Abstract
Tumor cell dissemination from the primary tumor site to distant organs is one of the characteristic properties of malignant tumors and represents a crucial step in the progression of disease. Although the pattern of spread may vary in different types of carcinomas, dissemination via the lymphatic system represents a common event in metastasis. The extent of lymph node metastasis is one of the major determinants for the prognosis of patients with gastrointestinal carcinomas and guides the therapeutically management. During the last decades, significant attention has been given to the molecular mechanisms that control lymphatic metastasis. The process of lymphangiogenesis has come into the focus. Lymphangiogenesis, the formation of newly lymphatics, comprises a series of complex cellular events and is controlled by a balance between pro- and anti-lymphangiogenic signals. This article will briefly describe the lymphatic system and then provide an overview of the molecular players involved in tumor lymphangiogenesis.
Keywords: Lymphangiogenesis, lymphatic metastasis, molecular mechanisms, gastrointestinal tumors, lymphangiogenic factors
Introduction
Solid tumors of the gastrointestinal tract such as esophageal, gastric, pancreatic colorectal cancer (CRC) are among the 10 most common malignancies worldwide. In Germany 2009 a total of ~215.000 people died suffering from cancer. Looking at the specific cancer sites colorectal cancer is the second leading cause of death, pancreatic cancer the fourth and gastric cancer the fifth in men and sixth in women (Cancer in Germany 2007/2008 8th edition 2012). Despite the existence of screening programs, prevention strategies and multimodality appro-aches in the treatment of malignant gastrointestinal tumors, they remain a major public health problem. Although the pattern of spread may vary in these different types of carcinomas, dissemination from the primary tumor site to distant organs via the lymphatic system represents a common step in metastasis [1]. The presence of regional lymph node metastasis and the number of metastatic regional lymph nodes are of crucial importance for prognosis of patients with gastrointestinal carcinomas. When lymphatic metastasis occurs patients have less favorable outcome (Table 1). Considering this and the high rate of incidence and mortality of gastrointestinal tumors it is critical to determine the molecular mechanisms of tumor dissemination. While the role of angiogenesis in cancer progression is well established, the role of the lymphatic system and the relation between lymphangiogenesis and tumor metastasis is still incomplete clarified. This article provides a brief review of current knowledge in this area.
Table 1.
The 5-year survival rate drops significantly from early tumors stage UICC I to advanced tumors stage UICC III, when lymphatic metastasis occurs
| Tumor type | UICC I | UICC III |
|---|---|---|
| Esophageal cancer | > 50% | < 15% |
| Gastric cancer | > 80% | < 30% |
| Colorectal cancer | > 90% | 30-60% |
Structure and function of the lymphatic system
The identification of lymph specific markers such as Prox-1, LYVE-1 or podoplanin in the last two decades has led to a broader understanding of functional anatomical, cellular and molecular aspects of the lymphatic system (LS). Also these lymphatic markers are not totally specific to the lymph endothelium they play a crucial role in the development of the LS. The lymphatic vascular system is formed by lymphatic endothelial cells (LEC’s). LEC’s of initial capillaries typically express Prox-1, VEGFR-3, podoplanin, LYVE-1 and can secrete chemokines. After arteriovenous differentiation, controlled by Notch/COUP-TFII, Sox18 activates Prox-1 and induces the lymphatic differentiation program in the anterior cardinal vein [2].
Prox-1, the prospero-related homedomain transcription factor is required for LEC specification. Downstream signaling of Prox-1 results in upregulation of LYVE-1 and VEGFR-3. The VEGF-C/VEGFR-3 is necessary for migration and survival of newly formed LEC’s.
LYVE-1, a homologue of the hyaluronic acid receptor CD44, is a member of the Link protein family and functions as a receptor for hyaluronan. Hyaluronan is a key mediator of cell migration. LYVE-1 is used as a marker for distinguishing lymphatic vessels (LV) from blood vessels in normal and tumor tissue. During embryogenesis LYVE-1 is expressed in cardinal vein endothelium, almost simultaneous with Prox-1 expression. In contrast to Prox-1, LYVE-1 is not necessary for normal development or function of LV [3,4].
