Abstract
Thyroid function regulates lipid metabolism. Despite the fact that T2DM is more prevalent in the elderly, often associates with thyroid dysfunction and increases cardiovascular risk both per se and via high TC and LDL-C levels, the association of the latter with FT3 and FT4 levels has not yet been fully investigated in T2DM. While trying to fill this gap in 296 elderly outpatients with T2DM, we found that TC and LDL-C correlated negatively with FT4 and positively with FT3. When divided according to treatment by oral hypoglycaemic agents (OHA) and insulin (IT), they reacted differently with respect to investigated associations: in the OHA's TC and LDL-C correlated negatively with FT4 and showed no association with FT3, whereas, in the IT's TC and LDL-C correlated positively with FT3 and negatively with FT4. When controlled for possible confounding factors, these associations did not change in the IT's but were missing in the OHA's. Recent literature reports upon complex hypothalamic and peripheral interactions between T2DM and thyroid, and suggests T3 to enhance cholesterol synthesis and to have a role in insulin resistance states. Further investigations are needed to understand the intimate mechanisms of lipid metabolism in T2DM with respect to thyroid function.
1. Introduction
Thyroid function affects both lipid profiles and metabolic parameters [1]. Thyroxine (T4) and triiodothyronine (T3), the two main thyroid hormones, are secreted after thyroglobulin macropinocytosis and hydrolysis under the stimulation of pituitary thyrotropin (TSH) and are present in the blood mainly in noncovalent interactions with thyroxine-binding globulin, prealbumin, and albumin. Only 0.4% T3 and 0.04% T4 dynamically escape binding and, since free to interact directly with peripheral organs and tissues, are called free T3 (FT3) and free T4 (FT4) and are the only fractions believed to be metabolically active [2].
FT4 levels are about 100 times higher than those of FT3, which is considered to be the most active form of thyroid hormone and into which FT4 is converted within the peripheral tissue [3]. Inside a variety of cells, both interact with different affinity with α1, α2, β1, and β2 nuclear receptors in regulating the expression of target genes through the so-called thyroid response elements (TRE's), thus exerting the typical physiological functions which characterize them. These are mostly represented by enhanced calorigenesis, increased stroke volume and heart rate, enhanced sensitivity to catecholamines, bone turnover, gluconeogenesis, glycogenolysis, and lipolysis. The latter is mainly due to enhanced lipoprotein-lipase activity and increased hepatic low-density lipoprotein (LDL) receptor concentrations [4]. Besides genomic effects, widespread rapid onset nongenomic effects of FT3 and FT4 have been reported involving membrane-signalling pathways [5], the real extent of which has not been well defined yet [6].
When dealing with lipid metabolism, circulating T4 concentrations have always been found to be inversely associated with total (TC), high-density lipoprotein (HDL-C), and LDL cholesterol (LDL-C) levels [7]. In fact, high TC levels in hypothyroidism are caused by a reduction in LDL receptors [8] and L-T4 administration has a hypolipidemic effect in hypothyroidism, which is characterized by high thyrotropin (TSH) concentrations, meant at compensating for low FT4 levels. Thus, there is a greater benefit in patients displaying higher pretreatment TC or LDL-C and TSH despite the difficulty to identify any well-defined cut-off threshold for the association of the latter with lipids [9]. As a matter of fact, 4.3% hypercholesterolemic patients have been reported to be hypothyroid [10].
A contributing factor to high cholesterol levels in hypothyroidism is represented also by low FT3. Under normal conditions, the latter has the control over sterol regulatory element-binding protein-1 (SREBP-1), a crucial step for the expression of the LDL receptor, rather than over SREBP-2 [11]. However, it also acts directly by inducing at least two LDL-receptor TRE's in the liver [12]. Finally, increased flow of bile acids as a result of FT3 and FT4 is known to reduce cholesterol levels by depletion of its hepatic pool; however, such effect is counterbalanced by enhanced synthesis and uptake in the liver, eventually increasing TC and LDL-C under unfavourable circumstances [13].
