Abstract
Upper tract urothelial carcinoma (UTUC) associated with Balkan endemic nephropathy (BEN) is characterized by a number of aberrations in cell-cycle regulation and apoptosis. The aim of this study was to detect angiogenesis-related marker(s) specific for BEN UTUC, and to examine the influence of HIF 1α upon angiogenesis and apoptosis in UTUC. Present investigation included 110 patients with UTUC, 50 from BEN region and 60 control tumors. Altered expression of VEGFR1 was more often present in control UTUC than in BEN tumors (p<0.005). It was associated with high grade, low and high stage, solid growth, and metaplastic change of control UTUC. Microvessel density assessed by CD31 (MVD CD31) was significantly higher in UTUC with lymphovascular invasion (p<0.05), and in BEN tumors with papillary growth (p<0.05). Discriminant analysis indicated that BEN and control tumors do not differ significantly in expression of angiogenesis related markers. The most important discriminant variable that determined control UTUC was expression of VEGFR1 (p=0.002). HIF 1α in UTUC significantly correlated with the low stage, papillary growth and expression of Bcl-2, Caspase-3 index, and MVD CD34 (p<0.001; 0.0005; 0.01; 0.005; 0.01, respectively). HIF-1α may be helpful marker in evaluation of UTUC, especially when combined with angiogenesis and apoptosis.
Keywords: Balkan endemic nephropathy, upper tract urothelial carcinoma, angiogenesis, VEGF, VEGFR1, VEGFR2, CD31, CD34, HIF 1α, apoptosis
Introduction
Upper tract urothelial carcinoma (UTUC) is rare but deadly disease due to its aggressive biological behavior and a late stage diagnosis [1]. An increased number of UTUC has been observed in the foci of Balkan endemic nephropathy (BEN) [2,3]. BEN is a bilateral chronic slow progressive tubulointerstitial disease with long evolution to terminal renal failure [4,5]. Environmental factors that include toxicants, such as herbs containing aristolochic acid (AA) [6,7], mycotoxins [8] and organic compounds leached from coal deposits [9], have influence in the pathogenesis of BEN [9,10]. Moreover, epidemiological and pedigree analysis revealed that genetic factors are important in BEN, and genetic factors interplay with the environmental factors [4]. Carcinomas may occur alone or in combination with BEN. Some authors suggest that the same factor have some influence in the development of the tubulointerstitial disease and UTUC [4]. We have demonstrated p53 pathway as the specific cell cycle marker involved in BEN related UTUC [11]. Our recent analysis of apoptosis related markers identified Bax as a specific marker of BEN-associated UTUC as well as alteration of Survivin. This finding may be indicative for specific disturbances of an intrinsic apoptotic pathway in UTUC arising in endemic areas [12].
Various growth factors and molecules have been reported to be associated with tumor growth, progression, and survival in urothelial cancer [13,14]. Urothelial cancer cells suffer aberrations in cell-cycle regulation, apoptosis, signal transduction and angiogenesis, particularly in BEN regions [10,15]. The importance of angiogenesis in human tumors is reflected by recent studies that demonstrated that the angiogenic phenotype, as measured by microvessel density (MVD), is an indicator of survival, locally advanced disease, stage, lymphovascular invasion and lymph node metastasis in patients with various types of cancer [16]. To date, limited number of studies investigated angiogenesis in UTUC, especially in UTUC associated with BEN. The aims of this study were to evaluate and correlate the expression of angiogenesis related markers-VEGF, VEGFR1, VEGFR2, HIF 1α, MVD CD31, and MVD CD34 with pathological characteristics of UTUC in BEN and control population; and to determine the influence of HIF 1α to angiogenesis and apoptosis in UTUC.
Materials and methods
Patient’s population
We studied 110 patients with UTUC who had undergone open type of nephroureterectomy with removal of bladder cuff as part of the nephroureterectomy procedure. Extended lymphadenectomy was not routinely performed. All cases of UTUC were diagnosed at the Institute of Pathology, Faculty of Medicine, Nis. The analysis included 80 transitional cell carcinomas with pelvic localization and 30 with ureteral. Patients were divided in two groups: 50 patients were from endemic settlements, the villages along the South Morava River basin (BEN tumors) and 60 consecutive control subjects, residents of rural and city areas free of BEN.
