Abstract
Interleukin-6 (IL6) is a pleiotropic inflammatory cytokine, which is implicated in the development and progression of several types of cancer. The -174G/C polymorphism of the IL6 gene controls serum levels of IL6 and may be associated with cancer risk, but the results from the published studies on the association between this polymorphism and cancer risk are conflicting. A comprehensive meta-analysis was conducted to assess the association of IL6 -174G/C with cancer risk. Studies were identified by searches of MEDLINE and HuGE Published Literature databases, with no restrictions. An eligible 83 articles involving 44,735 cancer patients and 60,747 controls were included. Combined odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the strength of the association between the IL6 -174 G/C polymorphism and cancer risk. Potential sources of heterogeneity were explored by meta-regression and sensitivity analysis. Overall, the IL6 -174G/C polymorphism was not significantly associated with cancer risk. However, cancer risk was increased for individuals with the CC genotype compared to those carrying the GG genotype in African populations (OR=1.83, 95% CI 1.26–2.67, P=0.002), but not in Caucasian populations (OR=1.00, 95% CI 0.92–1.08, P=0.938). The present meta-analysis provides the first evidence of the ethnic-specific association of the IL6 -174G/C polymorphism with cancer risk. Further investigations with a large number of cases and controls are required to confirm the associations between this polymorphism and cancer in Africans.
Keywords: interleukin-6, polymorphism, cancer risk, meta-analysis
Introduction
Clinical and epidemiological studies suggest that chronic inflammation predisposes individuals to different types of cancer, and inflammatory molecules promote the proliferation of malignant cells (1,2). The connection between inflammation and cancer is mediated by several mechanisms, including genetic and epigenetic alterations, that generate an inflammatory microenvironment that further reinforces the development of cancer (3). Moreover, functional polymorphisms of inflammatory cytokine genes are associated with cancer susceptibility (4–6).
Interleukin-6 (IL6) is a pleiotropic inflammatory cytokine that is important for immune responses, cell survival, proliferation and apoptosis (7). Elevated expression of IL6 and its major effector, signal transducer and activator of transcription-3 (STAT3), have been implicated in different stages of tumor development, including initiation, promotion, malignant conversion, invasion and metastasis (8–12). The best characterized genetic variants of IL6 is a G-to-C substitution at position -174, upstream of the transcription start site, which has been reported to influence IL6 levels in vitro and in vivo (13,14). Elucidation of an association, if any, between this polymorphism and cancer risk would support the hypothesis that genetic variants in IL6, resulting in aberrant IL6 expression, play a role in cancer development.
Individual studies and previously published meta-analyses regarding the association of IL6 -174G/C with cancer susceptibility (15,16) enrolled too few subjects to provide conclusive evidence for or against an association of this polymorphism with cancer risk. The aim of this study was to assess the association of IL6 -174G/C polymorphism with cancer risk by conducting a comprehensive meta-analysis of all eligible case-control studies.
Materials and methods
This meta-analysis was performed according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA statement) (17) and the reporting meta-analysis of observational studies in epidemiology (MOOSE) (18).
Data sources and study selection
To identify all studies on the association between the IL6 -174G/C polymorphism and cancer risk, we conducted a systematic search of the literature published before April 2011 using the MEDLINE database and the HuGE Published Literature database (HuGE Pub Lit) (19) with no restrictions. For MEDLINE, keywords ‘IL-6’ OR ‘IL 6’ OR ‘IL6’ OR ‘interleukin-6’ OR ‘interleukin 6’ AND ‘polymorphism’ AND ‘cancer’ were used; For HuGE Pub Lit, keywords ‘IL6’ AND ‘cancer’ were used for searching eligible studies. In addition, a manual review of references from primary or review articles was screened to trace additional relevant studies. Studies were included if they had a case-control design and the available frequency of three genotypes regarding the IL6 -174G/C polymorphism. Of the studies with overlapping data, we selected the ones with the largest number of subjects.
Data extraction
Three investigators independently extracted data and reached a consensus on all items. The following data were extracted from each study: the first author's last name, publication year, ethnicity of the subjects, cancer type, study design (retrospective case-control or prospective cohort study), and numbers of genotyped cases and controls with GG, GC or CC genotypes. Ethnic group was defined as African, Caucasian or ‘mixed’, including more than one ethnic category. Studies investigating more than one type of cancer with overlapping or same controls were regarded as individual data sets only in subgroup analyses by cancer type.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) analysis for the frequencies of GG, GC and CC genotypes among controls in each study was assessed using Pearson's Chi-square test. The strength of the association between IL6 -174G/C polymorphism and cancer risk was measured by odds ratio (OR) with its 95% confidence interval (CI). The pooled ORs for IL6 -174G/C genotypes CC, GC and C allele carriers (CC or GC) against GG genotype were calculated, respectively. The significance of the pooled OR was determined by the Z-test and P<0.05 was considered statistically significant. Subgroup analysis was performed using stratification by study character, cancer type, ethnicity and study design, respectively. If a cancer type contained less than three independent individual studies, it was categorized into the ‘other types’ group.
Testing for heterogeneity among studies was performed by a Chi-square-based Q-test (20). Since Q-test is poor at detecting true heterogeneity, heterogeneity was considered significant for P<0.10 rather than P<0.05 (21). Additionally, the magnitude of the between-study heterogeneity was also assessed by I2, which can be calculated from the basic results of a typical meta-analysis as I2 = 100% x (Q-df)/Q, ranges form 0 to 100%, and is typically considered low for I2<25%, modest for 25–50% and large for >50% (22). Meta-regression was carried out to investigate whether statistical heterogeneity between the results of the multiple studies was related to one or more characteristics of the studies (23). To identify the studies that led to significant heterogeneity, sensitivity analysis for between-study heterogeneity was implemented by the sequential algorithm proposed by Patsopoulos et al (24). Whenever the P-value of the Q-test was >0.10, the summarized OR estimate of each study was calculated by the fixed-effects model (Mantel-Haenszel method) (25). Otherwise, the random-effects model (DerSimonian and Laird method) was used (26). Funnel plots were used to examine whether the results of a meta-analysis may have been affected by publication bias (27). A modified version of Egger's test proposed by Harbord, Egger and Sterne was implemented to test funnel plot asymmetry (28). All statistical analyses were performed using Stata statistical software (Stata/SE version 10.1 for Windows; Stata Corp, College Station, TX, USA).
