Abstract
Treatment with glutamine has been shown to reduce myocardial damage associated with ischemia/reperfusion injury. However, the cardioprotective effect of glutamine specifically after burn injury remains unclear. The present study explores the ability of glutamine to protect against myocardial damage in rats that have been severely burned. Seventy-two Wistar rats were randomly divided into three groups: normal controls (C), burned controls (B) and a glutamine-treated group (G). Groups B and G were subjected to full thickness burns comprising 30% of total body surface area. Group G was administered 1.5 g/ (kg•d) glutamine and group B was given the same dose of alanine via intragastric administration for 3 days. Levels of serum creatine kinase (CK), lactate dehydrogenase (LDH), aspartate transaminase (AST) and blood lactic acid were measured, as well as myocardial ATP and glutathione (GSH) contents. Cardiac function indices and histopathological changes were analyzed at 12, 24, 48 and 72 post-burn hours. In both burned groups, levels of serum CK, LDH, AST and blood lactic acid increased significantly, while myocardial ATP and GSH contents decreased. Compared with group B, CK, LDH, and AST levels were lower and blood lactic acid, myocardial ATP and GSH levels were higher in group G. Moreover, cardiac contractile function inhibition and myocardial histopathological damage were significantly reduced in group G compared to B. Taken together, these results show that glutamine supplementation protects myocardial structure and function after burn injury by improving energy metabolism and by promotedthe synthesis of ATP and GSH in cardiac myocytes.
Keywords: Burn injury, glutamine, myocardial damage, energy metabolism, GSH, rat
Introduction
Ischemic/hypoxic damage is the primary cause of tissue and organ damage after burn injury [1]. Until recently, it was widely believed that burn injury did not cause this type of damage in heart tissues; however, the last two studies by our group showed that regional myocardial blood flow is significantly reduced 1 h after a severe burn, remaining considerably lower than in the control group up to 24 h post-burn. Furthermore, ATP synthesis in the myocardium is also significantly decreased under these conditions [2,3]. Therefore, ischemia/hypoxia and energy metabolism defects in cardiac myocytes can lead to cardiac dysfunction after severe burn injury. These findings highlight a potentially important therapeutic role for improving myocardial haemoperfusion and cardiac myocyte energy metabolism after burn injury, as well as protecting myocardial functions and reducing cardiac damage.
A number of recent studies have shown that several amino acids, such as glutamine, glycine, arginine and taurine, have cardioprotective properties, including a cytoprotective effect on cardiac myocytes [4-9] . Glutamine, in particular, is one of the principal free intracellular amino acids in mammalian cardiac myocytes, where it plays a central role in the biosynthesis of nucleotides and proteins by acting as an energy and nitrogen donor [10-12]. Moreover, previous studies have demonstrated that glutamine administration can improve cardiac myocyte energy metabolism, promote ATP biosynthesis, increase energy reserves and accelerate cardiac muscle functional recovery in an ischemia/reperfusion (I/R) injury model [13-15]. Glutamine is currently used to treat various short-bowel syndrome, as well as others [16]. However, there is no study has demonstrated the cytoprotective effect of glutamine on cardiac myocytes specifically after burn injury. Herein, we demonstrate the cardioprotective effects of glutamine in rats after severe burn injury and explore the possible mechanisms through which this protection occurs.
Materials and methods
Drugs and reagents
ATP and glutamine were obtained from Sigma Chemical Co. (St. Louis, MO). Blood lactic acid and glutathione (GSH) detection kits were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Alanine injections were purchased from Fujian Haiwang Pharmaceutical Ltd. (Fuzhou, China). All other chemicals and reagents were of analytical grade.
Experimental animals
Seventy-two male adult Wistar rats (weighing 205 g to 255 g) were obtained from the Laboratory Animal Centre, Third Military Medical University, and placed in individual wire-bottomed cages under controlled temperature and humidity with a 12 h light-dark cycle. The rats were acclimatised to the environment with a diet of standard rat pellets for 7 d prior to the experiment. They were then randomly divided into three groups: normal control (C), burned control (B) and glutamine-treated (G) groups. The eight rats in group C were shaved and received anaesthesia, but were not burned. The 64 rats in groups B and G were subjected to full thickness (third degree) burns comprising 30% of total body surface area under general anaesthesia (pentobarbital, 40 mg/kg of body weight) and analgesia (buprenorphine, 1 mg/kg of body weight). The rats were anaesthetized, shaved, napalm-burned for 18 s, and intraperitoneally injected with lactated Ringer’s solution (1.5 ml/kg per 1% of burned body surface area) for resuscitation. The eight rats were observed at each time points(12, 24, 48 and 72 post-burn hours) in each group .