Podoplanin is another specific marker for the lymphatic endothelium, due to the fact that it is expressed by developing and mature LEC’s but not by blood vessels. It is a transmembrane mucin like protein, which is expressed in normal human tissue predominantly in lymph endothelial cells but also e.g. by podocytes, osteoblasts, alveolar Type I cells. Under normal conditions podoplanin is involved in LV formation. Podoplanin knockout mice have lymphatic defects associated with dilated, malfunctioning lymphatic vessels and lymphedema and die at birth of respiratory failure [5,6]. The expression of podoplanin is regulated by Prox-1. Since podoplanin is differentially expressed in a number of neoplasms such as vascular tumors, germ cells tumors, esophageal squamous cell carcinomas and gastric carcinomas, a role for podoplanin in tumor invasion and metastasis has been suggested [7]. Recent findings indicate that podoplanin might favor invasion via its ability to remodel the cytoskeleton and thus increasing tumor cell motility [8].
The LS is a hierarchical network comprising circulating lymphocytes, blind ended capillaries, collector vessels, lymph nodes (LN) and lymphoid organs and it serves key physiological functions. It maintains fluid homeostasis by absorbing and draining e. g. interstitial fluid, plasma proteins, extravasated cells and returning them back into the blood circulation, and the LS is an essential part of the body’s immunological surveillance system. Gene expression profiles of LEC’s and blood endothelial cells (BEC’s) have been analyzed and compared (Figure 1). About 300 genes are differentially expressed and the most obvious differences were detected in genes coding for proinflammatory cytokines/chemokines, cytoskeletal and cell matrix organization [9-12]. The difference in gene coding for cytoskeletal and cell matrix organization is consistent with the fact that BEC’s are exposed to high blood flow/pressure and therefore need stronger adhesion.
Figure 1.

Scheme of differentially expressed genes between the lymphatic endothelium and the blood endothelium. Abbreviations: signal transducer and activator of transcription 6 (Stat6), monocyte chemotactic protein-1 (MCP-1), interleukin 6/8 (IL-6/8), intracellular adhesion molecule (ICAM), angiopoietin-1 (Ang-1), vascular endothelial growth factor/receptor (VEGF/R), cluster of Differentiation 44 (CD-44), insulin like growth factor 1/2 (IGF-1/2), fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), mesenchymal epithelial transition factor (c-MET), angiopoietin receptor 2 (Tie-2). Adapted from [46].
The function (fluid absorption and lymph transport) is reflected in the structure of LV and differs fundamentally from blood vessels (BV) (Figure 2). Initial lymphatic capillaries are about < 100 μm in diameter and characterized by loose intercellular junctions. In contrast to blood capillaries they have no or an incomplete basement membrane and the wall of LEC’s is jointed to the extracellular matrix by anchoring filaments. Anchoring filaments, which contain elastic fibers, help the vessels to function. They prevent vessel collapse in conditions of high interstitial pressure via opening overlapping cell junctions and thereby widening the capillary lumen. Lymphatic capillaries merge into collector lymphatic vessels. These collector LV consists of pericytes and have valves to help propel a unidirectional flow to LN. Tumor cells can take advantage of the structural LV design to promote their dissemination. The lack of a complete basement membrane or the loose intercellular junctions provides an easily accessible route for tumor cells. To facilitate tumor cell entry into the lymphatic vasculature alteration of the functional properties of the lymphatic endothelium could lead to adhesion and intravasation of tumor cells. Furthermore, chemokines secreted by LEC’s, can mediate detachment, migration or invasion of tumor cells into LV.
Figure 2.

Structure of lymphatic vessels compared to blood vessel. Blood vessels are characterized by a complete basement membrane and are surrounded by pericytes and smooth muscle cells. Initial lymphatic vessels in contrast have no or an incomplete basement membrane and are characterized by lose intercellular junctions and anchoring filaments, which makes them suitable for uptake of fluid, particles and tumor cells. Collecting afferent lymphatic vessel consist like blood vessels of pericytes, which help to reduce lymphatic fluid extravasation and draining lymph fluid to lymph nodes. Adapted from [46].