Results
Characteristics of the included studies
The detailed steps of our literature search are described in Fig. 1. Eighty-three independent articles that met the inclusion criteria were included in the final analysis. Of these articles, one study provided data on breast and prostate cancer using independent controls (29), therefore each group in this article was treated as an independent study in our meta-analysis. The characteristics of the included studies are summarized in Table I. Overall, the present meta-analysis is based on a total of 105,482 participants, including 44,735 cancer patients and 60,747 controls. The studies were published between April 2000 and March 2011. Seventeen studies were conducted with a prospective cohort design, and 67 were conducted with a retrospective case-control design. Approximately two-thirds of cases and controls (29,019 cases and 42,120 controls) were from 73 studies involving Caucasian populations, a fraction of the data (1,138 cases and 1,299 controls) from seven studies involving African populations, and nearly one-third of the data (14,578 cases and 17,328 controls) from four studies involving ‘mixed’ populations. As shown in Table I, there were two studies from Dossus et al involving Caucasians, African-Americans, Asians, Latinos and native Hawaiians; one study from Ognjanovic et al involving Caucasians, Asians and Hawaiians; and one study from Bushley et al involving Caucasians, Asians and other populations. In the controls, the frequency of the rare C allele among controls varied considerably between Caucasians and Africans (0.417±0.052 and 0.207±0.097, respectively; P<0.001). A significant deviation from HWE was noted in two studies in Africans, nine in Caucasians and four in mixed populations (Table I).
Figure 1.
Flow diagram of the study selection for the meta-analysis.
Table I.
Characteristics of all studies included in the meta-analysis.
| Author | Year | Ethnicity | Cancer type | Study designa | Cases
|
Controls
|
P-valueb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GC | CC | GG | GC | CC | ||||||
| Snoussi | 2005 | African | Breast cancer | Retrospective | 199 | 98 | 8 | 150 | 46 | 4 | 0.830 |
| Vishnoi | 2007 | African | Gallbladder cancer | Retrospective | 97 | 25 | 2 | 153 | 44 | 3 | 0.936 |
| Ahirwar | 2008 | African | Bladder cancer | Retrospective | 86 | 24 | 26 | 130 | 56 | 14 | 0.027 |
| Kesarwani | 2008 | African | Prostate cancer | Retrospective | 102 | 84 | 14 | 103 | 87 | 10 | 0.120 |
| Upadhyay | 2008 | African | Esophageal cancer | Retrospective | 135 | 28 | 5 | 131 | 62 | 8 | 0.845 |
| Badr El-Din | 2009 | African | Brain tumor | Retrospective | 6 | 27 | 12 | 5 | 87 | 6 | 0.000 |
| Gangwar | 2009 | African | Cervical cancer | Retrospective | 107 | 36 | 17 | 142 | 51 | 7 | 0.372 |
| Foster | 2000 | Caucasian | Kaposi sarcoma | Retrospective | 61 | 44 | 10 | 44 | 55 | 27 | 0.214 |
| Hulkkonen | 2000 | Caucasian | Leukemia | Retrospective | 8 | 13 | 14 | 81 | 201 | 118 | 0.785 |
| Zheng | 2000 | Caucasian | Multiple myeloma | Retrospective | 22 | 36 | 15 | 33 | 69 | 26 | 0.357 |
| Martinez-Escribano | 2002 | Caucasian | Melanoma | Retrospective | 14 | 26 | 2 | 20 | 26 | 2 | 0.071 |
| El-Omar | 2003 | Caucasian | Esophageal and gastric cancer | Retrospective | 88 | 91 | 34 | 83 | 98 | 28 | 0.913 |
| Howell | 2003 | Caucasian | Melanoma | Retrospective | 48 | 79 | 34 | 79 | 101 | 44 | 0.258 |
| Hwang | 2003 | Caucasian | Gastric cancer | Retrospective | 19 | 9 | 2 | 22 | 8 | 0 | 0.399 |
| Landi | 2003 | Caucasian | Colorectal cancer | Retrospective | 133 | 180 | 48 | 145 | 133 | 33 | 0.761 |
| Yakupova | 2003 | Caucasian | Multiple myeloma | Retrospective | 23 | 33 | 13 | 37 | 53 | 12 | 0.286 |
| Campa | 2004 | Caucasian | Lung cancer | Retrospective | 64 | 111 | 68 | 55 | 105 | 47 | 0.818 |
| Cozen | 2004 | Caucasian | Lymphoma | Retrospective | 41 | 37 | 8 | 25 | 39 | 14 | 0.858 |