The experimental process followed the regulations stipulated by the Third Military Medical University Animal Care Committee, according to the protocol outlined in the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication no. 85-23, revised 1996).
Treatments
Group G was supplemented with 1.5 g/kg of glutamine per day and group B was supplemented with the same dose of alanine via intragastric administration, both for 3 days. All treatments were administered twice per day. Rats were housed in individual cages and had free access to food and water. At each observation point, the cardiac contractile function index was measured, and myocardial tissue and blood plasma were harvested.
Histological and water content examination
The harvested cardiac apex was fixed in formalin. Tissues were dehydrated, embedded in paraffin wax, cut into 5 μm sections, and plated. The histological sections were stained with haematoxylin and eosin (HE) after removing the tissues from the paraffin. At the end of each experiment, a sample of the cardiac apex (0.1-0.2g) was dissected, weighed and stored in a separate glass container. After being dried in an oven at 100°C for 48 hours, each sample was reweighed on the same scale. Water content was calculated using the following formula: (wet weight - dry weight)/ wet weight x 100%.
Myocardial zymogram
Blood samples were collected in tubes and centrifuged at 4000 rpm at 4 °C for 10 min, after which the serum fraction was transferred to another clean tube. Myocardial injury was assessed by determining the activity levels of serum creatine kinase (CK), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) using an autoanalyser AU-800 (Olympus, Japan). Results are expressed in international units per litre.
Lactic acid levels in blood and GSH levels in tissues
Levels of lactic acid in the blood were measured using a blood lactic acid detection kit and a 721 spectrophotometer (Beckman, US). The procedure was performed according to the kit protocol. Briefly, blood (0.1 ml) was processed with protein precipitant (0.6 ml) in an anticoagulant tube. The mixture was then centrifuged at 4000 rpm at 4 °C for 8 min. The colour reaction was monitored by measuring absorbance at 530 nm. Results are expressed as millimoles of lactic acid per litre of whole blood.
Levels of GSH in tissues were determined using a GSH detection kit and a 721 spectrophotometer, according to the protocol provided by the kit. Briefly, approximately 100 mg of myocardial tissue was homogenized with 5% sulphosalicylic acid and 1 ml of PBS solution. The homogenate was kept on ice for 30 min, and then centrifuged at 18000 rpm at 4 °C for 20 min. Total GSH concentrations were determined by adding 2-nitrobenzoic acid into appropriate aliquots of supernatant. The ensuing colour reaction was monitored by measuring absorbance at 412 nm. Protein concentrations in the supernatant were determined using BCA reagent (Pierce, US). Results are expressed as micromoles of GSH per gram of protein.
ATP levels in tissues
The heart was arrested in vivo with a cold cardioplegic solution, and the myocardial tissue of the cardiac apex was immediately frozen using a metal splint precooled by liquid nitrogen. The frozen myocardial tissue was then mulled into a fine powder using a mortar and pestle, and the powder was transferred to a test tube containing 0.6 N cold perchloric acid. Metabolites were extracted, and the extract was neutralised with a mixture of KOH and K2CO3 and then centrifuged at 8000 rpm at 4 °C for 15 min. The supernatant (10 μl) was subjected to high performance liquid chromatography UV/VIS-152 (Gilson, France). Results are expressed as micromoles of ATP per milligram of protein.
Cardiac contractile function assay
Pentobarbital sodium (40 mg/kg) was used as an anaesthetic prior to exposing the right common carotid artery. A polyethylene catheter filled with 100 U/ml of heparin saline was then inserted into the left cardiac ventricle and the other end of the catheter was connected to the press transducer of a four channel physiological recorder (Nihon Kohden, Japan). After 5 min of stability, aortic systolic pressure (AOSP), aortic diastolic pressure (AODP), left ventricular systolic peak pressure (LVSP) and ±dp/dtmax were measured.