Lymphangiogenesis
Tumor lymphangiogenesis
Carcinogenesis is a complex multistep process and dissemination from the primary tumor site to target organs via the lymphatic system represents a common step in tumor cell spread. Gastric cancer frequently spreads to regional lymph nodes and gastrectomy with D1 and D2 lymphadenectomy has become a standard treatment procedure. Lymphangiogenesis, the process of formation of new lymphatic vessels, takes place in a variety of physiological and pathophysiological processes such as embryonic development, regeneration, inflammation, wound healing and lymph vascular malformation [13]. There is an ongoing considerable debate in the literature whether lymphangiogenesis can be induced by tumor cells, whether lymphatic invasion requires the formation of new lymphatic vessels or uses preexisting lymphatic vessels. Lymphatic metastasis of tumor was classically viewed as a passive process, where tumor cells spread by utilizing preexisting lymphatic vessels e.g. via open junctions or by tumor eroding and not through the process of active new lymphatic formation (lymphangiogenesis). Until now there is mounting evidence that lymphangiogenesis does occur in tumors and that it promotes tumor progression. By analogy with angiogenesis, a shift in the balance between pro lymphangiogenic and anti lymphangiogenic signaling, might lead to lymphangiogenesis (Figure 3). The most obvious molecular regulators are represented in the following.
Figure 3.
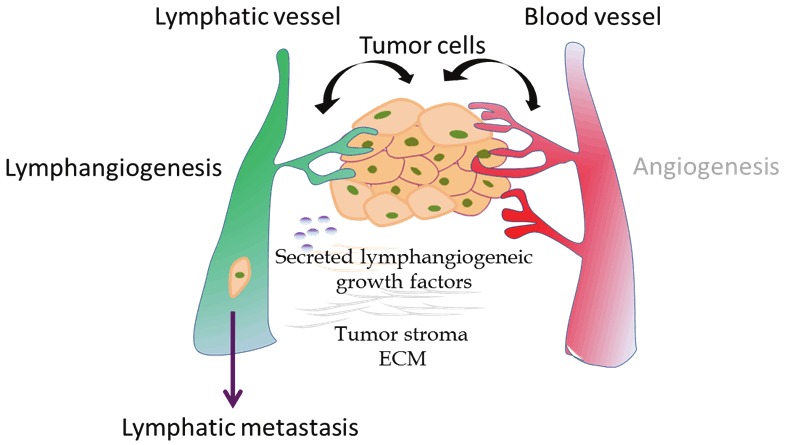
Schematic overview of processes involved in tumor lymphangiogenesis and metastasis. To facilitate tumor cell entry into the lymphatic vasculature tumor cells could secreted various lymphangiogenic growth factors.
Molecular regulators of tumor lymphangiogenesis
Vascular endothelial growth factors (VEGF)
The human VEGF family of growth factors includes five members VEGF-A, VEGF-B, VEGF-C, VEGF-D, PIGF, three tyrosine kinase receptors VEGFR-1, VEGFR-2, VEGFR-3 and two non protein kinase co-receptors (neutropilin -1 and neutropilin-2). VEGF-C and VEGF-D are the most important lymphangiogenic factors and exert this function via binding to VEGFR-2 and VEGFR-3 [14]. The rationale for this approach was derived from transgenic mouse models [15]. In VEGF-C deficient mice lymphatic vessels fail to develop, while transgenic induction of VEGF-C leads to hyperplasia of lymphatic vessels [16-18]. The VEGF-C/VEGF-D/VEGFR-3 axis in adult human tissues is mainly expressed by lymphatic endothelial cells but also by a variety of human carcinomas. Binding of growth factors leads to receptor dimerization, autophosphorylation of tyrosine residues, initiating of signal pathways and causes an increase in vascular permeability, proliferation, migration and survival of LEC’s in vitro and in vivo [19,20]. In solid human tumors the clinicopathological significance of VEGF-C/VEGF-D status currently remains controversial (Table 2). For CRC a significantly higher expression level of VEGF-C/VEGFR-3 compared to normal tissue is described [21]. Figure 4 shows a histopathological result of VEGF-C expression.
Table 2.