| Gazouli | 2004 | Caucasian | Kaposi sarcoma | Retrospective | 10 | 4 | 1 | 11 | 22 | 7 | 0.482 |
| Smith | 2004 | Caucasian | Breast cancer | Retrospective | 57 | 67 | 20 | 79 | 101 | 44 | 0.258 |
| Vasku | 2004 | Caucasian | Lymphoma | Retrospective | 19 | 35 | 9 | 36 | 46 | 23 | 0.259 |
| Basturk | 2005 | Caucasian | Renal cell carcinoma | Retrospective | 15 | 10 | 0 | 27 | 13 | 9 | 0.007 |
| Campa | 2005 | Caucasian | Lung cancer | Retrospective | 629 | 954 | 412 | 615 | 993 | 374 | 0.448 |
| Cordano | 2005 | Caucasian | Lymphoma | Retrospective | 134 | 197 | 77 | 106 | 184 | 59 | 0.167 |
| Festa | 2005 | Caucasian | Basal cell carcinoma | Retrospective | 57 | 126 | 58 | 62 | 130 | 68 | 0.993 |
| Hefler | 2005 | Caucasian | Breast cancer | Retrospective | 78 | 139 | 52 | 91 | 105 | 31 | 0.935 |
| Mazur | 2005 | Caucasian | Multiple myeloma | Retrospective | 11 | 31 | 12 | 16 | 28 | 6 | 0.239 |
| Seifart | 2005 | Caucasian | Lung cancer | Retrospective | 47 | 52 | 17 | 90 | 107 | 46 | 0.163 |
| Balasubramanian | 2006 | Caucasian | Breast cancer | Retrospective | 170 | 244 | 83 | 168 | 235 | 87 | 0.759 |
| Gonzalez-Zuloeta Ladd | 2006 | Caucasian | Breast cancer | Prospective | 55 | 86 | 30 | 1,286 | 1,733 | 632 | 0.246 |
| Gunter | 2006 | Caucasian | Colorectal cancer | Retrospective | 79 | 90 | 35 | 83 | 81 | 26 | 0.385 |
| Kamangar | 2006 | Caucasian | Gastric cancer | Prospective | 21 | 54 | 27 | 51 | 58 | 43 | 0.004 |
| Lan | 2006 | Caucasian | Lymphoma | Retrospective | 211 | 231 | 68 | 241 | 264 | 85 | 0.358 |
| Michaud | 2006 | Caucasian | Prostate cancer | Prospective | 170 | 223 | 91 | 230 | 293 | 90 | 0.832 |
| Morgan | 2006 | Caucasian | Aneurysm | Retrospective | 40 | 40 | 6 | 867 | 1,358 | 495 | 0.360 |
| Nogueira de Souza | 2006 | Caucasian | Cervical cancer | Retrospective | 24 | 32 | 0 | 148 | 102 | 3 | 0.001 |
| Rothman | 2006 | Caucasian | Lymphoma | Retrospective | 1,277 | 1,658 | 564 | 1,097 | 1,470 | 499 | 0.860 |
| Theodoropoulos | 2006 | Caucasian | Colorectal cancer | Retrospective | 111 | 76 | 35 | 64 | 86 | 50 | 0.055 |
| Vogel | 2006 | Caucasian | Breast cancer | Prospective | 108 | 167 | 86 | 98 | 177 | 86 | 0.728 |
| Wang | 2006 | Caucasian | Lymphoma | Retrospective | 486 | 474 | 174 | 393 | 410 | 138 | 0.068 |
| Berkovic | 2007 | Caucasian | GEP-NETs | Retrospective | 25 | 44 | 11 | 69 | 75 | 18 | 0.724 |
| Brenner | 2007 | Caucasian | Glioma | Retrospective | 222 | 332 | 100 | 319 | 503 | 211 | 0.621 |
| Deans | 2007 | Caucasian | Gastro-oesophageal cancer | Retrospective | 71 | 83 | 43 | 79 | 101 | 44 | 0.258 |
| Duch | 2007 | Caucasian | Multiple myeloma | Retrospective | 28 | 22 | 2 | 35 | 23 | 2 | 0.442 |
| Gatti | 2007 | Caucasian | Gastric cancer | Retrospective | 42 | 13 | 1 | 23 | 27 | 6 | 0.642 |
| Gonullu | 2007 | Caucasian | Breast cancer | Retrospective | 15 | 17 | 6 | 14 | 3 | 7 | <0.001 |
| Litovkin | 2007 | Caucasian | Breast cancer and uterine leiomyoma | Retrospective | 44 | 64 | 25 | 30 | 39 | 9 | 0.490 |
| Oliveira | 2007 | Caucasian | Osteosarcoma | Retrospective | 9 | 23 | 32 | 10 | 68 | 82 | 0.405 |
| Purdue | 2007 | Caucasian | Lymphoma | Retrospective | 177 | 245 | 91 | 157 | 210 | 90 | 0.194 |
| Slattery | 2007 | Caucasian | Colorectal cancer | Retrospective | 952 | 1,043 | 355 | 728 | 897 | 347 | 0.015 |
| Talseth | 2007 | Caucasian | Colorectal cancer | Retrospective | 36 | 58 | 24 | 25 | 53 | 22 | 0.542 |
| Vogel | 2007 | Caucasian | Basal cell carcinoma | Prospective | 65 | 176 | 63 | 89 | 157 | 69 | 0.988 |
| Vogel | 2007 | Caucasian | Colorectal cancer | Prospective | 98 | 168 | 89 | 204 | 364 | 185 | 0.371 |
| Zanke | 2007 | Caucasian | Colorectal cancer | Retrospective | 381 | 557 | 195 | 373 | 539 | 213 | 0.461 |
| Colakogullari | 2008 | Caucasian | Lung cancer | Retrospective | 10 | 29 | 5 | 27 | 22 | 9 | 0.222 |
| Crusius | 2008 | Caucasian | Gastric cancer | Prospective | 78 | 122 | 43 | 415 | 517 | 206 | 0.044 |