Statistical analysis
All values are expressed as mean±standard deviation (SD). Since our experimental design contained two variables, one being the treatment factor and the other the time factor, two-way analyses of variance (Two-Way ANOVAs) were performed on the data to simultaneously test the significance of the two variables and their interaction. All statistical analyses were done using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). A two-sided probability value of less than 0.05 was considered significant.
Results
Myocardium histology and water content
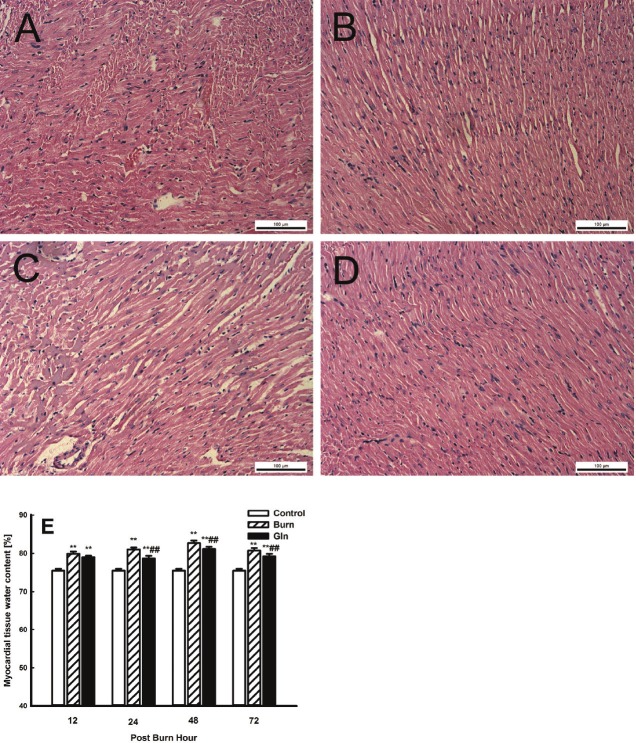
We observed a number of pathomorphological changes in rat myocardial tissues after severe burn injury. At 12 PBH, histological analysis revealed cardiac interstitial oedema, fibre engorgement, transverse striation defects, poorly defined cell boundaries and cytoplasm destruction. By 72 PBH, inflammatory characteristics such as engorged capillaries and exuded erythrocytes were also observed in myocardial tissues. Overall, the major pathomorphological features included cardiac interstitial engorgement and oedema (Figure 1A-D). In general, group G exhibited less severe structural damages than group B. Myocardial water content was significantly higher in groups B and G than in group C (P<0.01), and maximum water content was observed in group B at 48 PBH (Figure 1E). In contrast, glutamine supplementation was able to partially suppress some of the pathomorphological changes associated with burn injury, and the water content in group G was lower than that in group B at all time points (P<0.01).
Figure 1.
Cardiac pathological change with glutamine treatment after burn injury. (A-D) HE stained sections showing cardiac apex tissue of A. group B at 12 PBH, B. group G at 12 PBH, C. group B at 24 PBH, and D. group G at 72 PBH. Images were taken at 200x. E. Changes in the water content of myocardial tissue samples from the three groups at 12, 24, 48 and 72 PBH. Data are mean±SD. 2-Way ANOVA; **, P<0.01 compared with group C; ##, P<0.01 compared with group B.
Myocardial zymogram
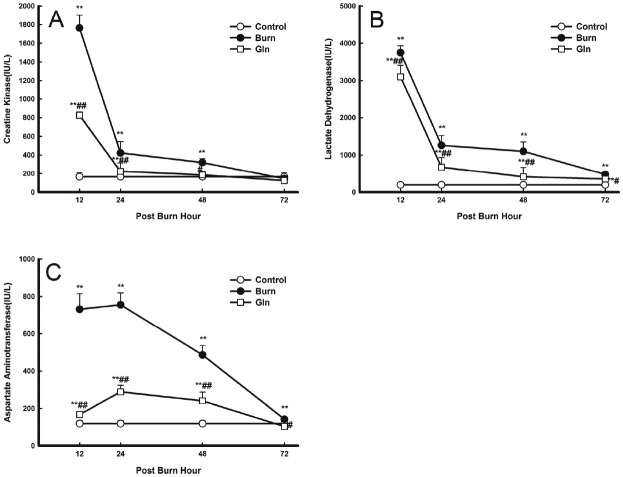
Serum activity levels of AST, CK and LDH were initially low in groups C, but increased dramatically in groups B and G after burn injury. Maximum levels were observed at 12 PBH (CK and LDH) and at 24 PBH (AST), after which activity decreased steadily back down to control levels at 72 PBH. Serum activities in group G decreased compared with those in group B (P<0.05, P<0.01) (Figure 2).