Korrelation of VEGF-C/VEGF-D/VEGFR-3 expression in gastrointestinal tumors and clinical outcome.
| Tumor Type | Findings | Reference |
|---|---|---|
| Esophageal cancer | Correlation VEGF-C/VEGF-D expression to tumor progression, lymphatic spread | [47,48] |
| Strong correlation between VEGF-C expression and lymph node metastasis in squamous cell carcinoma, but not in adenocarcinoma. | [49] | |
| Gastric cancer | Positive correlation between VEGF-C/VEGF-D expression and lymphatic metastasis | [50,51] |
| No association of vascular endothelial growth factor-A (VEGF-A) and VEGF-C expression with survival in patients with gastric cancer | [52] | |
| Pancreatic cancer | Expression VEGF-C/VEGF-D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. | [53,54] |
| Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis (no overexpression of VEGF-C/VEGF-D | [55] | |
| Esophageal cancer | Pos. correlation of VEGF-C/VEGF-D expression with lymphatic metastasis and poor prognosis | [25] |
| No correlation between VEGF-C/VEGF-D expression and clinicopathological variables | [56,57] |
Figure 4.
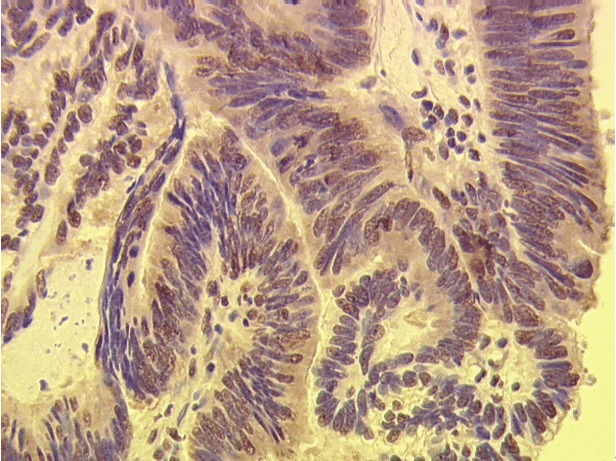
Immunohistochemistry for VEGF-C expression in CRC.
The majority of studies (such as esophageal, gastric, pancreatic or colorectal) have found a positive correlation between overexpression of VEGF-C/VEGF-D on the one hand and vascular invasion, lymph vessel and lymph node involvement, distant metastasis and poor clinical outcome on the other hand [22-27]. However, in other studies such correlation could not be confirmed. The VEGF-C/VEGF-D/VEGFR-3 axis exerts different biological effects on cancer cells to cause tumor progression. Some authors observed a lymphangiogenic switch in tumors, which means the ability of tumor cells to secrete their own growth factors leading to autocrine stimulation and via paracrine mechanisms tumor cells could induce the stimulation of other cells and thereby generating a (neo) vascularization of the tumor microenvironment. It is also of importance that the expression of VEGF-C/VEGF-D differs within the tumors. Some investigators showed that the expression of VEGF-C in the invasion front is significantly higher than in tumor center, suggesting that the invasive edge may play a more important role in inducing tumor associated lymphangiogenesis compared to the rest of the tumor [2,25,28]. In addition, these studies have reported that lymphatic vessels in the tumor margin were enlarged and dilated. Suggesting that tumor cells can easily use these gateways and intratumoral LV might be poor in function [29]. The debate about the dominant role of intratumoral vs. peritumoral LV is still controversial. Taken together, several studies have provided evidence of an active involvement of the VEGF-C/VEGF-D/VEGFR-3 axis in lymphatic metastasis in gastrointestinal carcinomas.
Angiopoietins (Angs)
Among the four distant Angs, Angiopoietin-1 (Ang-1) and Angiopoietin-2 (Ang-2) are the most intensive characterized members of the Ang family. Ang-1 acts via the receptor tyrosine kinase Tie2 as a constitutive Tie2 agonist, whereas Ang-2 is capable of acting as an agonist and antagonist in the interacting with Tie2. Tie2 is expressed on blood and lymph endothelial cells, but can also be found on cancers cells, monocytes and tumor associated macrophages [30]. While Ang-1 is widely expressed in adult tissues, where it promotes vessel maturation and stabilization, Ang-2 expression occurs during vascular remodeling and angiogenesis. About the role of Angs in lymphangiogenesis little information is available. Ang-1 is involved in LEC proliferation and lymphatic vessel sprouting and Ang-2 deficient mice exhibited severe lymphatic dysfunctions [31]. Overexpression of Angs and their receptor Tie2 in gastrointestinal tumor is associated with advanced disease and poor prognosis [32] [33,34]. Ang-2 e.g. drives lymphatic metastasis of pancreatic ductal adenocarcinoma via lymphatic vascularization in the tumor stroma and through enhancing the capacity of tumor cells for adherence to endothelial cells. The detailed function of Angs in lymphangiogenesis is still unclear.