| Ennas | 2008 | Caucasian | Leukemia | Retrospective | 17 | 16 | 6 | 64 | 43 | 5 | 0.506 |
| Fontanella | 2008 | Caucasian | Aneurysm | Retrospective | 144 | 157 | 34 | 66 | 71 | 19 | 0.989 |
| Gu | 2008 | Caucasian | Melanoma | Prospective | 69 | 106 | 32 | 69 | 102 | 33 | 0.646 |
| Kury | 2008 | Caucasian | Colorectal cancer | Retrospective | 363 | 489 | 171 | 435 | 504 | 182 | 0.079 |
| Vairaktaris | 2008 | Caucasian | Oral cancr | Retrospective | 42 | 102 | 18 | 90 | 60 | 6 | 0.298 |
| Vogel | 2008 | Caucasian | Lung cancer | Prospective | 105 | 202 | 96 | 204 | 361 | 179 | 0.437 |
| Wilkening | 2008 | Caucasian | Colorectal cancer | Prospective | 79 | 163 | 61 | 162 | 297 | 121 | 0.481 |
| Aladzsity | 2009 | Caucasian | Multiple myeloma | Retrospective | 37 | 43 | 17 | 36 | 49 | 14 | 0.681 |
| Birmann | 2009 | Caucasian | Multiple myeloma | Prospective | 21 | 46 | 10 | 52 | 82 | 28 | 0.655 |
| Cherel | 2009 | Caucasian | Breast cancer | Retrospective | 102 | 131 | 60 | 29 | 58 | 25 | 0.695 |
| Moore | 2009 | Caucasian | Prostate cancer | Prospective | 191 | 485 | 281 | 196 | 401 | 250 | 0.152 |
| Ozgen | 2009 | Caucasian | Papillary thyroid carcinoma | Retrospective | 21 | 14 | 7 | 143 | 171 | 26 | 0.009 |
| Pierce | 2009 | Caucasian | Prostate cancer | Prospective | 82 | 101 | 32 | 864 | 848 | 306 | 0.000 |
| Talar-Wojnarowska | 2009 | Caucasian | Pancreatic cancer | Retrospective | 13 | 19 | 9 | 22 | 19 | 9 | 0.191 |
| Tsilidis | 2009 | Caucasian | Colorectal cancer | Prospective | 68 | 93 | 39 | 113 | 170 | 71 | 0.627 |
| Vasku | 2009 | Caucasian | Colorectal cancer | Retrospective | 22 | 46 | 32 | 22 | 47 | 31 | 0.601 |
| Wang | 2009 | Caucasian | Prostate cancer | Prospective | 91 | 116 | 43 | 84 | 128 | 40 | 0.448 |
| Cacev | 2010 | Caucasian | Colorectal cancer | Retrospective | 64 | 70 | 26 | 68 | 75 | 17 | 0.582 |
| Guey | 2010 | Caucasian | Bladder cancer | Retrospective | 470 | 438 | 109 | 450 | 495 | 120 | 0.356 |
| Jakubowska | 2010 | Caucasian | Breast and ovarian cancer | Retrospective | 135 | 227 | 102 | 73 | 144 | 73 | 0.907 |
| MARIE-GENICA | 2010 | Caucasian | Breast cancer | Retrospective | 986 | 1,571 | 585 | 1,774 | 2,671 | 1,036 | 0.586 |
| Consortium | |||||||||||
| Schonfeld | 2010 | Caucasian | Breast cancer | Retrospective | 274 | 408 | 156 | 379 | 487 | 211 | 0.017 |
| Giannitrapani | 2011 | Caucasian | Hepatocellular carcinoma | Retrospective | 63 | 36 | 6 | 51 | 37 | 10 | 0.402 |
| Grimm | 2011 | Caucasian | Cervical cancer | Retrospective | 55 | 51 | 25 | 85 | 96 | 27 | 0.990 |
| Bushley | 2004 | Mixed | Ovarian cancer | Retrospective | 5 | 34 | 143 | 9 | 46 | 163 | 0.020 |
| Dossus | 2010 | Mixed | Prostate cancer | Prospective | 3,594 | 3,218 | 1,125 | 3,832 | 3,402 | 1,274 | <0.001 |
| Dossus | 2010 | Mixed | Breast cancer | Prospective | 2,847 | 2,523 | 820 | 3,707 | 3,324 | 1,035 | <0.001 |
| Ognjanovic | 2010 | Mixed | Colorectal cancer | Retrospective | 173 | 74 | 22 | 357 | 136 | 43 | <0.001 |
Retrospective case-control or prospective cohort study.
P-value for Hardy-Weinberg equilibrium analysis among controls. References provided upon request.
Test of heterogeneity
There was a significant heterogeneity in overall comparison of the CC genotype vs. GG genotype (P<0.001 and I2=43.8%). The meta-regression showed that the strong heterogeneity could not be traditionally explained by cancer types, ethnicities or study designs (P=0.285, 0.129 and 0.306, respectively). Furthermore, the 15 studies that deviate from HWE showed similar heterogeneity with that of studies that were in HWE (Table II), suggesting that the remarkable heterogeneity among the overall analysis was not due to the variability of the control quality. Therefore, we carefully assessed the association of the IL6 -174G/C polymorphism with cancer risk in several subgroups, and carried out sensitivity analyses of between-study heterogeneity to detect studies that have remarkable influence on homogeneity.
Table II.