Based on the considerations mentioned above, the relationship between thyroid function and the metabolic syndrome (MetS) has become a subject of interest for many research groups during the last few years [14], leading to the conclusion that even low-normal T4 levels may contribute to increased cardiovascular risk associated with lipoid abnormalities in people with the MetS [15]. Some of these studies reported that T3 behaved in a different way, being positively associated with body mass index (BMI) and waist girth [16–18]. A higher compensatory conversion of T4 to T3 to increase thermogenesis in obesity was the hypothesized mechanism [19, 20].
Diabetes mellitus (DM), in particular type 2 (T2DM), which is mostly associated with lipoid abnormalities and displays an increasing prevalence with age [21], is also known to dramatically increase cardiovascular risk (CVR) at any age [22]. Age does not seem to affect FT3 and FT4 levels in the human [23], whereas people with DM have been reported to suffer from thyroid dysfunction twice as much as the nondiabetic population [24]. Nevertheless, despite the fact that TC and LDL-C are major CVR factors identified as crucial targets in all current diabetes treatment guidelines [25], no extensive investigation can be found in the literature concerning the relation between thyroid and lipids in T2DM. This link is missing in the clinical management of people with DM now that carbohydrate response element-binding protein (ChREBP)—a newly identified lipogenic glucose-sensing transcription factor controlling hepatic lipogenesis—is known to be positively controlled by T3 in mammals [26] through its binding to thyroid receptor β-1. In fact, T3 has been shown to modulate hepatic lipogenesis through reciprocal regulation of SREBP-1c and ChREBP gene expression [27]. Moreover, T3 is endowed with a lipogenic effect via ChREBP enhancement in white adipose tissue, where both α and β thyroid receptors are expressed but only the β isoform is active with regard to that effect [28]. Thus, glucose, lipids, and thyroid hormones seem to interact according to a more complex mathematical function than as previously expected.
Taking into account the considerations mentioned above, with the present study, we evaluated the relations among FT3, FT4, TC, and LDL-C in elderly euthyroid people with T2DM.
2. Materials and Methods
We retrospectively evaluated clinical records from 350 outpatients, ages 70 years and older, referred to our clinic for T2DM during the last three years. All of them had thyroid function routinely evaluated in terms of FT3, FT4, and TSH concentrations. The study protocol was approved by the ethical committee. Exclusion criteria included smoking and any complications worse than background retinopathy, microalbuminuria, low-grade neuropathy, and nonobstructive arteriopathy. We also did not accept for this study patients with overt heart, liver or kidney failure, hypo/hyperthyroidism, or those on any medications possibly interfering with thyroid function. Thus, 296 people were qualified for study (245 women, 51 men), all taking statins since the time of diagnosis (4,5 ± 2,7 years) in order to prevent cardiovascular complications according to Italian Diabetes Guidelines [29]. They were either under oral hypoglycaemic agents (OHA subgroup, n = 196), invariably consisting of sulphonylureas and metformin, or under insulin treatment (IT subgroup, n = 100, of which 63 on 4 basal-bolus injections, the others with 2 or 3 injections as needed). Both subgroups followed a thorough self-monitoring blood glucose supervision associated with a strong empowering strategy [30].
Blood was drawn in our laboratory in the morning, after a 12-hour overnight fast. Chemistry was measured by Kodak Blood Multiple Analyzer and thyroid hormones by Immulite 2000 Immunoassay System.
Data evaluation was based upon SPSS 13.0 for descriptive (mean ± SD) and correlation analysis. Correlation analysis was performed first on all cases and then completed by partial correlation analysis applied to each subgroup (namely, OHA and IT), controlling for possible confounding factors. The least statistical significance of the differences among the means and of the associations was set at P < 0.05.
For clarification concerning the abbreviations used within the text, please refer to the list at the end of the paper.
3. Results
The means and the standard deviations (S.D.) of all recorded clinical parameters are presented in Table 1.
Table 1.