Chemokines
Chemokines are a super family of chemoattractant cytokines and they are involved in a variety of immune responses. Chemokines bind to G-protein coupled receptors and they are grouped, according to the position of the cysteine residue, into four subfamilies: CXC, CXCR3, CC and C. Like mentioned before, LEC’s can secrete lymphangiocrine cytokines such as CCL21/CCL19, which act via CCR7 and CXCL12, which binds to CXCR4. They mediate homing of lymphocytes and migration of dendritic cells into lymphatic vessels. To date, several sets of chemokines and their receptors have been suggested to play crucial roles in LN metastasis. High levels of expression of chemokine receptors such CCR7 in gastric cancer, CCR7/CXCR4 in esophageal cancer, CXCR3/CXCR4 and CCR7 in colorectal and CXCR4 in pancreatic cancer incre-ase the efficiency of tumor cells homing to metastatic target sites [13,35,36]. Patients with CXCR3 positive colon cancer showed a significant shorter survival compared to those without CXCR3 expression.
Fibroblast growth factor (FGF)
The fibroblast growth factor family consists of structurally related ligands and four receptors (FGFR-1, FGFR-2, FGFR-3, FGFR-4). FGFR-1 and FGFR-2 are expressed in endothelial cells. The role of FGF-2 in angiogenesis has been well characterized, but FGF-2 is also supposed to promote lymphangiogenesis. It might act directly via its receptor FGFR, which is upregulated by Prox-1, or through the protein kinase B (Akt)/mammalian target of rapamycin (mTOR)/p70 ribosomal S6 protein kinase (p70S6K) signaling pathway [37,38]. Upregulation of FGFR-2 has been reported in pancreatic, gastric and colorectal cancer [39,40].
Insulin like growth factor (IGF)
The IGF system consists of the ligands insulin like growth factor 1 (IGF-1) and insulin like growth factor 2 (IGF-2) and their receptors IGF-1R, IGF-2R and 6 IGF binding proteins (IGFBP’s). IGF-1 and IGF-2 are polypeptide hormones. The IGF axis exerts diverse biological functions such as cell growth, differentiation and survival. Receptor signaling leads to activation of PI3K, Akt and various downstream networks. Recent findings indicate, that IGF-1/IGF-2 promote lymphangiogenesis in a dependent and independent manner of the VEGF-C/VEGF-D/VEGFR-3 axis [41]. The role of the IGF system in tumorigenesis, cancer proliferation and survival is well documented [42,43]. IGF-1R is frequently over expressed in several types of carcinomas (pancreatic, breast, colorectal, primary liver, sarcomas) and associated with invasion and metastasis.
Hepatocyte growth factor (HGF)
HGF belongs to the plasminogen prothrombin gene superfamily and its activities are mediated by binding to the tyrosine kinase receptor c-Met. HGF is supposed to be involved in proliferation, migration and tube formation of LEC’s via downstream ERK 1/2 and PI3K signaling. Several studies have shown a differentially expression of c-Met in solid tumors (colorectal cancer) and have indicated a positive correlation between c-Met expression metastatic spread and poor outcome [44,45].
Conclusion
In human gastrointestinal cancer, lymph node metastasis is the hallmark of cancer progression. Lymph node status not only provides the most important prognostic indicator, it also forms the basis for rational treatment options (neoadjuvant/adjuvant therapy). A Pubmed search for the term “lymphangiogenesis” identifies 1783 results versus 58778 results for the term “angiogenesis”. This clearly shows that lymphangiogenesis is fairly behind in research compared to blood vessel formation. In the last decades, since the identification of LEC specific markers, our knowledge of mechanisms controlling lymphatic metastasis has increased significantly (although each of these specific markers has limitations) and lymphangiogenesis seems to play an important role in tumor spread. Tumor lymphangiogenesis is a complex multi step process, which is characterized by a chain of events in which organ specific factors (f.e. lymph flow/lymph node density) and lymphangiogenic factors are implicated, such as VEGF-C, VEGF-D, IGFs, HGF, angiopoietins and the list will undoubtedly continue to grow (Table 3).
Table 3.