Figure 2.
Effects of glutamine treatment on myocardial zymogram activity at 12, 24, 48, and 72 PBH. Changes in the serum levels of various proteins over 72 PBH: A. CK, B. LDH and C. AST. Data are expressed as mean±SD. *, P<0.05 and **, P<0.01 compared with group C; #, P<0.05 and ##, P<0.01 compared with group B.
Blood lactic acid content
Consistent with the abovementioned findings, blood lactic acid levels were initially low and increased significantly after burn injury. Maximal lactic acid concentrations were observed at 24 PBH and decreased steadily over time, but had not yet returned to control levels at 72 PBH. Blood lactic acid levels in group G were significantly lower than in group B at 12, 24 and 48 PBH (P<0.05, P<0.01), but remained higher than in group C at all time points (P<0.01) (Figure 3).
Figure 3.
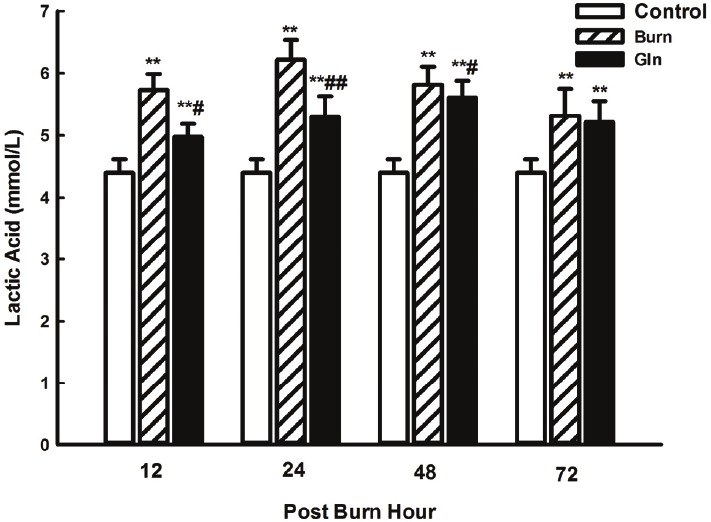
Effects of glutamine treatment on lactic acid content in the blood at 12, 24, 48, and 72 PBH. Data are expressed as mean±SD. *, P<0.05 and **, P<0.01 compared with group C; #, P<0.05 and ##, P<0.01 compared with group B.
ATP levels in myocardial tissue
Levels of ATP in myocardial tissue decreased continuously after burn injury in group B compared with group C (P<0.01). Minimum ATP levels in group B were observed at 72 PBH. However, glutamine supplementation for 3 days dramatically suppressed this effect, and ATP levels in group G were significantly higher than in group B at 24, 48 and 72 PBH (P<0.05, P<0.01) (Figure 4).
Figure 4.
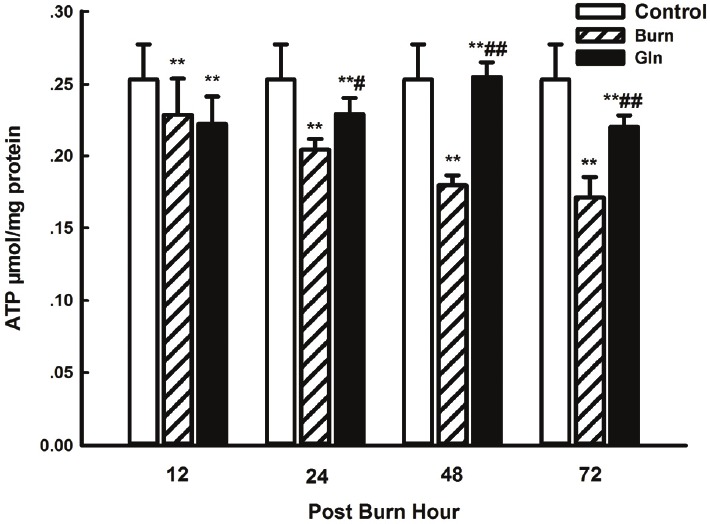
Effects of glutamine treatment on myocardial ATP content at 12, 24, 48, and 72 PBH. Data are expressed as mean±SD. *, P<0.05 and **, P<0.01 compared with group C; #, P<0.05 and ##, P<0.01 compared with group B.