Clinical parameters (mean ± SD) in the 296 patients under evaluation.
| Mean | SD | |
|---|---|---|
| Age (years) | 76.4 | 5.2 |
| BMI (Kg/m2) | 31.5 | 7.4 |
| Dis-Dur (years) | 4.47 | 2.69 |
| HbA1c (%) | 7.95 | 2.08 |
| TC (mg/dL) | 188.5 | 40.4 |
| HDL-C (mg/dL) | 47.6 | 14.3 |
| LDL-C (mg/dL) | 111.0 | 35.5 |
| TG (mg/dL) | 148.6 | 81.4 |
| SBP (mmHg) | 136.1 | 16.5 |
| DBP (mmHg) | 74.8 | 11.4 |
| FT3 (pg/mL) | 2.76 | 0.77 |
| FT4 (pg/mL) | 12.5 | 5.9 |
| TSH (mU/L) | 1.44 | 1.35 |
When analysing all the cases, we observed a positive correlation of FT3 with both TC (r = 0.144, P < 0.05) and LDL-C (r = 0.161, P < 0.02). Conversely, FT4 correlated negatively with TC and LDL-C (r = −0.131, P < 0.05 and r = −0.134, P < 0.05, resp.). The disease duration and HbA1c were found to correlate negatively with LDL-C (r = −0.108, P < 0.05 and r = −0.144, P < 0.02, resp.). To avoid confounding effects, we introduced them together with other potentially interfering factors that are possibly related to thyroid function (age, BMI, and blood pressure) into partial correlation analysis of total and LDL-C with FT3 and FT4. As a result, no changes in correlation coefficient signs or significances were observed.
At this point, when we proceeded to further analyze the variance of observed parameters between OHA and IT subgroups, we found that the two were statistically homogenous with each other, as shown in Table 2.
Table 2.
Clinical parameters recorded in the IT and OHA patients: none of them differs significantly between subgroups.
| IT subgroup | OHA subgroup | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 76.2 | 4.9 | 76.5 | 5.2 |
| BMI (Kg/m2) | 30.9 | 6.4 | 31.9 | 7.9 |
| Dis-Dur (years) | 5.01 | 2.80 | 4.13 | 2.47 |
| HbA1c (%) | 8.0 | 2.5 | 7.9 | 2.1 |
| TC (mg/dL) | 184.6 | 41.6 | 191.0 | 40.3 |
| HDL-C (mg/dL) | 46.1 | 13.2 | 48.6 | 14.8 |
| LDL-C (mg/dL) | 107.5 | 35.1 | 113.2 | 36.2 |
| TG (mg/dL) | 150.2 | 67.5 | 144.1 | 74.4 |
| SBP (mmHg) | 137.1 | 16.6 | 135.7 | 16.6 |
| DBP (mmHg) | 73.8 | 12.4 | 75.4 | 11.1 |
| FT3 (pg/mL) | 2.73 | 0.94 | 2.80 | 0.65 |
| FT4 (pg/mL) | 11.9 | 2.1 | 12.8 | 7.1 |
| TSH (mU/L) | 1.45 | 1.40 | 1.37 | 1.29 |
Though, in terms of correlation analysis, the two subgroups behaved differently in terms of thyroid hormones. In fact, in the OHA, total and LDL cholesterol showed no correlation with FT3 (r = 0.037 and r = 0.098, resp., nonsignificant) but correlated negatively with FT4 (r = −0.144, P < 0.05 and r = −0,145, P < 0,05). Conversely, in the IT, total, and LDL cholesterol correlated positively with FT3 (r = 0.291, P < 0.01 and r = 0.275, P < 0.02, resp.) while maintaining the same negative correlation with FT4, as observed in the OHA (r = −0.239, P < 0.05 and r = −0.186, P < 0.05). Moreover, the IT revealed a previously “hidden” negative correlation between HDL cholesterol and FT4 (r = −0.302, P < 0.01).
We then repeated the same correlation analysis controlling lipid to thyroid relationship for age, BMI, HbA1c, blood pressure, and disease duration within each subgroup. As clearly shown in Figure 1, we could therefore confirm the positive correlation of FT3 with LDL cholesterol. Moreover, as summarized in Table 3, all the correlations previously described before were confirmed, including the one linking FT4 to HDL cholesterol.
Figure 1.
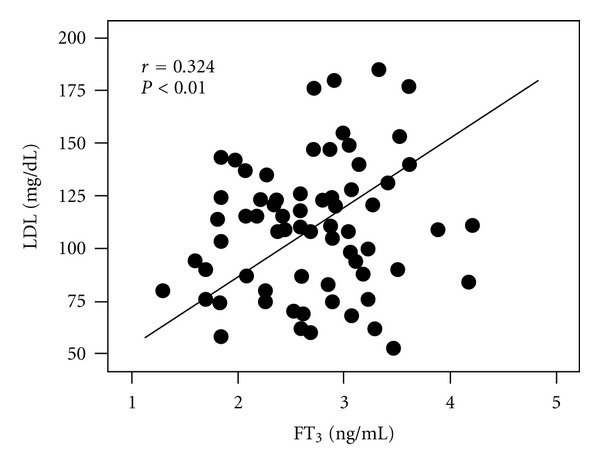
Association between FT3 and LDL cholesterol in IT-treated T2DM subjects controlling for age, BMI disease duration, blood pressure, and HbA1c.
