Abstract
The present study investigated the relationship between the expression of p-mTOR, p-4EBP1 and p-p70S6K in the cytoplasm and nucleus of ameloblastoma (AB) cells and the invasiveness of ABs. Immunohistochemistry was performed to detect the expression of p-mTOR, p-4EBP1 and p-p70S6K in ABs and the level of these proteins in the nucleus and cytoplasm was scored. There was ectopic expression of p-mTOR in ABs. 27 AB patients were positive for p-mTOR expression in the nucleus and 47 for p-mTOR expression in the cytoplasm. The ectopic expression of p-4EBP-1 was also noted. 23 patients (27%) were positive for p-4EBP-1 expression in the nucleus and 55 (64.7%) for p-4EBP-1 expression in the cytoplasm. The ectopic expression of p-p70S6K was also noted. Of these patients, 33 were positive for p-p70S6K expression in the nucleus (38.8%) and 45 for p-p70S6K expression in the cytoplasm (52.9%). Statistical analysis showed the expression of three proteins in the nucleus of patients with recurrent cancer was markedly higher than that in those with primary cancer. The expression of p-mTOR, p-4E-BP1 and p-p70S6K in the nucleus was related to the invasiveness of ABs. Multivariable analysis with Cox proportional hazards model showed the p-mTOR expression had influence on AB recurrence (OR: 6.417, 95%CI: 1.428-28.824). The possibility of AB recurrence in patients with nuclear p-mTOR expression was 6.417 folds higher than that in those with cytoplasmic p-mTOR expression. The nuclear expression of p-mTOR, p-4E-BP1 and p-p70S6K was associated with biological behaviors (invasiveness) of ABs, and p-mTOR was an independent predictor of AB.
Keywords: Phosphorylated mTOR, ameloblastoma, biological behavior
Introduction
Ameloblastoma (AB) is an odontogenic tumor derived from the jaw. In China, the AB accounts for 36% of odontogenic tumors [1]. The AB mainly consists of enamel-like structure, but has no enamel and other hard tissues. The majority of AB occurs in the jaw resulting in jaw enlargement and facial deformation. Although AB is a benign tumor, the incidence of postoperative recurrence of AB is still higher than that of other odontogenic tumors. In 2005, WHO classified AB into 4 variants with different pathological characteristics including solid/polycystic AB, extra-osseous/peripheral AB, desmoplastic AB and unicystic AB. Patients with AB of different variants presents with differences in the age, site of AB, imaging findings and clinical prognosis. Previously, curettage was used for the treatment of AB. Currently, extensive resection of AB is usually applied in the treatment of AB due to the high incidence of recurrence. However, a small number of patients have the tendency to recurrence. The focal invasive growth of ABs is a major cause of post-operative recurrence, and the mechanism underlying the invasive growth of ABs has become a hot topic in research on AB [2-3]. In recent years, studies on the invasive growth of ABs focus on the protein and gene levels, and attention has been paid to the proliferation, apoptosis, matrix degradation and expression of oncogenes and tumor suppressor genes [4-5].
mTOR signaling pathway is one of important pathways closely related to the proliferation and apoptosis of cells. In recent years, studies in depth reveal that mTOR signaling pathway plays important roles in the cellular biological processes (cell apoptosis, transcription, translation, metabolism, angiogenesis and regulation of cell cycle) which are associated with the occurrence and development of numerous tumors [6-8]. The genetic alteration of mTOR signaling pathway and biochemical activation of this pathway are often found in the cancer of early phase or progressive phase. In addition, the extent of activation of this pathway is also used as an indicator to determine the prognosis of cancer patients. Thus, the mTOR signaling pathway has been a target in the treatment and prevention of cancers [9-10].
