zhao Y, et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila Pa) 2009;2:557–565. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 14.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prevention Research. 2011 doi: 10.1158/1940-6207.CAPR-10-0381. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Hursting SD. Inflammatory talk: linking obesity, NF-κB and aromatase. Cancer Prevention Research. 2011 doi: 10.1158/1940-6207.CAPR-11-0056. In Press. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC. Dietary fat plays a major role in obesity: no. Obes Rev. 2002;3:59–68. doi: 10.1046/j.1467-789x.2002.00060.x. [DOI] [PubMed] [Google Scholar]
- 18.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 19.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, Sarafi MN, Lavigne F, Hamid Q, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613–5626. [PubMed] [Google Scholar]
- 22.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 23.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 24.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, et al. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes. 2009;58:104–115. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adlercreutz H, Mazur W, Bartels P, Elomaa V, Watanabe S, Wahala K, et al. Phytoestrogens and prostate disease. J Nutr. 2000;130:658S–659S. doi: 10.1093/jn/130.3.658S. [DOI] [PubMed] [Google Scholar]
- 26.Borriello SP, Setchell KD, Axelson M, Lawson AM. Production and metabolism of lignans by the human faecal flora. J Appl Bacteriol. 1985;58:37–43. doi: 10.1111/j.1365-2672.1985.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin X, Switzer BR, Demark-Wahnefried W. Effect of mammalian lignans on the growth of prostate cancer cell lines. Anticancer Res. 2001;21:3995–3999. [PubMed] [Google Scholar]
- 28.Chen LH, Fang J, Li H, Demark-Wahnefried W, Lin X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol Cancer Ther. 2007;6:2581–2590. doi: 10.1158/1535-7163.MCT-07-0220. [DOI] [PubMed] [Google Scholar]
- 29.Chen LH, Fang J, Sun Z, Li H, Wu Y, Demark-Wahnefried W, et al. Enterolactone inhibits insulin-like growth factor-1 receptor signaling in human prostatic carcinoma PC-3 cells. J Nutr. 2009;139:653–659. doi: 10.3945/jn.108.101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MTt, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17:3577–3587. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demark-Wahnefried W, George SL, Switzer BR, Snyder DC, Madden JF, Polascik TJ, et al. Overcoming challenges in designing and implementing a phase II randomized controlled trial using a presurgical model to test a dietary intervention in prostate cancer. Clin Trials. 2008;5:262–272. doi: 10.1177/1740774508091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 33.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II Trial of Infusional Fluorouracil, Irinotecan, and Bevacizumab for Metastatic Colorectal Cancer: Efficacy and Circulating Angiogenic Biomarkers Associated With Therapeutic Resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanrahan EO, Lin HY, Kim ES, Yan S, Du DZ, McKee KS, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28:193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 36.Huang CY, Lee CY, Chen MY, Yang WH, Chen YH, Chang CH, et al. Stromal cell-derived factor-1/CXCR4 enhanced motility of human osteosarcoma cells involves MEK1/2, ERK and NF-kappaB-dependent pathways. J Cell Physiol. 2009;221:204–212. doi: 10.1002/jcp.21846. [DOI] [PubMed] [Google Scholar]
- 37.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, et al. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4:215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aljada A, Mohanty P, Ghanim H, Abdo T, Tripathy D, Chaudhuri A, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr. 2004;79:682–690. doi: 10.1093/ajcn/79.4.682. [DOI] [PubMed] [Google Scholar]
- 42.Taylor CT, Cummins EP. The role of NF-kappaB in hypoxia-induced gene expression. Ann N Y Acad Sci. 2009;1177:178–184. doi: 10.1111/j.1749-6632.2009.05024.x. [DOI] [PubMed] [Google Scholar]
- 43.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 44.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 45.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:s843–s857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 46.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 47.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 49.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11–19. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tymchuk CN, Barnard RJ, Heber D, Aronson WJ. Evidence of an inhibitory effect of diet and exercise on prostate cancer cell growth. J Urol. 2001;166:1185–1189. [PubMed] [Google Scholar]
- 51.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318:665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 52.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 53.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 54.Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, et al. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.