Table 3.
Correlation coefficients of cholesterol and its LDL and HDL fractions to FT3 and FT4 in T2DM elderly patients controlling for age, BMI, disease duration, and HbA1c.
| Control variables | Thyroid hormone | Group | Statistics | Total cholesterol | LDL cholesterol | HDL cholesterol |
|---|---|---|---|---|---|---|
| Age, BMI, disease duration, blood pressure, and HbA1c | FT3 | IT | r | 0.346 | 0.324 | 0.147 |
| p | 0.006 | 0.009 | n.s. | |||
| OHA | r | −0.014 | 0.043 | −0.038 | ||
| p | n.s. | n.s. | n.s. | |||
| FT4 | IT | r | −0.295 | −0.241 | −0.310 | |
| p | 0.019 | 0.050 | 0.013 | |||
| OHA | r | −0.149 | −0.142 | −0.119 | ||
| p | n.s. | n.s. | n.s. |
IT: insulin treated; OHA: treated by oral hypoglycaemic agents.
4. Discussion
Before discussing our results, we will try to summarize them. TC and LDL-C displayed opposite correlations with FT3 (positive) and FT4 (negative) in elderly euthyroid subjects with T2DM. While a negative correlation would have been easy to accept and understand for thyroid hormones, a positive one was totally unexpected according to current concepts. When repeating the analysis in the two different subgroups, controlling them for confounding factors, we confirmed the above findings in the IT subgroup but not in the OHA subgroup.
However, regarding the clinical implications of our results, a crucial point is that elderly people with T2DM carry the burden of a high CVR and have to be treated as carefully as their younger counterpart since morbility/mortality increases together with their LDL-C levels. With this in mind, diabetologists often concentrate on glucose, lipids, and blood pressure and generally do not take into account thyroid hormones in the absence of typical symptoms of thyroid dysfunction [31]. On the other hand, subclinical hypo- and hyperthyroidism are not rare at all. As geriatric endocrinologists, we prefer to assay thyroid hormones in diabetic elderly patients in order to rule out any subtle thyroid malfunction during the course of overall evaluation.
Such a habit allowed us to collect data from a number of euthyroid elderly people with T2DM and to perform a correlation analysis between cholesterol and thyroid hormones. With regard to FT4, the analysis confirmed that thyroid function negatively correlates with TC and LDL-C, a trend commonly found in the general population. However, quite unexpectedly FT3 behaved the opposite way, being positively associated with TC and LDL-C. We then analyzed the association between thyroid hormones and all possible confounding factors. We found that age correlated positively and HbA1c and negatively to TC and LDL-C. Therefore, we introduced these two factors and other potentially interfering parameters including BMI, blood pressure, and disease duration as confounding factors, confirming their associations by performing partial correlation analysis.
After we divided our subjects into OHA and IT subgroups, the analysis of variance showed that they were fully homogeneous in terms of recorded clinical parameters. The only difference was in their treatment, namely, metformin/sulphonylureas versus insulin. This made us more confident in trying to reveal eventual differences occurring in relations between lipid profile and thyroid hormones. In fact, this would allow us to check whether different associations within subgroups are possibly linked to different treatment regimens.
In the OHA group, TC and LDL-C displayed no correlation with FT3 but correlated negatively with FT4 (P < 0.05), whereas in the IT group, TC and LDL-C correlated positively with FT3 (P < 0.02) and negatively with FT4 (P < 0.05). Once again we performed partial correlation analysis in the IT subgroup and found that the positive association of lipids with FT3 became even stronger while that with FT4 remained the same (see Table 3); however, both vanished in the OHA subgroup.