GSH levels in myocardial tissue
GSH levels in myocardial tissue were high in group C and markedly reduced in group B at all time points (P<0.05, P<0.01), with the lowest level being observed at 48 PBH. Compared with group B, the decrease in GSH levels in group G was much less pronounced at 12 and 24 PBH (P<0.01) (Figure 5).
Figure 5.
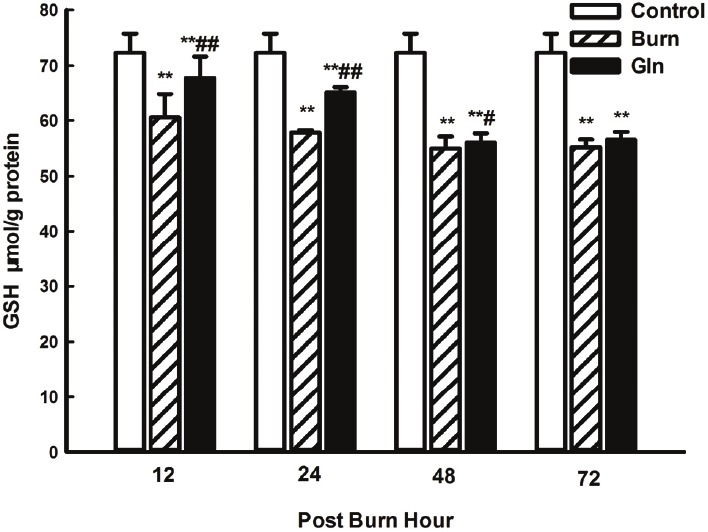
Effects of glutamine treatment on myocardial GSH content at 12, 24, 48, and 72 PBH. Data are expressed as mean±SD. *, P<0.05 and **, P<0.01 compared with group C; #, P<0.05 and ##, P<0.01 compared with group B.
Cardiac contractile function assay
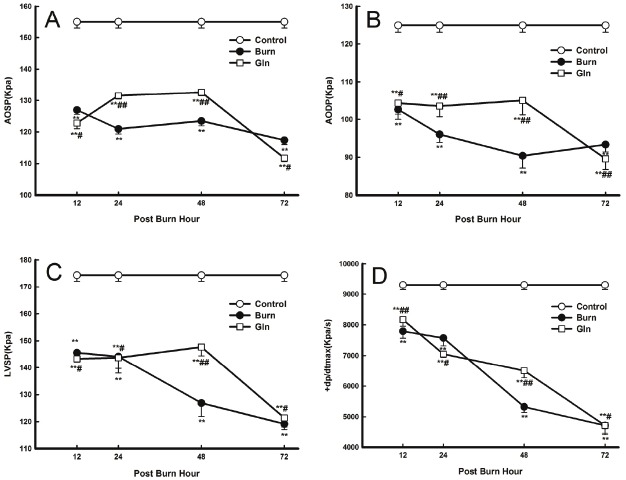
Cardiac contractile dysfunction was observed as of 12 PBH and persisted throughout the duration of the observations. AOSP, AODP, LVSP and +dp/dtmax were all significantly decreased at all time points in groups B and G compared with group C. Minimum values were observed at 48 PBH (AODP) and at 72 PBH (AOSP, LVSP, and +dp/dtmax). Cardiac function indices were significantly improved in group G compared with group B (P<0.05, P<0.01) (Figure 6).
Figure 6.
Effects of glutamine treatment on cardiac function at 12, 24, 48, and 72 PBH. Changes in the various cardiac function indices: A. AOSP, B. AODP, C. LVSP and D. +dp/dtmax. Data are expressed as mean±SD. *, P<0.05 and **, P<0.01 compared with group C; #, P<0.05 and ##, P<0.01 compared with group B.