Summary of ORs and 95% CIs for the IL6 -174G>C polymorphism and cancer risk and heterogeneity test for studies of each group.
| Variables | No.a | Cases/controls | CC vs. GG
|
GC vs. GG
|
GC/CC vs. GG
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)b | PHc | I2 (%) | OR (95% CI)b | PHc | I2 (%) | OR (95% CI)b | PHc | I2 (%) | |||
| Total | 84 | 44,735/60,747 | 1.01 (0.95–1.08) | <0.001 | 43.8 | 1.01 (0.96–1.07) | <0.001 | 52.5 | 1.01 (0.96–1.07) | <0.001 | 57.0 |
| Ethnicities | |||||||||||
| African | 7 | 1,138/1,299 | 1.83 (1.26–2.67)e | 0.294 | 17.7 | 0.80 (0.55–1.16) | 0.002 | 71.0 | 0.94 (0.67–1.31) | 0.004 | 68.9 |
| Caucasian | 73 | 29019/42120 | 1.00 (0.92–1.08) | <0.001 | 43.8 | 1.02 (0.96–1.09) | <0.001 | 52.4 | 1.02 (0.96–1.08) | <0.001 | 58.2 |
| Mixed | 4 | 14,578/17,328 | 0.98 (0.92–1.05) | 0.488 | 0.0 | 1.00 (0.96–1.05) | 0.839 | 0.0 | 1.00 (0.95–1.04) | 0.789 | 0.0 |
| Cancer typesd | |||||||||||
| Breast cancer | 13 | 12,640/20,281 | 1.01 (0.94–1.09) | 0.374 | 7.2 | 1.06 (0.96–1.17) | 0.033 | 46.4 | 1.05 (0.95–1.15) | 0.043 | 44.3 |
| Colorectal cancer | 13 | 6,798/7,502 | 0.97 (0.82–1.14) | 0.014 | 52.4 | 1.01 (0.89–1.13) | 0.030 | 47.2 | 1.00 (0.87–1.13) | 0.002 | 61.9 |
| Prostate cancer | 6 | 10,043/12,438 | 0.99 (0.91–1.07) | 0.254 | 24.0 | 1.03 (0.97–1.09) | 0.361 | 8.6 | 1.01 (0.96–1.07) | 0.357 | 9.3 |
| Lymphomad | 7 | 6,213/5,586 | 0.96 (0.86–1.07) | 0.574 | 0.0 | 0.96 (0.89–1.04) | 0.609 | 0.0 | 0.96 (0.89–1.03) | 0.621 | 0.0 |
| Multiple myelomad | 6 | 422/601 | 1.23 (0.82–1.83) | 0.609 | 0.0 | 1.07 (0.80–1.41) | 0.717 | 0.0 | 1.10 (0.84–1.44) | 0.731 | 0.0 |
| Lung cancerd | 5 | 2,801/3,234 | 1.06 (0.92–1.23) | 0.715 | 0.0 | 1.05 (0.83–1.33) | 0.071 | 53.7 | 1.01 (0.90–1.13) | 0.153 | 40.2 |
| Gastric cancerd | 5 | 554 /1,585 | 1.06 (0.78–1.44) | 0.114 | 46.2 | 0.98 (0.55–1.73) | 0.001 | 78.8 | 0.95 (0.54–1.76) | <0.001 | 80.4 |
| Melanomad | 3 | 410/476 | 1.13 (0.75–1.68) | 0.790 | 0.0 | 1.18 (0.88–1.58) | 0.721 | 0.0 | 1.17 (0.88–1.54) | 0.672 | 0.0 |
| Cervical cancer | 3 | 347/661 | 1.85 (1.12–3.08)f | 0.320 | 12.2 | 1.11 (0.68–1.82) | 0.068 | 62.7 | 1.22 (0.92–1.61) | 0.195 | 38.7 |
| Other types | 28 | 4,723/9,403 | 1.10 (0.85–1.43) | <0.001 | 67.6 | 0.94 (0.79–1.11) | <0.001 | 67.1 | 0.97 (0.82–1.16) | <0.001 | 71.5 |
| Study design | |||||||||||
| Prospective | 17 | 18,759/28,718 | 1.01 (0.95–1.07) | 0.893 | 0.0 | 1.03 (0.98–1.07) | 0.150 | 26.6 | 1.02 (0.98–1.06) | 0.316 | 11.8 |
| Retrospective | 67 | 25,976/32,029 | 1.00 (0.91–1.11) | <0.001 | 51.9 | 0.98 (0.92–1.06) | <0.001 | 56.3 | 0.99 (0.92–1.06) | <0.001 | 61.9 |
| HWE | |||||||||||
| >0.05 | 69 | 26,067/36,098 | 1.01 (0.93–1.09) | <0.001 | 44.7 | 1.00 (0.94–1.07) | <0.001 | 50.8 | 1.00 (0.94–1.07) | <0.001 | 59.0 |
| <0.05 | 15 | 18,668/24,649 | 1.02 (0.90–1.15) | 0.043 | 42.3 | 1.06 (0.95–1.18) | 0.001 | 61.4 | 1.04 (0.96–1.13) | 0.019 | 48.3 |
No. of comparisons.
Random-effect model was used when P-value for heterogeneity test was <0.10; otherwise, the fixed-effect model was used.
P-value of Q-test for heterogeneity.
All the studies were from Caucasian populations.
P=0.002.
P=0.017.
For ethnic-specific subgroup analysis, no heterogeneity was detected within African population studies (P=0.294), but there were significant heterogeneity within Caucasian population studies (P<0.001). Sensitivity analysis of between-study heterogeneity revealed that five studies (30–34) mainly contributed to the heterogeneity within Caucasian population studies. After performing cancer type-specific analyses, we found no heterogeneity in studies of breast, prostate, lung, gastric cancer, lymphoma, multiple myeloma, melanoma and cervical cancer (Table II). However, there was strong heterogeneity for colorectal cancer, which was due to one Caucasian study (32).