Such findings might seem contradictory, but, in fact, they fit well with the role played by FT3 on SREBP-1 and SREBP-2 control, and consequently on the expression of LDL receptor, with its ability to indirectly enhance cholesterol synthesis and uptake by the liver. Moreover, in some previous studies an unexpected positive association between FT3 and lipids was mentioned without indepth explanation, and therefore remained underestimated. For instance, De Pergola et al. [32] found that FT3 correlated negatively with HDL-C levels (P < 0.001), and, in multiple correlation analysis, maintained an independent positive association with age (P < 0.001), waist girth (P < 0.05) and insulin levels (P < 0.001), a proxy for insulin resistance. It is worthwhile noting that in the study of De Pergola et al., FT3 was also associated with smoking habits. Our study took into account only nonsmokers, thus ruling out a priori a possible strong confounding factor. Others confirmed the previously mentioned association between FT3 and MetS components [15] suggesting insulin-resistance to be the link between the thyroid and lipids. Therefore, T3 may act as a strong independent metabolic signal in euthyroid insulin-resistant T2DM patients [33, 34].
Interestingly enough, according to recent reports, T3 added to diets containing peanut oil increased serum lipids in rats, sometimes even up to 20-fold [35], whereas T3 was found to increase cholesterol biosynthesis in the liver through the activation of de novo protein synthesis [36, 37]. Furthermore, T3, insulin, and their combination markedly stimulate cholesterol synthesis in cultured human skin fibroblasts [38], and in recent studies FT3 has been even shown to exert a beta-cell protective effect [39, 40]. All above considerations might explain the association we found between FT3 and TC and LDL-C in our IT patients and not in our OHA patients. In fact, it seems as if in the presence of insulin resistance T3 may not be able to act as fully as in the case of an unopposed insulin signal, such as when metabolically effective exogenous insulin levels are attained. Still another possible explanation for our findings result from an eventual compensatory increase in FT3. In fact, based upon widely accepted lipid metabolism regulation mechanisms by thyroid hormones, it might be hypothesized that in our patients cholesterol synthesis might have been enhanced by exogenous insulin and by peripheral T3 conversion from T4 [41, 42].
Another apparently anomalous finding, regarding the negative correlation of HDL-C with FT4 levels (P < 0.01) but not with FT3, deserves more discussion. Since early studies in the field, HDL-C was found to decrease both in hypo- and in hyperthyroidism, thus indicating that such association followed a U-shaped curve and could, therefore, only be analyzed within the physiological range of FT4 concentrations [43, 44]. In fact, slightly different behaviour between the two hormones has been reported in the literature, including a stronger enhancing effect of HDL-C on T4 target cell penetration in comparison to T3 target cell penetration [45]. A pro-HDL and anti-LDL effect of T3 was also described [46]. These observations have been often overlooked, despite their potential pharmacologic utilization in the metabolic syndrome.
We are aware of the intrinsic limits of the retrospective cross-sectional character of our study, which refers to subjects over 70 years of age with T2DM and, therefore, allows no definite conclusions with regard to younger people or in terms of cause-effect relationships. Nevertheless, due to the different association patterns found in IT patients as compared to OHA patients, we feel it is worthwhile to further investigate the topic.
Specifically, reported data prompts some reappraisal regarding the relationship among some hidden aspects of cholesterol, insulin, and thyroid hormone metabolism, eventually fostering new controlled studies concerning the role of T3 in T2DM and the possible pharmacological interferences that different drugs may have on it.
Based on our data, it seems more prudent to treat elderly hypothyroid patients with T4, without any T3 integration if they are on insulin. This might prevent the risk of letting their LDL-C increase and thus of increasing statin dosage. We still do not fully understand the meaning of the association we found between FT3 and cholesterol in IT people with T2DM. In other terms, the positive association might be the expression of a compensatory hormone response to spontaneous LDL-C increase, as already hypothesized for patients with the MetS [15, 33, 34] and in line with the complex DM-thyroid interactions occurring via hypothalamic glucose sensing mechanisms [47] (as recently reviewed by Duntas et al. [48]).
In conclusion, in order to clarify the pathophysiological mechanisms underlying the observed results further experimental and clinical follow-up studies are needed. Should the observed associations be confirmed by future investigations, in older T2DM patients it might be useful to include FT3 once again in thyroid test panels which today are mostly limited to screening TSH and confirmation FT4.