The PI3K/Akt/mTOR signaling pathway is closely associated with proliferation and growth of cells [11-12]. PI3K/Akt signaling pathway and LKB1 /AMPK signaling pathway function via the TSC. Akt can phosphorylate TSC2, which blocks the influence of TSC complex on Rheb activity leading to the activation of mTOR. On the contrary, AMPK phosphorylates TSC2, which blocks the mTOR induced activation of two target proteins (ribosomal p70S6 kinase [S6K1] and 4E-BP1) downstream of mTOR. 4E-BP1 is an inhibitor of eukaryotic translation initiation factor (eIF4E). After being released from the phosphorylated 4E-BP, eIF4E is activated and promotes the translation of mRNA. S6K1 is widely expressed in cells and is the ribosomal 40S subunit S6 protein kinase. S6K1 functions to phosphorylate ribosomal 40S subunit S6 protein, which facilitates the translation of 5 ’TOP (tract of py2rimidine) in mRNA. The products of mRNA containing 5 ’TOP are mainly ribosome, initiation factor and elongation factor which are essential for the translation.
However, few studies have been conducted to investigate the mTOR signaling pathway in the odontogenic tumors. In our previous study, results showed ABs were strong positive for Akt, a regulatory factor upstream of mTOR. Only one study was carried out to investigate the mTOR and related regulatory genes in ABs [13]. In this study, immunohistochemistry was done to detect the expression of mTOR and related regulatory genes in 32 AB patients, and results confirmed that AB was positive for these proteins. The phosphorylation of mTOR, 4E-BP1 and P70S6K may lead to their activation. In the present study, immunohistochemistry was employed to detect the expression of p-mTOR, p-4EBP1 and p-p70S6K in AB from 85 patients and to explore the relationship between these proteins and the invasiveness of AB.
Materials and methods
Clinical information
Among 600 patients with AB admitted into affiliated hospital of China Medical University from 2006 to 2010, 85 patients were selected of whom 50 had primary AB, 33 had recurrent AB and 2 developed malignant transformation. There were 55 males and 30 females with the male to female ratio of 1.83:1. The median age was 44.3 years (range: 7~76 years). Patients aged 33-52 years accounted from 50.5% (43/85). The longest course of disease was 16 years, and the shortest one was 7 days. Among these patients, 85.8% of patients developed AB in the mandible (73/85): left mandible: n=34; right mandible: n=33; mandibular symphysis: n=5. The 11.8% of patients had AB in the maxillary: left maxillary: n=7; right maxillary: n=3. In addition, AB was found in the gum in 1 patient and in the rapharyngeal space in 1 patient. The interval to recurrence ranged from 4 months to 30 years. The pathological examination showed solid/polycystic AB in 49.4% (66/85) of patients, unicystic AB in 17.6% (15/85), desmoplastic AB in 2.35% (2/85) and peripheral AB in 2.35% (2/85). Among 66 patients with solid AB, follicular pattern was found in 19 patients, plexiform pattern in 37, acanthomatous pattern in 6, basal cell pattern in 3, and mixed follicular/plexiform pattern in 1. In addition, the diagnosis of malignant AB was based on the criteria for the classification of odontogenic tumor (WHO, 2005), and basal cell pattern and plexiform pattern of malignant AB was found in 1 and 1 patient, respectively (Table 1). Among patients with recurrence, 21 developed recurrent AB after curettage and 12 had recurrent AB after resection (1 received partial resection of right mandible and iliac bone grafting). In the normal control group, the oral mucosa was collected.
Table 1.
Histopathological patterns of AB (Immunohistochemsitry)
| Pattern | N |
|---|---|
| Solid/polycystic | 66 |
| follicular | 19 |
| plexiform | 37 |
| mixed follicular/plexiform | 1 |
| acanthomatous | 6 |
| basal cell | 3 |
| Unicystic | 15 |
| Peripheral | 2 |
| Desmoplastic | 2 |
Immunohistochemistry
Tissues were consecutively cut into sections (4μm). The sections were selected and used for immunohistochemistry with SP method under the same condition according to the manufacturer’s instructions (p-mTOR: 1:100; p-4E-BP1: 1:50; p-p70S6K: 1:100). Visualization was done with DAB. In the negative control group, the primary antibody was replaced with 0.01 mol/L PBS. The positive control was provided by Maixin. Cells with brown granules were regarded as positive for target proteins. Protein expression in the membrane and/or cytoplasm or the nucleus alone was regarded as abnormal.