Lymphatic vascular factors and receptors. Adapted from [46]
| Lymphatic vascular factor | Biological activity | Localization/Expression pattern |
|---|---|---|
| VEGF-C/VEGF-D/VEGFR-3 | - growth factor/receptor | - secreted dimeric glycoprotein growth factors |
| - LEC: sprouting, migration proliferation, survial | - proteolytic procession of VEGF-C/-D precursors dictate angiogenic/lymphangiogenic potential | |
| - VEGFR -3 is predominantly expressed in LECs that line inner surface of lymphatic vessels | ||
|
| ||
| LYVE-1 | - endocytotic receptor for hyaluronan (major component of ECM and involved in cell migration, differentiation) | - type I integral membrane glycoprotein, luminal/abluminal surface of LECs |
|
| ||
| Podoplanin | - glomerular podocyte mucoprotein | - single transmembrane protein |
| - expressed on lymphatic but not on blood vessel endothelium, osteoblasts, renal podocytes and lung alveolar cells→cell motility | ||
|
| ||
| Prox-1 | - homeobox gene for embryologic lymphatic development and differentiation | - subcellular localization controlled by competition between nuclear localization signal and nuclear export signal |
| - expression: f.e. lens, heart, liver, pancreas | ||
|
| ||
| IGF | - growth factor | - produced in many tissue, function via autocrine/paracrine mechanisms, circulating in the plasma in association with IGFBPs |
|
| ||
| FGF | - LEC migration and proliferation | - FGF-1/FGF-2 lack signal sequences for export out of the producer cell, most of the other members of the FGF family are secreted |
|
| ||
| HGF | - growth factor | - growth promoting activity of HGF requires proteolytic cleavage by extracellular serine proteinases |
|
| ||
| Ang1/Ang2/Tie2 | - growth factor | - Tie2 expressed on surface of endothelialcells |
A thorough understanding of these mechanisms will define the concepts for future therapeutic targeting of gastrointestinal tumors and will hopefully improve patient outcomes.
Acknowledgements
This study was supported by the ELAN-Fond University of Erlangen-Nuremberg and the German Research Foundation (DFG CR 136/2-1).
References
- 1.Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, Giuliano R, Archie V, Calvin D, Mensha L, Shivers S, Cox C, Werner JA, Kitagawa Y, Kitajima M. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25:221. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, Harris AL, Dirix LY, Vermeulen PB. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006;95:1611. doi: 10.1038/sj.bjc.6603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S, Shimoda H, Ji RC, Miura M. Lymphangiogenesis and expression of specific molecules as lymphatic endothelial cell markers. Anat Sci Int. 2006;81:71. doi: 10.1111/j.1447-073X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, Watanabe M, Hirohashi S. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009;77:53. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]
- 6.Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997. [PubMed] [Google Scholar]
- 7.Raica M, Ribatti D. Targeting tumor lymphangiogenesis: an update. Curr Med Chem. 2010;17:698. doi: 10.2174/092986710790514471. [DOI] [PubMed] [Google Scholar]
- 8.Cueni LN, Hegyi I, Shin JW, Albinger-Hegyi A, Gruber S, Kunstfeld R, Moch H, Detmar M. Tumor lymphangiogenesis and metastasis to lymph nodes induced by cancer cell expression of podoplanin. Am J Pathol. 2010;177:1004. doi: 10.2353/ajpath.2010.090703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tammela T, Petrova TV, Alitalo K. Molecular lymphangiogenesis: new players. Trends Cell Biol. 2005;15:434. doi: 10.1016/j.tcb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Makinen T, Norrmen C, Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell Mol Life Sci. 2007;64:1915. doi: 10.1007/s00018-007-7040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011;71:1214. doi: 10.1158/0008-5472.CAN-10-3277. [DOI] [PubMed] [Google Scholar]
- 14.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Ribatti D. Transgenic mouse models of angiogenesis and lymphangiogenesis. Int Rev Cell Mol Biol. 2008;266:1. doi: 10.1016/S1937-6448(07)66001-8. [DOI] [PubMed] [Google Scholar]