Acknowledgments
The publication of the present paper has been made possible by research funds provided by the Italian Space Agency (ASI) through INRCA, Rome, research Contract I/010/11/0 for the study of the endocrine and metabolic effects of isolation-confinement (experiment MARS 500).
Abbreviations
- BMI:
Body mass index
- ChREBP:
Carbohydrate response element-binding protein
- DBP:
Diastolic blood pressure
- Dis-Dur:
Disease duration
- DM:
Diabetes mellitus
- FT3:
Free triiodothyronine
- FT4:
Free thyroxine
- HbA1c:
Glycated haemoglobin
- HDL:
High density lipoprotein
- HDL-C:
HDL cholesterol
- IT:
Insulin treated
- LDL:
Low-density lipoprotein
- LDL-C:
LDL cholesterol
- OHA:
Oral hypoglycaemic agents
- SBP:
Systolic blood pressure
- SREBP:
Sterol regulatory element-binding protein
- T2DM:
Type 2 diabetes mellitus
- T3:
Triiodothyronine
- T4:
Thyroxine
- TC:
Total cholesterol
- TG:
Triglycerides
- TRE:
Thyroid response element
- TSH:
Thyroid stimulating hormone.
References
- 1.Risal P, Maharjan BR, Koju R, Makaju RK, Gautam M. Variation of total serum cholesterol among the patient with thyroid dysfunction. Kathmandu University Medical Journal. 2010;8(30):265–268. doi: 10.3126/kumj.v8i2.3573. [DOI] [PubMed] [Google Scholar]
- 2.Gershengorn MC, Glinoer D, Robbins J. Transport and metabolism of thyroid hormones. In: De Visscher M, editor. The Thyroid Gland. New York, NY, USA: Raven Press; 1980. pp. 81–121. [Google Scholar]
- 3.Bernal J, DeGroot LJ. Mode of action of thyroid hormones. In: De Visscher M, editor. The Tyroid Gland. New York, NY, USA: Raven Press; 1980. pp. 123–143. [Google Scholar]
- 4.Yen PM. Physiological and molecular basis of Thyroid hormone action. Physiological Reviews. 2001;81(3):1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 5.Davis PJ, Davis FB. Nongenomic actions of thyroid hormone. Thyroid. 1996;6(5):497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- 6.Bassett JHD, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Molecular and Cellular Endocrinology. 2003;213(1):1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Celi FS, Zemskova M, Linderman JD, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. Journal of Clinical Endocrinology & Metabolism. 2011;96(11):3466–3474. doi: 10.1210/jc.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimberg M, Olubadewo JO, Wilcox HG. Plasma lipoproteins and regulation of hepatic metabolism of fatty acids in altered thyroid states. Endocrine Reviews. 1985;6(4):590–607. doi: 10.1210/edrv-6-4-590. [DOI] [PubMed] [Google Scholar]
- 9.Barbagallo CM, Averna MR, Liotta A, et al. Plasma levels of lipoproteins and apolipoproteins in congenital hypothyroidism: effects of L-thyroxine substitution therapy. Metabolism. 1995;44(10):1283–1287. doi: 10.1016/0026-0495(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 10.Tagami T, Kimura H, Ohtani S, et al. Multi-center study on the prevalence of hypothyroidism in patients with hypercholesterolemia. Endocrine Journal. 2001;58(6):449–457. doi: 10.1507/endocrj.k11e-012. [DOI] [PubMed] [Google Scholar]
- 11.Gnoni GV, Rochira A, Leone A, Damiano F, Marsigliante S, Siculella L. 3,5,3߰triiodo-L-thyronine induces SREBP-1 expression by non-genomic actions in human HEP G2 cells. Journal of Cellular Physiology. 2012;227(6):2388–2397. doi: 10.1002/jcp.22974. [DOI] [PubMed] [Google Scholar]
- 12.Lopez D, Abisambra Socarrás JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochimica et Biophysica Acta. 2007;1771(9):1216–1225. doi: 10.1016/j.bbalip.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Walton KW, Scott PJ, Dykes PW, Davies JW. The significance of alterations in serum lipids in thyroid dysfunction. II. Alterations of the metabolism and turnover of 131-I-low-density lipoproteins in hypothyroidism and thyrotoxicosis. Clinical science. 1965;29(2):217–238. [PubMed] [Google Scholar]