The pathology was evaluated by two experienced pathologists and consensus was obtained. The scoring of expression of p-mTOR, p-4E-BP1 and p-p70S6K was performed with criteria for IRS score: IRS=∑Si×PP where SI is staining intensity (0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) and PP is proportion of positive cells (0, no positive cells; 1, 10% positive cells; 2, 11-50% positive cells; 3, 51-81% positive cells; 4, >81% positive cells).
Statistical analysis
Statistical analysis was done with SPSS version 13.0. Chi square test and one way analysis of variance were used for comparisons. Kaplan-meier method was employed to delineate survival curve. Log-rank test was used to comparisons between groups. Multivariable survival analysis was done with Cox proportional risk model. A value of two-tailed P<0.05 was considered statistically significant.
Results
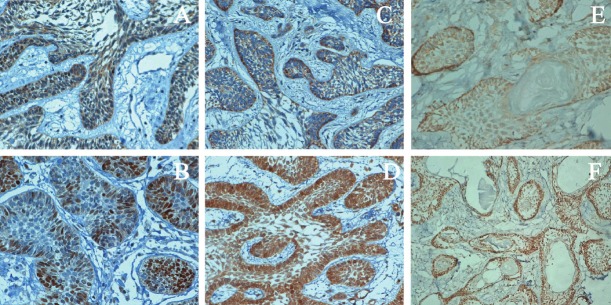
The p-mTOR positive cells in normal oral mucosa were stained brown. Protein was found in the membrane and nucleus. In the AB, abnormal expression of p-mTOR was noted, and the staining intensity increased. The p-mTOR expression in the membrane reduced. Among 63 AB patients, 47 were positive for cytoplasmic p-mTOR expression (55.3%) and 27 for nuclear p-mTOR expression (32%). Patients with abnormal nuclear p-mTOR expression also presented with abnormal cytoplasmic p-mTOR expression. In the normal oral mucosa, p-4E-BP1 positive cells had brown granules, and protein was mainly found in the cytoplasm. Abnormal expression was found in the nucleus and membrane. In the AB patients, abnormal p-4E-BP1 expression was found, 55 (64.7%) were positive for cytoplasmic p-4E-BP1 expression and 23 for nuclear p-4E-BP1 expression (27.1%). In normal oral mucosa, cells were negative for p-p70S6K and p- p70S6K expression was not found in the cytoplasm and nucleus. In the AB patients, abnormal p- p70S6K expression was found in the cytoplasm and nucleus, 45 were positive for cytoplasmic p- p70S6K expression and 33 for nuclear p- p70S6K expression (38.8%) (Figure 1).
Figure 1.
A: Cytoplasmic p-mTOR expression in AB; B: Nuclear p-mTOR expression in AB; C: Cytoplasmic p-4E-BP1 expression in AB; D: Nuclear p-4E-BP1 expression in AB; E: Cytoplasmic p-p70S6K expression in AB; F: Nuclear p-p70S6K expression in AB.
The expression of p-mTOR, p-4E-BP1 and p-p70S6K was scored. The cytoplasmic expression was defined as group 1 and nuclear expression as group 2. Chi square test showed the invasiveness of AB was closely related to the expression of these proteins in the cytoplasm or nucleus. Among patients with recurrent AB, the nuclear expression of p-mTOR, p-4E-BP1 and p-p70S6K was markedly higher than that in patients with primary AB (Table 2, 3, 4; X2=43.275, 41.150 and 19.276, respectively, P<0.0001).
Table 2.
Nuclear and cytoplasmic expression of p-mTOR in primary and recurrent AB
| Group | Cytoplasmic | Nuclear | Total |
|---|---|---|---|
| Primary | 45 (88.2%) | 6 (11.8%) | 51 |
| Recurrent | 2 (8.7%) | 21 (91.3%) | 23 |
| Total | 47 | 27 | 74 |
Table 3.