Discussion
Glutamine treatment after burn injury improves myocardial energy metabolism
Although glucose and fatty acids are the main sources of energy for cardiac myocytes, several studies have shown that glutamine can improve energy synthesis under pathological conditions [12,13,15,17]. The results of the present study demonstrate striking defects in energy synthesis and reserves in cardiac tissues after burn injury. Indeed, ATP levels in myocardial tissue were markedly reduced in groups G and B compared with group C, for the entire length of the experimental period. However, glutamine supplementation was able to partially block these effects, as evidence by the fact that ATP levels were significantly higher in group G than in group B from 24 PBH to 72 PBH.
Concurrently, lactic acid levels in the blood also increased significantly soon after burn injury, suggesting that aerobic oxidation was impaired and anaerobic glycolysis increased. Here again, glutamine administration partially suppressed the observed defects, improving aerobic oxidation, inhibiting anaerobic glycolysis and reducing lactic acid accumulation. These results demonstrate that burn injury can cause myocardial ischemia, oxygen uptake insufficiency and oxidative metabolism blockage. They also show that, under these circumstances, glutamine supplementation can improve myocardial tissue energy metabolism and promote ATP synthesis.
Glutamine is one of the principal free intracellular amino acids in mammalian cardiac myocytes, where it maintains normal energy metabolism and influences cellular activities [18]. Glutamine is quickly transported into cardiac myocytes via high capacity, saturable, stereospecific and sodium-dependent transporters in the cardiac myocyte membrane [12,17,19,20]. Moreover, a previous study has shown that the rate of glutamine catabolism is at least four times higher in cardiac muscle than in skeletal muscle [18]. Thus, glutamine is best suited for use in myocardial cells.
Post-burn glutamine treatment promotes GSH synthesis
During reperfusion after myocardial ischemia, molecular oxygen is reintroduced into the myocardium and converted to oxygen free radicals, which can oxidize protein sulfhydryl groups as well as promote further tissue injury by inducing lipid peroxidation of cell membranes [21]. High concentrations of free radicals, produced by the post-burn inflammatory reaction, can cause considerable damage to the myocardium [22,23]. GSH functions as a major antioxidant, and can therefore protect mammalian cells against ischemic injury and maintain the structural integrity of cells [24]. Our present results indicate that myocardial GSH levels were significantly reduced soon after burn injury and were consistently higher in group G than in group B from 24 to 72 PBH. These results demonstrate that glutamine supplementation after burn injury can considerably improve myocardial GSH synthesis, abate lipid peroxidation injury in cardiac myocytes, protect mitochondrial respiratory function and ameliorate cardiac energy metabolism.
Glutamine supplementation after burn injury alleviates myocardial damage and improves cardiac function
LDH, CK, and AST are the major metabolic enzymes in the heart and are released into the serum in response to cardiac damage. Our results show that serum levels of AST, CK, and LDH increased quickly after burn injury, generally reaching maximum values at 12 PBH. From 12 to 48 PBH, serum AST, CK, and LDH levels were significantly higher in groups B and G than in group C, and lower in group G than in group B.
A number of histopathological changes were also observed 12 hours after severe burn injury, including cardiac interstitial oedema, fibre engorgement, transverse striation defects, poorly defined cell boundaries and cytoplasm destruction. By 24 PBH, inflammatory features such as capillary engorgement and erythrocyte exudation were also seen in myocardial tissues. Pathomorphological changes, including punctiform and lamellar necroses, were most obvious at 72 PBH. Overall structural damage was significantly less in group G compared with group B.
Our previous study showed that regional myocardial blood flow declines significantly 1 hour after severe burn injury and remains markedly lower than control at 24 PBH [2]. The structural damage induced by ischemia/hypoxia in myocardial tissue in turn leads to cardiac dysfunction. Consistently, the results of our present study show that myocardial mechanics parameters were significantly altered in response to severe burn injury. Compared to control, AOSP, AODP, LVSP, and +dp/dtmax values were significantly decreased at all time points. These results suggest that soon after burn injury, cardiac contractile function, cardiac afterload, peripheral vascular resistance and maximal isolength tension of the myocardial fibres all decrease significantly. It also suggests that the contractile element velocity of the myocardium is decrease. Cardiac contractile function was improved in group G compared with group B, as evidenced by the significantly increased AOSP, AODP, LVDP, and +dp/dtmax values. Moreover, changes in contractility parameters were closely correlated with myocardial energy metabolism; glutamine treatment after burn injury increased myocardial GSH levels, and improved energy metabolism as well as cardiac contractile function.