- 16.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 17.Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am J Pathol. 2008;173:1891. doi: 10.2353/ajpath.2008.080378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 19.Roskoski R Jr. VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 20.Birk DM, Barbato J, Mureebe L, Chaer RA. Current insights on the biology and clinical aspects of VEGF regulation. Vasc Endovascular Surg. 2008;42:517. doi: 10.1177/1538574408322755. [DOI] [PubMed] [Google Scholar]
- 21.Omachi T, Kawai Y, Mizuno R, Nomiyama T, Miyagawa S, Ohhashi T, Nakayama J. Immunohistochemical demonstration of proliferating lymphatic vessels in colorectal carcinoma and its clinicopathological significance. Cancer Lett. 2007;246:167. doi: 10.1016/j.canlet.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Tai XH, Ji WY, Sun XH. [Relationship among lymphangiogenesis, vascular endothelial gro wth factor-C mRNA expression and cervical lymphatic metastasis in laryngeal carcinoma] . Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006;41:622. [PubMed] [Google Scholar]
- 23.Gou HF, Chen XC, Zhu J, Jiang M, Yang Y, Cao D, Hou M. Expressions of COX-2 and VEGF-C in gastric cancer: correlations with lymphangiogenesis and prognostic implications. J Exp Clin Cancer Res. 2011;30:14. doi: 10.1186/1756-9966-30-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TB, Chen ZG, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C and lymphoangiogenesis are associated with the lymph node metastasis and prognosis of patients with colorectal cancer. ANZ J Surg. 2011;81:694. doi: 10.1111/j.1445-2197.2010.05539.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin M, Lin HZ, Ma SP, Ji P, Xie D, Yu JX. Vascular endothelial growth factor-A and -C: expression and correlations with lymphatic metastasis and prognosis in colorectal cancer. Med Oncol. 2011;28:151. doi: 10.1007/s12032-010-9427-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhu C, Qi X, Chen Y, Sun B, Dai Y, Gu Y. PI3K/ Akt and MAPK/ERK1/2 signaling pathways are involved in IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res Clin Oncol. 2011;137:1587. doi: 10.1007/s00432-011-1049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werynska B, Dziegiel P, Jankowska R. Role of lymphangiogenesis in lung cancer. Folia Histochem Cytobiol. 2009;47:333. doi: 10.2478/v10042-009-0090-3. [DOI] [PubMed] [Google Scholar]
- 28.Acs G, Paragh G, Rakosy Z, Laronga C, Zhang PJ. The extent of retraction clefts correlates with lymphatic vessel density and VEGF-C expression and predicts nodal metastasis and poor prognosis in early-stage breast carcinoma. Mod Pathol. 2012;25:163. doi: 10.1038/modpathol.2011.138. [DOI] [PubMed] [Google Scholar]
- 29.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, Rupasinghe TW, Tull DL, Baldwin ME, Sloan EK, Fox SB, Achen MG, Stacker SA. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 31.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 32.Schulz P, Fischer C, Detjen KM, Rieke S, Hilfenhaus G, von Marschall Z, Bohmig M, Koch I, Kehrberger J, Hauff P, Thierauch KH, Alves F, Wiedenmann B, Scholz A. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;25:3325. doi: 10.1096/fj.11-182287. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Wu K, Zhang D, Tang H, Xie H, Hong L, Pan Y, Lan M, Hu S, Ning X, Fan D. Expressions and clinical significances of angiopoietin-1, -2 and Tie2 in human gastric cancer. Biochem Biophys Res Commun. 2005;337:386. doi: 10.1016/j.bbrc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 34.Jo MJ, Lee JH, Nam BH, Kook MC, Ryu KW, Choi IJ, Kim YW, Bae JM. Preoperative serum angiopoietin-2 levels correlate with lymph node status in patients with early gastric cancer. Ann Surg Oncol. 2009;16:2052. doi: 10.1245/s10434-009-0474-9. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Lillard JW Jr, Singh S. Chemokines: key players in cancer progression and metastasis. Front Biosci (Schol Ed) 2011;3:1569. doi: 10.2741/246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui Y, Madeddu P. The role of chemokines, cytokines and adhesion molecules in stem cell trafficking and homing. Curr Pharm Des. 2011;17:3271. doi: 10.2174/138161211797904109. [DOI] [PubMed] [Google Scholar]