Nuclear and cytoplasmic expression of p-4E-BP1 in primary and recurrent AB
| Group | Cytoplasmic | Nuclear | Total |
|---|---|---|---|
| Primary | 50 (92.6%) | 4 (7.4%) | 54 |
| Recurrent | 5 (9.1%) | 19 (82.6%) | 24 |
| Total | 55 | 23 | 78 |
Table 4.
Nuclear and cytoplasmic expression of p-p70S6K in primary and recurrent AB
| Group | Cytoplasmic | Nuclear | Total |
|---|---|---|---|
| Primary | 40 (74.1%) | 14 (25.9%) | 54 |
| Recurrent | 5 (20.8%) | 19 (79.2%) | 24 |
| Total | 45 | 33 | 78 |
In the present study, patients received follow up, and log-rank test and Cox proportional risk model were employed for univariable and multivariable survival analysis. Then, Kaplan-Meier method was employed to delineate the survival curve. Figure 2, 3 and 4 display the relationship between the p-mTOR expression and the interval to AB recurrence, between the p-4E-BP1 expression and the interval to AB recurrence and between the p-p70S6K expression and the interval to AB recurrence, respectively. The medial survival time of patients with nuclear and cytoplasmic p-mTOR expression was 7.74 years and 18.75 years, respectively, that of patients with nuclear and cytoplasmic p-4E-BP1 expression was 8.14 years and 12.71 years, respectively, and that of patients with nuclear and cytoplasmic p-P70S6K expression was 7.69 years and 17.13 years, respectively, showing significant difference (P<0.05). In the multivariable analysis showed nuclear expression Table 2. Nuclear and cytoplasmic expression of p-mTOR in primary and recurrent AB Group Cytoplasmic Nuclear Total Primary 45(88.2%) 6(11.8%) 51 Recurrent 2(8.7%) 21(91.3%) 23 Total 47 27 74 of p-mTOR was an independent factor affecting the recurrence of AB (OR: 6.417, 95%CI: 1.428-28.824). The nuclear p-mTOR expression increased the risk for the recurrence of AB by 6.417 folds as compared to the cytoplasmic p-mTOR expression.
Figure 2.
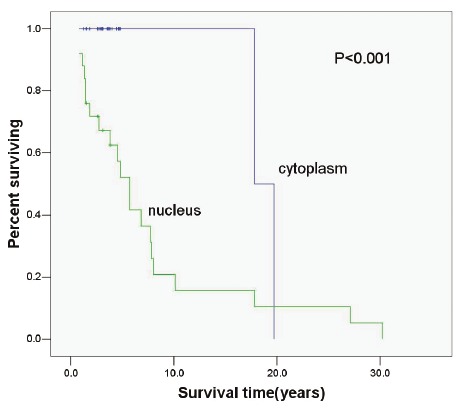
Relationship between p-mTOR expression and biological behavior of AB (P<0.001).
Figure 3.
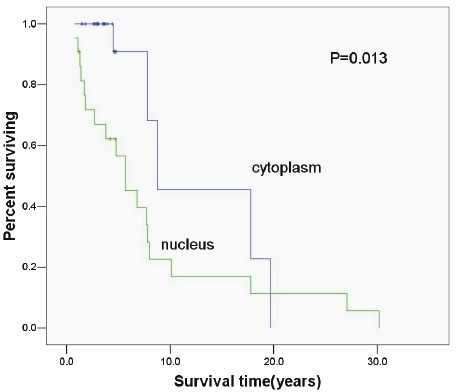
Relationship between p-4EBP1 expressionand biological behavior of AB (P=0.013).
Figure 4.