Based on the findings presented herein, we propose a mechanism whereby glutamine treatment after burn injury protects cardiac function by promoting improved GSH synthesis and cellular energy metabolism, as well as by reducing lipid peroxidation damage in cardiac myocytes. However, we found that oral administration of glutamine limits myocardial protection to some extent, because the effective period of post-burn protection is relatively short. The decline in efficacy after 48 PBH is most likely due to more serious intestinal injury occurring 48-72 hours after burn injury [25]. Necrosis and shedding of intestinal cells during this period may lead to intestinal epithelial cell glutamine transporter damage, and intestinal absorptive capacity may ultimately decline. A similar phenomenon has been observed in the sepsis model [26]. Thus, the effects of orally administered glutamine on myocardial protection are not obvious in later periods of burn injury. In order to overcome these limitations and improve efficacy, future experiments will focus on intravenous administration of glutamine.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 30971075), the Clinical Research Foundation of TMMU (Grant No. 2009XLC10) and the CPLA Scientific Research Fund (BWS11J039).
Conflict of interest statement
The authors report no conflict of interest.
References
- 1.Latenser BA. Critical care of the burn patient: the first 48 hours. Crit Care Med. 2009;37:2819–2826. doi: 10.1097/CCM.0b013e3181b3a08f. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Li Z, Yang Z. Roles of ischemia and hypoxia and the molecular pathogenesis of post-burn cardiac shock. Burns. 2003;29:828–833. doi: 10.1016/s0305-4179(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 3.Huang YS, Yang ZC, Yan BG, Yang JM, Chen FM, Crowther RS, Li A. Pathogenesis of early cardiac myocyte damage after severe burns. J Trauma. 1999;46:428–432. doi: 10.1097/00005373-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L, Qin X, Zhong X, Liu L, Lu Y, Fan L, He Z, Chen Q. Glycine-induced cytoprotection is mediated by ERK1/2 and AKT in renal cells with ATP depletion. Eur J Cell Biol. 2011;90:333–341. doi: 10.1016/j.ejcb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Sahin MA, Yucel O, Guler A, Doganci S, Jahollari A, Cingoz F, Arslan S, Gamsizkan M, Yaman H, Demirkilic U. Is there any cardioprotective role of Taurine during cold ischemic period following global myocardial ischemia? J Cardiothorac Surg. 2011;6:31. doi: 10.1186/1749-8090-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg S, Chernin G, Shapira I, George J, Wollman Y, Laniado S, Keren G. Captopril and L-arginine have a synergistic cardioprotective effect in ischemic-reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol Ther. 2000;5:281–290. doi: 10.1054/JCPT.2000.18021. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Young LH, Scalia R, Ross CR, Lefer AM. PR-39, a proline/arginine-rich antimicrobial peptide, exerts cardioprotective effects in myocardial ischemia-reperfusion. Cardiovasc Res. 2001;49:69–77. doi: 10.1016/s0008-6363(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 8.Lomivorotov VV, Efremov SM, Shmirev VA, Ponomarev DN, Lomivorotov VN, Karaskov AM. Glutamine Is Cardioprotective in Patients with Ischemic Heart Disease following Cardiopulmonary Bypass. Heart Surg Forum. 2011;14:E384–388. doi: 10.1532/HSF98.20111074. [DOI] [PubMed] [Google Scholar]
- 9.Ugurlucan M, Erer D, Karatepe O, Ziyade S, Haholu A, Gungor Ugurlucan F, Filizcan U, Tireli E, Dayioglu E, Alpagut U. Glutamine enhances the heat shock protein 70 expression as a cardioprotective mechanism in left heart tissues in the presence of diabetes mellitus. Expert Opin Ther Targets. 2010;14:1143–1156. doi: 10.1517/14728222.2010.521500. [DOI] [PubMed] [Google Scholar]