Histological analysis
The histological sections were processed from tissue fixed in 10% formalin by standard techniques, and stained with haematoxylin and eosin (H&E). H&E-stained slides were used to assess histological grade (low and high grade) [17], pathologic stage (pT) [18], growth of tumor (papillary/solid), lymphovascular invasion (LVI) and the presence of necrosis and metaplastic changes (squamous or glandular) within the tumor. The low stage non-muscle invasive tumors (pTa-pT1) and high stage muscle invasive tumors (pT2-pT4) were compared [19]. The presence of tumor necrosis was evaluated on microscopic examination of tumor. According to the WHO criteria for the diagnosis of histological variants of urothelial cancer squamous differentiation was defined as the presence of intercellular bridges or keratinization [17]. The conventional criteria for squamous metaplastic change in UTUC include abundant eosinophilic cytoplasm, large oval nuclei with an open chromatin pattern and prominent nucleoli. Glandular differentiation was defined as the presence of true glandular spaces and gland-like lumina within tumor cell nests.
Immunohistochemistry and scoring
Tumors were analyzed using the mouse monoclonal antibodies against VEGF (A-20: sc-152/Santa Cruz Biotechnology), anti-human VEGF R1 antibody (AF321, R&D sistem), anti-human VEGF R2 antibody (AF357, R&D sistem), monoclonal antibodies against CD31 (Clone JC70A, Dako), CD34 (Clone QBEnd Ready to use, Dako), and HIF1 alpha (H-206: sc-10790/Santa Cruz Biotechnology) at dilution 1: 250, 1: 20, 1: 6, 1: 20, ready to use, 1: 250 respectively. Apoptotic activity was assessed with the following antibodies: Bcl-2 (Clone 124, M 0887/Dako), Survivin (FL-142: sc-10811/ Santa Cruz Biotechnology), Bax (Code A 3533/ Dako), Fas (C-20: sc-715/ Santa Cruz Biotechnology) and Caspase-3 cleaved (ACR 229 A, B, C/ Biocare medical) at dilution of 1:50, 1:500, 1:1000, 1:100, 1:200 respectively. Before quantifying the immunohistochemical results, the technique quality was assessed, and those areas with greater positivity were selected, avoiding peripheral area measurement, necrosis or artifact. Slides were reviewed independently by three investigators (LJV, ARP, SS). Interobserver discrepancies were resolved using a double headed microscope. Nuclear expression was recorded for HIF1 alpha and Survivin, and cytoplasmic for VEGF, VEGF R1, VEGF R2, Bcl-2, Bax, and Fas [12].
Immunohistochemical reaction was scored as follows: negative if ≤10% of cells were stained, and positive if ≥10% were stained. Cytoplasmic staining intensity was scored using a scale of 0 to 3 (0, no staining; 1, weak; 2, moderate; and 3, intense). Tumor cell nuclear immunoreactivity for HIF-1alpha was scored in accordance with a previous study [20].
All markers were placed in one of two categories, altered or not altered (normal). Cytoplasmic immunoreactivity was considered altered when samples demonstrated positivity in >10% of tumor cells with an intensity of 2 or 3 [20]. Microvessels were identified by immunostaining of endothelial cells for CD31 and CD34. Active areas of angiogenesis (“hot spots”) were selected using low magnification. Images from five high power fields (400×) in the hot spot area were recorded for each sample. Any stained endothelial cell was considered to represent a single, countable microvessel if it was isolated from adjacent microvessels, tumor cells and other connective tissue elements. The total number of microvessels so obtained, was then divided by the number of the counted hotspots, and the result was used to denote the MVD [21]. Cleaved Caspase 3 index was calculated as number of positive cells x100 per total number of cells in ten random high power fields (x400) in each tumor. This index was established by counting at least 2000 cells in fields distant from necrotic areas [12].