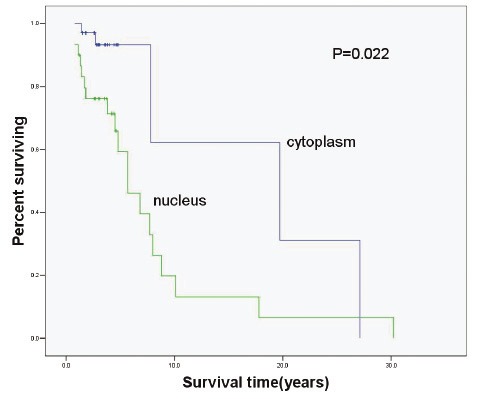
Relationship between p-p70S6K expressionand biological behavior of AB (P=0.022).
Discussion
mTOR and its regulatory genes play important roles in the occurrence and development of some tumors [14-16]. The activated mTOR can promote the proliferation of cancer cells, increase the secretion of oncoprotein, facilitate the cell cycle, shorten the G1 phase and promote the rapid progression of cancers [17]. The activation of mTOR signaling pathway can inhibit the apoptosis following multiple stimulations, promote the progression of cell cycle and then facilitate the survival and proliferation of cells. In addition, activation of this pathway also involves in the angiogenesis playing important role in the carcinogenesis and participates in the invasion and metastasis of cancers. The invasion and metastasis are two important factors affecting the prognosis of cancer patients. The degradation of extracellular matrix is essential for the invasion and metastasis of cancers. The PI3K/Akt/mTOR pathway has been found to be related to the expression of matrix metalloproteinase (MMP) and can upregulate the mRNA and protein expression of MMP-2. In the colon cancer cells (KM20 cells) [18] and highly invasive Lewis lung cancer cells (H259 cells) [19], results showed PI3K/Akt/mTOR activation could induce the invasion of cancer cells in a membrane type 1 MMP dependent manner. MT1-MMP belongs to the MMP family and is a major activator of MMP-2. PI3K inhibitor, mTOR inhibitor and Akt dominant negative mutant over-expression can block the MT1-MMP expression and then significantly reduce the invasion of cancer cells [20-21]. In the hepatocellular carcinoma (HCC), hepatitis B virus X protein (HBX) can stimulate the activation of PI3K/Akt signaling pathway to regulate the transcription of MMP-29, which then increases the invasion of cancer cells [22]. In AB, previous studies showed the mRNA expression of MMP-2 and its inhibitor TIMP-2 was markedly increased as compared to normal dental follicle tissue and that in recurrent AB and solid AB was significantly higher than that in primary AB and cystic AB. In addition, to silence MMP-2 expression via RNAi or reduce MMP-2 expression with MMP-2 inhibitor (Ro31-9790) may dramatically compromise the invasiveness of cancer cells. Our results showed mTOR and its downstream factors had high expression in AB, which may increase the MMP-2 expression, resulting in invasion of cancers.
Early studies on PI3K/Akt/mTOR signaling pathway showed this pathway was abnormal in numerous cancers including esophageal cancer, cholangiocarcinoma, prostate cancer, etc [23-26]. In the oral and maxillofacial tumors, similar findings have also been noted. Liu et al [27] found that the expression of mTOR and its substrate P70S6Kα1, α2, β1 and β2 increased with the increase in malignancy, suggesting that mTOR is a central regulator of cell growth. p70S6K is a direct substrate of mTOR and a key signal molecule regulating protein synthesis. Thus, mTOR/ p70S6K signaling pathway might play important regulator roles in the occurrence of oral squamous cell carcinoma. Chakraborty et al [28] found mTOR/ p70S6K signaling pathway was abnormal in oral squamous cell carcinoma. Thus, they selected 3 important molecules (TSC1, TSC2 and PTEN) in this pathway, and heterozygous deletion as well as was methylation and demethylation were introduced. Results showed TSC1, TSC2 and PTEN presented with heterozygous deletion at different sites, methylation was found in the promoter of TSC2 and PTEN. After administration of methylation inhibitor 5 - N -2 deoxycytidine, the methylation was absent, suggesting that the pathway plays crucial roles in the occurrence and development of oral squamous cell carcinoma. Liu et al investigated this pathway in the malignant parotid tumors, in which immunohistochemistry and western blot assay were employed to detect the expression of p70S6K in acinar cell carcinoma and parotid adenocarcinoma. Western blot assay showed the P70 S6K expression in malignant parotid tumors was markedly increased when compared with normal tissues (P<0.05). Immunohistochemistry revealed the p70S6K expression in malignant parotid tumors was also significantly higher than that in normal tissues.