Quantitative data synthesis
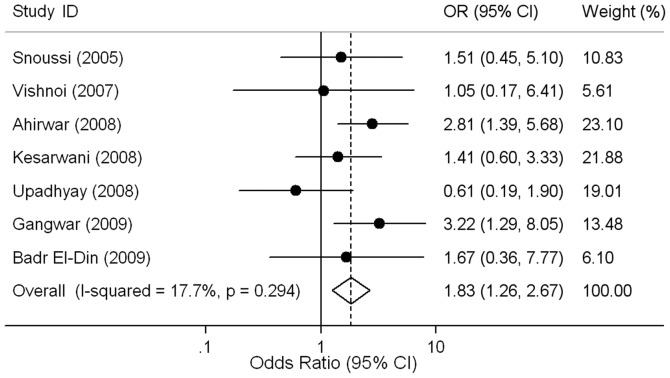
Overall, the CC genotype was not significantly associated with cancer risk when compared to the GG genotype (OR=1.01, 95% CI 0.95–1.08, P=0.698). Ethnic-specific ORs showed that cancer risk was increased for individuals carrying the CC genotype compared to those with the GG genotype in African populations (OR=1.83, 95% CI 1.26–2.67, P=0.002; Fig. 2), but not in Caucasian populations (OR=1.00, 95% CI 0.92–1.08, P=0.938; Table II). After excluding the studies (30–34) responsible for heterogeneity, we found that Caucasian individuals carrying the CC genotype had no remarkable effect on risk of cancer compared to GG genotype individuals (OR=1.02, 95% CI 0.97–1.07, P=0.561) with no significant between-study heterogeneities (P=0.196, I2=12.6%). Although there were nine data sets in which the genotype distribution did not follow HWE, the corresponding meta-analysis was qualitatively similar with or without excluding them.
Figure 2.
Risk of cancer for IL6-174 CC vs. GG genotype in African population. The circles and horizontal lines correspond to the study-specific odds ratio (OR) and 95% confidence interval (CI). The combined ORs and their 95% CIs are indicated by the diamonds.
Subsequently, we stratified the association between the IL6 -174G/C polymorphism and cancer risk by cancer types. When compared to individuals with the GG genotype, those with the CC genotype were associated with increased risk of cervical cancer (OR=1.85, 95% CI 1.12–3.08, P=0.017), but not with that of other types of cancer (Table II). Furthermore, no significant association of the IL6 -174G/C polymorphism with risk of breast, colorectal and prostate cancer was observed in individuals with Caucasian ancestry (Fig. 3). A few studies involving African populations did not allow us to perform subgroup analysis in Africans (Table I).
Figure 3.
Risk of breast, colorectal and prostate cancer for IL6 -174 CC vs. GG genotype in Caucasians. The circles and horizontal lines correspond to the study-specific odds ratio (OR) and 95% confidence interval (CI). The combined ORs and their 95% CIs are indicated by the diamonds for each type of cancer.
Lastly, we also assessed the ORs of cancer for individuals with the GC genotype or C allele carriers (GC or CC genotype) compared to those with the GG genotype, and found no significant association in overall and subgroup analyses (Table II).
Publication bias
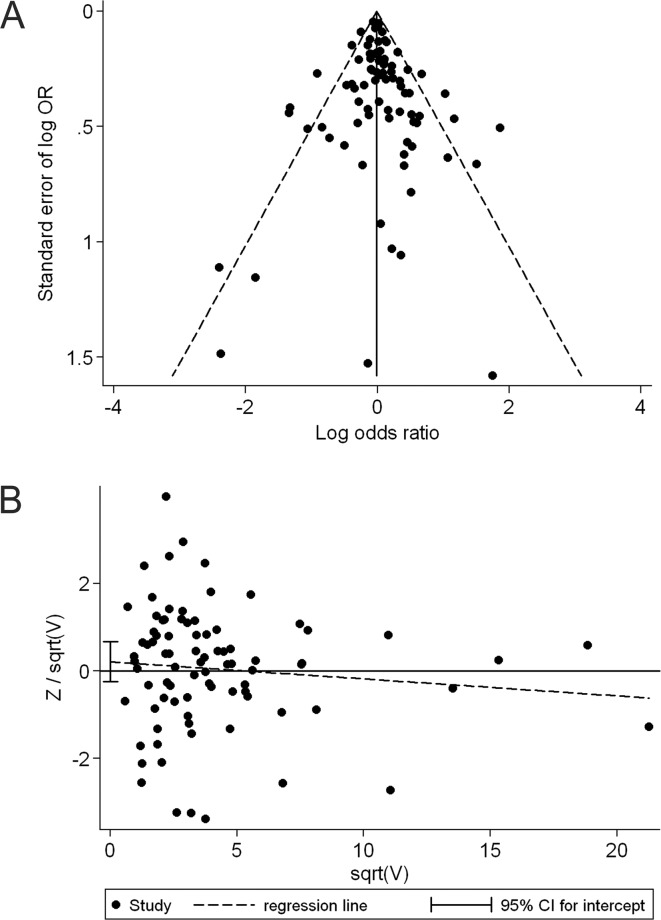
The shape of the funnel plot did not reveal any evidence of obvious asymmetry in comparison of the CC genotype vs. GG genotype (Fig. 4A). Then, we used Harbord's test to provide statistical evidence of funnel plot symmetry, and the result did not show evidence of publication bias (t=0.91, P=0.366; Fig. 4B). Subgroup analyses by ethnicity and cancer type did not provide any evidence of publication bias (t=−1.59, P=0.172 for African populations; t=0.27, P=0.786 for Caucasian populations; t=−0.58, P=0.575 for breast cancer and t=1.02, P=0.329 for colorectal cancer). Similarly, no publication bias was detected when comparing the GC genotype to the GG genotype (t=0.18, P=0.861), the GC/CC genotype to the GG genotype (t=0.42, P=0.677) and in any comparison of the corresponding subgroup analyses.
Figure 4.