Statistical analysis
For purposes of analysis, pathological tumor stage (low vs. high), grade (low vs. high), growth pattern (papillary vs. solid), LVI (yes vs. no), and clinical parameters-sex (M vs. F), localization (pelvis vs. ureter) were evaluated as dichotomized variables. The Fisher’s exact test and the χ2 test were used to estimate the expression of VEGF, VEGFR1, VEGFR2, and HIF1α in regard to pathological parameters (stage, grade, growth, lymphovascular invasion, necrosis, metaplastic differentiation) of tumors, and Mann-Whitney U test in evaluation of MVD with investigated parameters.
Discriminant analysis was used to investigate differences between examined groups. At the first step an F test (Wilks’ lambda) is used to test if the discriminant model as a whole is significant. At the second step, the covariance matrices, coefficients of canonical correlation and the standardized canonical discriminant function coefficients were used to classify the dependent variable. The standardized canonical discriminant function coefficients were used to compare the relative importance of the independent variables (VEGF, VEGFR1, VEGFR2, CD31, CD34, HIF 1α).
The result was considered statistically significant if p<0.05. All analyses were performed with the SPSS statistical package (SPSS version 10.0 for Windows).
Results
Clinical features in UTUC
The age in 110 patients with UTUC ranged from 22 to 87 years, with a mean age of 64.4 ± 8.51 years for tumors in BEN regions, and 64.02 ± 11 years for control tumors. There were 23 male (46%) and 27 female (54%) patients in BEN-associated UTUC group with ratio M: F = 1:1.2 while in the control group there were 36 men (60%) and 24 women (40%), with ratio M: F = 1.5:1. Tumor localization was more frequent on the left side in both BEN and control UTUC, however without any statistical difference between these two groups (32/18 versus 37/23).
Immunohistochemical evaluation of angiogenesis-related biomarkers and association with pathological characteristics in BEN and control UTUC
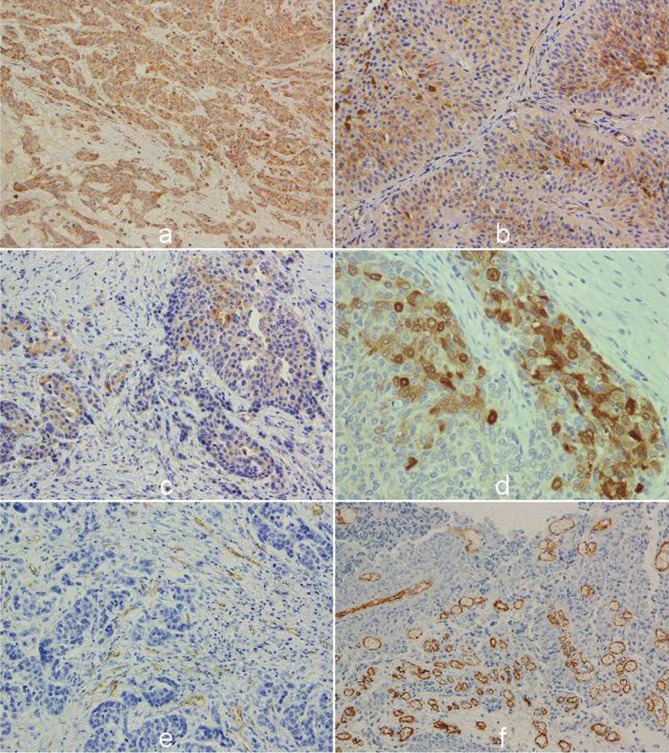
The immunohistochemical staining of VEGF, VEGFR1, VEGFR2, and HIF 1α was detected in 107 (97.3 %), 93 (84.5 %), 78 (70.9%), and 110 (100 %) UTUC, respectively. Angiogenic markers - VEGF, VEGFR1, VEGFR2, and HIF 1α were altered in 78 (70.9%), 72 (65.5%), 29 (26.4 %), 50 (45.45%) UTUC, respectively (Figure 1A, 1B, 1C, 1D). Investigation of relationships between conventional pathological parameters and immunohistochemical staining of VEGF, VEGFR1, VEGFR2, HIF 1α, MVD CD34 and MVD CD32 in UTUC showed that VEGF significantly associated with presence of tumor necrosis in UTUC [yes 32/44 (72.7%) versus no 26/66 (39.4%), χ2=11.66, p<0.001]; VEGFR2 with high stage [low stage 9/40 (22.5%) versus high stage 40/70 (57.1%), χ2=12.25, p<0.0005], and HIF 1α with stage and growth pattern of UTUC [low stage 25/40 (62.5%) versus high stage 25/70 (35.7%), χ2=7.30, p<0.001; papillary 27/42 (64.3%) versus solid 23/68 (33.8%), χ2=9.63, p<0.0005]. MVD CD31 was significantly higher in UTUC with LV invasion (yes 7.21±9.72 versus no 4.16±8.71, p<0.05) (Figure 1E). MVD CD34 was not significantly associated with investigated pathological characteristics in UTUC (Figure 1F).