Scheper et al [13] employed immunohistochemistry to investigate the expression and biological importance of mTOR in AB. Results showed the mTOR expression was found in both cytoplasm and membrane and showed similar level. In the present study, more samples were collected, and immunohistochemistry was done to detect the expression of p-mTOR, p-4E-BP1 and p-p70S6K. Results showed abnormal expression of these proteins. Of 65 patients, 47 were positive for cytoplasmic p-mTOR expression (55.3%) and 27 for nuclear p-mTOR expression (32%); 55 were positive for cytoplasmic p-4E-BP1 expression (64.7%) and 23 for nuclear p-4E-BP1 expression (27.1%); 45 were positive for cytoplasmic p-p70S6K expression (52.9%) and 33 for nuclear p-p70S6K expression (38.8%). These findings indicate that, in AB, there is abnormal aggregation of mTOR in the nucleus, which may activate the transcription of mTOR downstream factors and lead to the abnormal aggregation of these factors. Our results showed the p-mTOR, p-4E-BP1 and p-p70S6K entered from the cytoplasm to the nucleus, and this abnormality was related to the biological behavior of AB. In the multivariable analysis, results showed p-mTOR could serve as an independent factor predicting the prognosis of AB patients. The presence of nuclear p-mTOR expression in AB increased the risk for recurrence of AB by 6.417 folds.
It has been widely accepted that the expression of mTOR and its downstream factors is mainly found in the cytoplasm, and these proteins compose of several intracellular membranous structure. mTOR functions via two multi-protein complexes: mTORC1 and mTORC2. The downstream factors of mTORC1 include 4E-BP1 and p70S6K, and the phosphorylation of mTORC2 by Akt may lead to mTORC2 activation. The mTORC1 activation may increase the invasiveness of cancer cells, which may be attributed to the regulation of translation by 4E-BP1 and p70S6K. Usually, 4E-BP1 and p70S6K are found in the cytoplasm of cancer cells. Previous studies [29-30] showed the nuclear p-4E-BP1 expression was related to the poor differentiation of cancers. In the breast cancer and ovarian cancer, p-4E-BP1 expression is frequently found in poorly differentiated cancers and also predicts a poor prognosis. These findings support our results that nuclear expression of p-mTOR and p-4E-BP1was related to the invasiveness of cancers. There is evidence showing that mTORC1 locates in the cytoplasm of fibroblasts, but its distribution in cancer cells is poorly understood. Study showed the nuclear distribution of mTOR was helpful for the signal transduction of 4E-BP1 and p70S6K in the cytoplasm, but the specific mechanism is unclear. In recent years, Bechman et al [31] found that the nuclear expression of mTOR required the signals from cytoplasmic p70S6K, and p70S6K over-expression was associated with poor prognosis of cancers, which was consistent with our findings.