- 37.Matsuo M, Yamada S, Koizumi K, Sakurai H, Saiki I. Tumour-derived fibroblast growth factor- 2 exerts lymphangiogenic effects through Akt/mTOR/p70S6kinase pathway in rat lymphatic endothelial cells. Eur J Cancer. 2007;43:1748. doi: 10.1016/j.ejca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 39.Sundlisaeter E, Rosland GV, Hertel JK, Sakariassen PO, Almas B, Dicko A, Sondenaa K. Increased lymphatic vascular density is seen before colorectal cancers reach stage II and growth factor FGF-2 is downregulated in tumor tissue compared with normal mucosa. APMIS. 2009;117:212. doi: 10.1111/j.1600-0463.2008.00025.x. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, Kobrin MS, Korc M. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993;53:5289. [PubMed] [Google Scholar]
- 41.Da MX, Wu Z, Tian HW. Tumor lymphangiogenesis and lymphangiogenic growth factors. Arch Med Res. 2008;39:365. doi: 10.1016/j.arcmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinol Metab Clin North Am. 2012;41:335. doi: 10.1016/j.ecl.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao QG, Xie JX, Wong MS, Chen WF. IGF-I receptor signaling pathway is involved in the neuroprotective effect of genistein in the neuroblastoma SK-N-SH cells. Eur J Pharmacol. 2012;677:39. doi: 10.1016/j.ejphar.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 44.Kammula US, Kuntz EJ, Francone TD, Zeng Z, Shia J, Landmann RG, Paty PB, Weiser MR. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248:219. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3:S7. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langheinrich M, Schellerer V, Oeckl K, Stürzl M, Naschberger E, Croner R. Molecular Mechanisms of Lymphatic metastasis. Colorectal Cancer Book 1. 2011;285:298. [Google Scholar]
- 47.Noguchi T, Takeno S, Shibata T, Uchida Y, Yokoyama S, Muller W. VEGF-C expression correlates with histological differentiation and metastasis in squamous cell carcinoma of the esophagus. Oncol Rep. 2002;9:995. [PubMed] [Google Scholar]
- 48.Kimura Y, Watanabe M, Ohga T, Saeki H, Kakeji Y, Baba H, Maehara Y. Vascular endothelial growth factor C expression correlates with lymphatic involvement and poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep. 2003;10:1747. doi: 10.3892/or.10.6.1747. [DOI] [PubMed] [Google Scholar]
- 49.Mobius C, Freire J, Becker I, Feith M, Brucher BL, Hennig M, Siewert JR, Stein HJ. VEGF-C expression in squamous cell carcinoma and adenocarcinoma of the esophagus. World J Surg. 2007;31:1768. doi: 10.1007/s00268-006-0373-1. [DOI] [PubMed] [Google Scholar]
- 50.Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413. doi: 10.1016/s0959-8049(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Wu R, Sun G, Li X, Yuan W, Tang L. [Expression of VEGF-C and VEGF-D in gastric carcinoma and its relationship with lymph node metastases] . Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:335. doi: 10.3969/j.issn.1672-7347.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Lee SJ, Kim JG, Sohn SK, Chae YS, Moon JH, Kim SN, Bae HI, Chung HY, Yu W. No association of vascular endothelial growth factor-A (VEGF-A) and VEGF-C expression with survival in patients with gastric cancer. Cancer Res Treat. 2009;41:218. doi: 10.4143/crt.2009.41.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider M, Buchler P, Giese N, Giese T, Wilting J, Buchler MW, Friess H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int J Oncol. 2006;28:883. [PubMed] [Google Scholar]
- 54.Zhang B, Zhao WH, Zhou WY, Yu WS, Yu JM, Li S. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pan creatic adenocarcinoma. Cancer Detect Prev. 2007;31:436. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Sipos B, Kojima M, Tiemann K, Klapper W, Kruse ML, Kalthoff H, Schniewind B, Tepel J, Weich H, Kerjaschki D, Kloppel G. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. J Pathol. 2005;207:301. doi: 10.1002/path.1840. [DOI] [PubMed] [Google Scholar]
- 56.George ML, Tutton MG, Janssen F, Arnaout A, Abulafi AM, Eccles SA, Swift RI. VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia. 2001;3:420. doi: 10.1038/sj.neo.7900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanni O, Volpi A, Frassineti GL, De Paola F, Granato AM, Dubini A, Zoli W, Scarpi E, Turci D, Oliverio G, Gambi A, Amadori D. Role of biological markers in the clinical outcome of colon cancer. Br J Cancer. 2002;87:868. doi: 10.1038/sj.bjc.6600569. [DOI] [PMC free article] [PubMed] [Google Scholar]