Publication bias test for the comparison of the CC genotype vs. GG genotype. (A) Funnel plot (with pseudo 95% percent confidence limits) for publication bias test. The natural logarithm of odds ratio (OR) and its standard error were used in the funnel plot. The points correspond to the log OR from individual trials, and the diagonal lines show the expected 95% confidence intervals (CIs) around the summary estimate. (B) Harbord's modified test for funnel plot asymmetry: regress Z/sqrt(V) on sqrt(V) -174G/C, where Z is efficient score and V is score variance.
Discussion
To the best of our knowledge, the present meta-analysis of 83 studies, involving 44,735 cases and 60,747 controls (counting every study's cases and controls only once), provides the most comprehensive assessment of an association of the IL6 -174G/C polymorphism with cancer risk. It provides evidence that African individuals with the CC genotype have higher odds of cancer than individuals with the GG genotype; the findings of our meta-analysis do not show any association of the IL6 -174G/C with cancer risk in Caucasian populations. These findings suggest an ethnic-specific effect of IL6 -174G/C polymorphism on risk of cancer. The discrepancies among different populations suggest a possible role of ethnic differences in genetic backgrounds and the environment they lived in (35).
Recent studies have shown that IL6 and its major effector STAT3 play a role in the epigenetic switch from non-transformed epithelia to cancer cell (36,37). Elevated expression of IL6 via autocrine and paracrine mechanisms leading to subsequent chronic inflammation also exhibits a strong association with cancer (38–41). In the present study, we found that the IL6 -174 CC genotype was significantly associated with increased risk of cervical cancer compared to the GG genotype. However, the smaller number of individuals genotyped in these studies precludes any formal conclusion. As compared to previous analyses based on substantially less data (15,16), the present analysis essentially shows null associations between IL6 -174G/C and several common types of cancer, including breast, colorectal, prostate, lung, gastric cancer, lymphoma, multiple myeloma and melanoma.
Assessment of the between-study heterogeneity and identification of its sources are essential requirements in meta-analyses (23,42). In this study, we systematically examined the effect of IL6 -174G/C on cancer risk across all reliable studies, and the results of the overall analysis showed a strong heterogeneous effect among the 83 studies. Given the fact that clinical characteristics of studies, including study population, design approach and type of cancer, are likely to be potential sources of heterogeneity, we first used meta-regression to detect whether any of the characteristics could explain the between-study variation. However, none of the potential sources considered were able to systematically explain the observed variation across studies. It seems likely that there exist more than one answer to the nature of overall heterogeneity. We, therefore, induced a new approach to perform sensitivity analysis of between-study heterogeneity (24), and detected that several studies with different clinical characteristics were responsible for the heterogeneity (30–34).
Apart from between-study heterogeneity, publication bias has also been recognized as a major concern in robust meta-analyses. Thereby, in this study, we used funnel plots to assess whether the studies included could be affected by publication bias. According to the recommendations by Sterne and Egger (43), the log OR and its standard error are used for the horizontal and vertical axis, respectively. No evidence of publication bias was found when testing by a visual inspection of funnel plots. In support of this, Harbord's linear regression test confirmed the evidence of funnel plot symmetry across all constituent data sets.
Notably, social factors are believed to interact with genetic variants to govern complex human phenotypes (44,45). Cole et al recently demonstrated a strong interaction between the IL6 -174G/C polymorphism and social environment factors, which may further affect the risk of inflammation-related disease (46). However, in the absence of the original data of the reviewed studies, our evaluation of potential interactions of gene-environment with cancer risk was limited. This may explain why previous genetic association studies and some subgroup analyses in our meta-analysis, especially the Caucasian studies, failed to show an association between the IL6 -174G/C polymorphism and risk of cancer. Furthermore, two other polymorphisms (-6331T/C and -572G/C) and several haplotypes in the IL6 promoter affect the transcriptional activity of IL6 and may influence susceptibility to inflammation-related diseases (47–49). However, most studies included in our meta-analysis restricted their analysis to the IL6 -174G/C polymorphism and few carried out the IL6 haplotypic analysis on cancer susceptibility. It is difficult to estimate the role of a particular haplotype on cancer risk in the present meta-analysis.
Despite these limitations, our meta-analysis provides a leap in knowledge when compared to a previous study (15) that reviewed the association between the IL6 -174G/C polymorphism and risk of cancer. First, our updated review is more comprehensive than the previous, as we identified 83 independent articles with a total of 105,482 individuals on the association of IL6 -174G/C with cancer risk compared to 47 articles with 67,116 individuals in the previous report. Thus, our meta-analysis had significantly higher statistical power. Second, we noticed the potentially different roles of the IL6 -174G/C polymorphism in the development of cancer among various populations, and found different associations of this polymorphism with cancer risk between Africans and Caucasians. Third, sensitivity analysis of heterogeneity was used to detect studies that were responsible for between-study heterogeneity (24). Fourth, we assessed the pooled effect of the IL6 -174G/C polymorphism on cancer risk within or without the studies that did not follow HWE, and qualitatively similar results were found, suggesting that the estimations of our analyses are stable and convincing. Finally, for publication bias analysis, a modified method for testing funnel plot asymmetry was used (28), which maintains better control of the false-positive rate than the commonly used Egger's test. No publication bias was detected, suggesting that the pooled results should be unbiased.
In summary, the present meta-analysis provides evidence of the ethnic-specific association of the IL6 -174G/C polymorphism with cancer risk. More sophisticated gene-environment interactions should be considered in future analyses, which may result in better understanding of the relevance between the IL6 -174G/C polymorphism and risk of cancer. Moreover, this study reinforces the need to undertake investigations with very large number of cases and controls (including updated meta-analyses) to provide conclusive evidence for the associations between high-frequency genetic variants in low-penetrance genes and complex diseases, such as cancer.