References
- 1.Luo HY, Li TJ. Odontogenic tumors: A study of 1309 cases in a Chinese population. Oral Oncol. 2009;45:706–711. doi: 10.1016/j.oraloncology.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Gomes CC, Oliveira CS, Castro WH. Clonal nature of odontogenic tumours. J Oral Pathol Med. 2009;38:397–400. doi: 10.1111/j.1600-0714.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Migaldi M, Sartori G, Rossi G. Tumour cell proliferation and microsatellite alterations in human ameloblastoma. Oral Oncol. 2008;44:50–60. doi: 10.1016/j.oraloncology.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, Wang J, Pan C. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro. BMC Cancer. 2008;8:182–192. doi: 10.1186/1471-2407-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nodit L, Barnes L, Childers E, Finkelstein S, Swalsky P, Hunt J. Allelic loss of tumor suppressor genes in ameloblastic tumors. Mod Pathol. 2004;17:1062–1067. doi: 10.1038/modpathol.3800147. [DOI] [PubMed] [Google Scholar]
- 6.Dai DL, Majrtinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J. Clin. Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 7.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer. 2005;103:307–312. doi: 10.1002/cncr.20774. [DOI] [PubMed] [Google Scholar]
- 9.Roca S, Quivy A, Gross-Goupil M, Bernhard JC, De Clermont H, Ravaud A. Efficacy of rechallenging metastatic renal cell carcinoma with mTOR inhibitors. Acta Oncol. 2011;50:1135–1136. doi: 10.3109/0284186X.2011.592149. [DOI] [PubMed] [Google Scholar]
- 10.Min YH, Cheong JW, Kim JY, Eom JI, Lee ST, Hahn JS, Ko YW, Lee MH. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004;64:5225–5231. doi: 10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- 11.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB /AKT- a major therapeutic target. Biochim Biophys Acta. 2004:1697–1703. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome C from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheper MA, Chaisuparat R, Nikitakis NG, Sauk JJ. Expression and alterations of the PTEN / AKT / mTOR pathway in ameloblastomas. Oral Dis. 2008;14:561–8. doi: 10.1111/j.1601-0825.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon V, St-Germain ME, Parent S, Asselin E. Akt activity in endometrial cancer cells: regulation of cell survival through AP21. Int J Oncol. 2003;23:803–10. [PubMed] [Google Scholar]
- 15.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:295–302. doi: 10.1016/j.bbrc.2005.03.166. [DOI] [PubMed] [Google Scholar]
- 16.Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst) 2004;3:927–34. doi: 10.1016/j.dnarep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signaling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 18.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, Schmidt M, Mills GB, Mendelsohn J, Fan Z. HER2 /PI23K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–12. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 19.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 20.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR /p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281–91. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- 21.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Brodt P. Type 1 insulin2like growth factor regulatesMT12 MMP synthesis and tumor invasion via PI 32kinase /Akt signaling. Oncogene. 2003;22:974–982. doi: 10.1038/sj.onc.1206197. [DOI] [PubMed] [Google Scholar]
- 23.Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrixmetalloproteinase 29 gene expression through activation of ERK and PI3K/AKT pathways: involvement of invasive potential. FASEB J. 2004;18:1123–1125. doi: 10.1096/fj.03-1429fje. [DOI] [PubMed] [Google Scholar]
- 24.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butzal M, Loges S, Schweizer M, Fischer U, Gehling UM, Hossfeld DK, Fiedler W. Rapamycin inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Exp Cell Res. 2004;300:652–671. doi: 10.1016/j.yexcr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, Gartel A, Hay N. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Yang ML, Zhang Y, Yu BZ. The expression of mTOR and its substrates in oral squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi. 2004;22:331–3. [PubMed] [Google Scholar]
- 28.Chakraborty S, Mohiyuddin SM, Gopinath KS, Kumar A. Involvement of TSC genes and differential expression of other members of the mTOR signaling pathway in oral squamous cell carcinoma. BMC Cancer. 2008;8:163–175. doi: 10.1186/1471-2407-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Shu L, Hosoi H, Murti KG, Houghton PJ. Predominant nuclear localization of mammalian target of rapamycin innormal and malignant cells in culture. J Biol Chem. 2002;277:28127–34. doi: 10.1074/jbc.M202625200. [DOI] [PubMed] [Google Scholar]
- 30.Sabatini DM. MTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann RA, Kim JH, Wu AL, Park IH, Chen J. A nuclear transport signal in mammalian target of rapamycin is critical for its cytoplasmic signaling to S6 kinase 1. J Biol Chem. 2006;281:7357–63. doi: 10.1074/jbc.M512218200. [DOI] [PubMed] [Google Scholar]