- 10.Venturini A, Ascione R, Lin H, Polesel E, Angelini GD, Suleiman MS. The importance of myocardial amino acids during ischemia and reperfusion in dilated left ventricle of patients with degenerative mitral valve disease. Mol Cell Biochem. 2009;330:63–70. doi: 10.1007/s11010-009-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen MA, van de Poll MC, Ligthart-Melis GC, Dejong CH, van den Tol MP, Boelens PG, van Leeuwen PA. Specific amino acids in the critically ill patient--exogenous glutamine/arginine: a common denominator? Crit Care Med. 2007;35:S568–576. doi: 10.1097/01.CCM.0000278600.14265.95. [DOI] [PubMed] [Google Scholar]
- 12.Bolotin G, Raman J, Williams U, Bacha E, Kocherginsky M, Jeevanandam V. Glutamine improves myocardial function following ischemia-reperfusion injury. Asian Cardiovasc Thorac Ann. 2007;15:463–467. doi: 10.1177/021849230701500603. [DOI] [PubMed] [Google Scholar]
- 13.McGuinness J, Neilan TG, Cummins R, Sharkasi A, Bouchier-Hayes D, Redmond JM. Intravenous glutamine enhances COX-2 activity giving cardioprotection. J Surg Res. 2009;152:140–147. doi: 10.1016/j.jss.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stottrup NB, Kristiansen SB, Lofgren B, Hansen BF, Kimose HH, Botker HE, Nielsen TT. L-glutamate and glutamine improve haemodynamic function and restore myocardial glycogen content during postischaemic reperfusion: A radioactive tracer study in the rat isolated heart. Clin Exp Pharmacol Physiol. 2006;33:1099–1103. doi: 10.1111/j.1440-1681.2006.04497.x. [DOI] [PubMed] [Google Scholar]
- 16.Song JX, Tu XH, Wang L, Li CJ. Glutamine dipeptide-supplemented parenteral nutrition in patients with colorectal cancer. Clinical Nutrition. 2004:49–53. [Google Scholar]
- 17.Khogali SE, Pringle SD, Weryk BV, Rennie MJ. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123–126. doi: 10.1016/s0899-9007(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 18.Boelens PG, Nijveldt RJ, Houdijk AP, Meijer S, van Leeuwen PA. Glutamine alimentation in catabolic state. J Nutr. 2001;131:2569S–2577S. doi: 10.1093/jn/131.9.2569S. discussion 2590S. [DOI] [PubMed] [Google Scholar]
- 19.Rennie MJ, Khogali SE, Low SY, McDowell HE, Hundal HS, Ahmed A, Taylor PM. Amino acid transport in heart and skeletal muscle and the functional consequences. Biochem Soc Trans. 1996;24:869–873. doi: 10.1042/bst0240869. [DOI] [PubMed] [Google Scholar]
- 20.Schachter D. L-glutamine in vitro regulates rat aortic glutamate content and modulates nitric oxide formation and contractility responses. Am J Physiol Cell Physiol. 2007;293:C142–151. doi: 10.1152/ajpcell.00589.2006. [DOI] [PubMed] [Google Scholar]
- 21.Tsai MS, Sun S, Tang W, Ristagno G, Chen WJ, Weil MH. Free radicals mediate postshock contractile impairment in cardiomyocytes. Crit Care Med. 2008;36:3213–3219. doi: 10.1097/CCM.0b013e31818f23f0. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Privratsky JR, Yang X, Dong F, Carlson EC. Metallothionein alleviates glutathione depletion-induced oxidative cardiomyopathy in murine hearts. Crit Care Med. 2008;36:2106–2116. doi: 10.1097/CCM.0b013e31817bf925. [DOI] [PubMed] [Google Scholar]
- 23.Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, Klimberg VS. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009;25:812–817. doi: 10.1016/j.nut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg JM, Davis JA, Venkatachalam MA. Cytosolic-free calcium increases to greater than 100 micromolar in ATP-depleted proximal tubules. J Clin Invest. 1997;100:713–722. doi: 10.1172/JCI119584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Liu L, Hu T, Lei W, Wan F, Zhang P, Xu J, Zhu H, Zhu Z, Yang Y, Hu X, Xu L, Wang S. Protective effect of glucose-insulin-potassium (GIK) on intestinal tissues after severe burn in experimental rats. Burns. 2012 doi: 10.1016/j.burns.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Li Y, Zhang W, Zhang F, Li J. Changes of plasma glutamine concentration and hepatocyte membrane system N transporters expression in early endotoxemia. J Surg Res. 2011;166:290–297. doi: 10.1016/j.jss.2009.08.027. [DOI] [PubMed] [Google Scholar]