Acknowledgments
This study was, in part, supported by grants from the National Natural Science Foundation of China (81171894, 30973425 and 30672400 to H.T. Zhang), the Program for New Century Excellent Talents in University (NCET-09-0165 to H.T. Zhang), the Science and Technology Committee of Jiangsu Province (BK2008162 to H.T. Zhang), the SRF for ROCS, State Education Ministry (2008890 to H.T. Zhang), the Qing-Lan Project of Education Bureau of Jiangsu Province (to H.T. Zhang), the ‘333’ Project of Jiangsu Province Government (to H.T. Zhang), and the Soochow Scholar Project of Soochow University (to H.T. Zhang). This study was also supported by grants #2R01GM069430-06, CA 137000, CA 112520 and CA 108741 from the NIH (to B. Pasche).
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Engels EA, Wu X, Gu J, Dong Q, Liu J, Spitz MR. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67:6520–6527. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- 6.Tindall EA, Severi G, Hoang HN, et al. Comprehensive analysis of the cytokine-rich chromosome 5q31.1 region suggests a role for IL-4 gene variants in prostate cancer risk. Carcinogenesis. 2010;31:1748–1754. doi: 10.1093/carcin/bgq081. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto T. Interleukin-6: from basic science to medicine – 40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 8.Katsumata N, Eguchi K, Fukuda M, et al. Serum levels of cytokines in patients with untreated primary lung cancer. Clin Cancer Res. 1996;2:553–559. [PubMed] [Google Scholar]
- 9.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belluco C, Olivieri F, Bonafe M, et al. -174 G>C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clin Cancer Res. 2003;9:2173–2176. [PubMed] [Google Scholar]
- 15.Xu B, Niu XB, Wang ZD, et al. IL-6 -174G>C polymorphism and cancer risk: a meta-analysis involving 29,377 cases and 37,739 controls. Mol Biol Rep. 2011;38:2589–2596. doi: 10.1007/s11033-010-0399-1. [DOI] [PubMed] [Google Scholar]
- 16.Yu KD, Di GH, Fan L, Chen AX, Yang C, Shao ZM. Lack of an association between a functional polymorphism in the interleukin-6 gene promoter and breast cancer risk: a meta-analysis involving 25,703 subjects. Breast Cancer Res Treat. 2010;122:483–488. doi: 10.1007/s10549-009-0706-5. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Yu W, Gwinn M, Clyne M, Yesupriya A, Khoury MJ. A navigator for human genome epidemiology. Nat Genet. 2008;40:124–125. doi: 10.1038/ng0208-124. [DOI] [PubMed] [Google Scholar]
- 20.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 21.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;14:154–176. doi: 10.1093/oxfordjournals.epirev.a036084. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet. 1998;351:123–127. doi: 10.1016/S0140-6736(97)08468-7. [DOI] [PubMed] [Google Scholar]
- 24.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 29.Dossus L, Kaaks R, Canzian F, et al. PTGS2 and IL6 genetic variation and risk of breast and prostate cancer: results from the Breast and Prostate Cancer Cohort Consortium (BPC3) Carcinogenesis. 2010;31:455–461. doi: 10.1093/carcin/bgp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster CB, Lehrnbecher T, Samuels S, et al. An IL6 promoter polymorphism is associated with a lifetime risk of development of Kaposi sarcoma in men infected with human immunodeficiency virus. Blood. 2000;96:2562–2567. [PubMed] [Google Scholar]
- 31.Morgan L, Cooper J, Montgomery H, Kitchen N, Humphries SE. The interleukin-6 gene -174G>C and -572G>C promoter polymorphisms are related to cerebral aneurysms. J Neurol Neurosurg Psychiatry. 2006;77:915–917. doi: 10.1136/jnnp.2005.081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theodoropoulos G, Papaconstantinou I, Felekouras E, et al. Relation between common polymorphisms in genes related to inflammatory response and colorectal cancer. World J Gastroenterol. 2006;12:5037–5043. doi: 10.3748/wjg.v12.i31.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vairaktaris E, Yapijakis C, Serefoglou Z, et al. Gene expression polymorphisms of interleukins-1 beta, -4, -6, -8, -10, and tumor necrosis factors-alpha, -beta: regression analysis of their effect upon oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134:821–832. doi: 10.1007/s00432-008-0360-z. [DOI] [PubMed] [Google Scholar]
- 34.Slattery ML, Wolff RK, Herrick JS, Caan BJ, Potter JD. IL6 genotypes and colon and rectal cancer. Cancer Causes Control. 2007;18:1095–1105. doi: 10.1007/s10552-007-9049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 39.Yeh HH, Lai WW, Chen HH, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–4309. doi: 10.1038/sj.onc.1209464. [DOI] [PubMed] [Google Scholar]
- 40.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ. 1994;309:1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 44.Robinson GE. Genomics. Beyond nature and nurture Science. 2004;304:397–399. doi: 10.1126/science.1095766. [DOI] [PubMed] [Google Scholar]
- 45.Frazer KA, Murray SS, Schork NJ, Topol EJ. Human genetic variation and its contribution to complex traits. Nat Rev Genet. 2009;10:241–251. doi: 10.1038/nrg2554. [DOI] [PubMed] [Google Scholar]
- 46.Cole SW, Arevalo JM, Takahashi R, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci USA. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu W, Du DY, Huang J, et al. Identification of interleukin-6 promoter polymorphisms in the Chinese Han population and their functional significance. Crit Care Med. 2008;36:1437–1443. doi: 10.1097/CCM.0b013e31816a0adb. [DOI] [PubMed] [Google Scholar]
- 48.Smith AJ, D'Aiuto F, Palmen J, et al. Association of serum interleukin-6 concentration with a functional IL6 -6331T>C polymorphism. Clin Chem. 2008;54:841–850. doi: 10.1373/clinchem.2007.098608. [DOI] [PubMed] [Google Scholar]
- 49.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]