- 14.Kumar HK, Yadav RK, Prajapati J, Reddy CV, Raghunath M, Modi KD. Association between thyroid hormones, insulin resistance, and metabolic syndrome. Saudi Medical Journal. 2009;30(7):907–911. [PubMed] [Google Scholar]
- 15.Taneichi H, Sasai T, Ohara M, et al. Higher serum free triiodothyronine levels within the normal range are associated with metabolic syndrome components in type 2 diabetic subjects with euthyroidism. Tohoku Journal of Experimental Medicine. 2011;224(3):173–178. doi: 10.1620/tjem.224.173. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. Journal of Clinical Endocrinology and Metabolism. 2005;90(7):4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 17.Sestoft L. Metabolic aspects of the calorigenic effect of thyroid hormone in mammals. Clinical Endocrinology. 1980;13(5):489–506. doi: 10.1111/j.1365-2265.1980.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 18.De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clinical Endocrinology. 2007;67(2):265–269. doi: 10.1111/j.1365-2265.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 19.Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clinical Endocrinology. 2003;59(2):258–262. doi: 10.1046/j.1365-2265.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. American Journal of Clinical Nutrition. 2000;71(6):1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 21.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia. 2009;52(10):2001–2014. doi: 10.1007/s00125-009-1454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robuschi G, Safran M, Braverman LE, Gnudi A, Roti E. Hypothyroidism in the elderly. Endocrine Reviews. 1987;8(2):142–153. doi: 10.1210/edrv-8-2-142. [DOI] [PubMed] [Google Scholar]
- 24.Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. International Journal of Clinical Practice. 2010;64(8):1130–1139. doi: 10.1111/j.1742-1241.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(supplement 34):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iizuka K, Horikawa Y. ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocrine Journal. 2008;55(4):617–624. doi: 10.1507/endocrj.k07e-110. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto K, Ishida E, Matsumoto S, et al. Carbohydrate response element binding protein gene expression is positively regulated by thyroid hormone. Endocrinology. 2009;150(7):3417–3424. doi: 10.1210/en.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauthier K, Billon C, Bissler M, et al. Thyroid hormone receptor β(TRβ) and liver X receptor (LXR) regulate carbohydrate-response element-binding protein (ChREBP) expression in a tissue-selective manner. Journal of Biological Chemistry. 2010;285(36):28156–28163. doi: 10.1074/jbc.M110.146241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruno G, De Micheli A, Frontoni S, Monge L. Highlights from “Italian Standards of Care for Diabetes Mellitus 2009-2010”. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(4):302–314. doi: 10.1016/j.numecd.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Kruger DF, Lorenzi GM, Dokken BB, Sadler C, Mann K, Valentine V. Managing diabetes with integrated teams: maximizing your efforts with limited time. Postgraduate Medicine. 2012;124(2):64–76. doi: 10.3810/pgm.2012.03.2538. [DOI] [PubMed] [Google Scholar]
- 31.Papaleontiou M, Haymart MR. Approach to and treatment of thyroid disorders in the elderly. Medical Clinics of North America. 2012;96(2):297–310. doi: 10.1016/j.mcna.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Pergola G, Ciampolilloi A, Alò D, Sciaraffia M, Guida P. Free triiodothyronine is associated with smoking habit, independently of obesity, body fat distribution, insulin, and metabolic parameters. Journal of Endocrinological Investigation. 2010;33(11):815–818. doi: 10.1007/BF03350348. [DOI] [PubMed] [Google Scholar]
- 33.Lambadiari V, Mitrou P, Maratou E, et al. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine. 2011;39(1):28–32. doi: 10.1007/s12020-010-9408-3. [DOI] [PubMed] [Google Scholar]
- 34.Ruhla S, Arafat AM, Weickert MO, et al. T3/rT3-ratio is associated with insulin resistance independent of TSH. Hormone and Metabolic Research. 2011;43(2):130–134. doi: 10.1055/s-0030-1267997. [DOI] [PubMed] [Google Scholar]
- 35.Fayek KI. Effect of dietary fats and triiodothyronine administration on the lipid components of serum and tissues in the rat. Zeitschrift fur Ernahrungswissenschaft. 1979;18(4):269–274. doi: 10.1007/BF02020517. [DOI] [PubMed] [Google Scholar]
- 36.Lopez JP, Sancho MJ, Marino A, Macarulla JM. Cholesterol biosynthesis in chicken liver: effect of triiodothyronine. Experimental and Clinical Endocrinology. 1984;84(1):45–51. doi: 10.1055/s-0029-1210365. [DOI] [PubMed] [Google Scholar]
- 37.Cocco T, Petragallo VA, Gnoni GV. Short term stimulation of lipogenesis by triiodothyronine in rat hepatocyte cultures. Bollettino della Societa Italiana di Biologia Sperimentale. 1985;61(4):555–562. [PubMed] [Google Scholar]
- 38.Amorosa LF, Khachadurian AK, Harris JN. The effects of triiodothyronine, hydrocortisone and insulin on lipid synthesis by cultured fibroblasts preincubated in a serum-free medium. Biochimica et Biophysica Acta. 1984;792(2):192–198. doi: 10.1016/0005-2760(84)90222-4. [DOI] [PubMed] [Google Scholar]
- 39.Falzacappa CV, Mangialardo C, Madaro L, et al. Thyroid hormone T3 counteracts STZ induced diabetes in mouse. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019839.e19839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falzacappa CV, Mangialardo C, Raffa S, et al. The thyroid hormone T3 improves function and survival of rat pancreatic islets during in vitro culture. Islets. 2010;2(2) doi: 10.4161/isl.2.2.11170. [DOI] [PubMed] [Google Scholar]
- 41.Fadeyev VV, Morgunova TB, Melnichenko GA, Dedov II. Combined therapy with L-Thyroxine and L-Tiiodothyronine compared to L-Thyroxine alone in the treatment of primary hypothyroidism. Hormones. 2010;9(3):245–252. doi: 10.14310/horm.2002.1274. [DOI] [PubMed] [Google Scholar]
- 42.Heimberg M, Olubadewo JO, Wilcox HG. Plasma lipoproteins and regulation of hepatic metabolism of fatty acids in altered thyroid states. Endocrine Reviews. 1985;6(4):590–607. doi: 10.1210/edrv-6-4-590. [DOI] [PubMed] [Google Scholar]
- 43.Agdeppa D, Macaron C, Mallik T, Schnuda ND. Plasma high density lipoprotein cholesterol in thyroid disease. Journal of Clinical Endocrinology and Metabolism. 1979;49(5):726–729. doi: 10.1210/jcem-49-5-726. [DOI] [PubMed] [Google Scholar]
- 44.Boberg J, Dahlberg PA, Vessby B, Lithell H. Serum lipoprotein and apolipoprotein concentrations in patients with hyperthyroidism and the effect of treatment with carbimazole. Acta Medica Scandinavica. 1984;215(5):453–459. doi: 10.1111/j.0954-6820.1984.tb17678.x. [DOI] [PubMed] [Google Scholar]
- 45.Benvenga S, Alesci S, Trimarchi F. High-density lipoprotein-facilitated entry of thyroid hormones into cells: a mechanism different from the low-density lipoprotein-facilitated entry. Thyroid. 2002;12(7):547–556. doi: 10.1089/105072502320288384. [DOI] [PubMed] [Google Scholar]
- 46.Ness GC, Lopez D, Chambers CM, et al. Effects of L-triiodothyronine and the thyromimetic L-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor 3-hydroxy-3-methylglutaryl coenzyme A reductase, and Apo A-I gene expression. Biochemical Pharmacology. 1998;56(1):121–129. doi: 10.1016/s0006-2952(98)00119-1. [DOI] [PubMed] [Google Scholar]
- 47.Schöfl C, Schleth A, Berger D, Terkamp C, Von zur Mühlen A, Brabant G. Sympathoadrenal counterregulation in patients with hypothalamic craniopharyngioma. Journal of Clinical Endocrinology and Metabolism. 2002;87(2):624–629. doi: 10.1210/jcem.87.2.8193. [DOI] [PubMed] [Google Scholar]
- 48.Duntas LH, Orgiazzi J, Brabant G. The Interface between thyroid and diabetes mellitus. Clinical Endocrinology. 2011;75(1):1–9. doi: 10.1111/j.1365-2265.2011.04029.x. [DOI] [PubMed] [Google Scholar]