Figure 1.
The representative immunohistochemical staining of angiogenic markers in upper tract urothelial carcinoma, with strong cytoplasmic VEGF activity in high grade (A); altered cytoplasmic expression of VEGFR1 and (B); VEGFR2 in invasive tumors with (C), nuclear and cytoplasmic staining of HIF 1α (D). Immunostaining of endothelial cells for CD31 and (E). active areas of angiogenesis detected with CD34 in urothelial cancer (F) (original magnification: x400).
Tables 1 and 2 display relationships between conventional pathological parameters in BEN and control UTUC and angiogenesis related biomarkers. Control tumors have significantly higher cytoplasmic expression of VEGFR1, which is associated with high grade, stage - low and high, solid growth and metaplastic change in UTUC than BEN associated UTUC with the same pathological features (χ2=11.56, p<0.001; χ2=4.2, p<0.05; χ2=4.8, p<0.05; χ2=6.34, p<0.01; χ2=7.72, p<0.005, respectively). Investigation of MVD CD31 showed that BEN tumors with papillary growth had a higher value than control tumors with the same growth (p<0.05). The other angiogenesis related markers- VEGF, VEGFR2, HIF 1α, and MVD CD34, were not significantly associated with the phenotypic characteristics of BEN and control associated UTUC.
Table 1.
Correlation of angiogenesis-related biomarkers expression with pathological characteristics in BEN and control UTUC
| UTUC | N 110 | Altered expression of biomarker | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| VEGF | p < | VEGFR1 | p < | VEGFR2 | p < | ||
| Low grade | N.S. | N.S. | N.S. | ||||
| BEN | 20 | 14 | 11 | 5 | |||
| Control | 24 | 13 | 16 | 4 | |||
| High grade | N.S. | 0.001 | N.S. | ||||
| BEN | 30 | 20 | 14 | 10 | |||
| Control | 36 | 31 | 31 | 10 | |||
| Low stage | N.S. | 0.05 | N.S. | ||||
| BEN | 22 | 14 | 10 | 4 | |||
| Control | 18 | 11 | 14 | 5 | |||
| High stage | N.S. | 0.05 | N.S. | ||||
| BEN | 28 | 20 | 15 | 11 | |||
| Control | 42 | 33 | 33 | 29 | |||
| Papillary growth | N.S. | N.S. | N.S. | ||||
| BEN | 16 | 11 | 8 | 4 | |||
| Control | 26 | 18 | 20 | 8 | |||
| Solid growth | N.S. | 0.01 | N.S. | ||||
| BEN | 34 | 23 | 17 | 11 | |||
| Control | 34 | 26 | 27 | 6 | |||
| LV invasion-yes | N.S. | N.S. | N.S. | ||||
| BEN | 11 | 8 | 7 | 4 | |||
| Control | 20 | 17 | 16 | 5 | |||
| Necrosis - yes | N.S. | N.S. | N.S. | ||||
| BEN | 19 | 13 | 13 | 6 | |||
| Control | 25 | 19 | 19 | 4 | |||
| Nonmetaplastic | N.S. | N.S. | N.S. | ||||
| BEN | 37 | 25 | 21 | 10 | |||
| Control | 47 | 33 | 36 | 12 | |||
| Metaplastic | N.S. | 0.005 | N.S. | ||||
| BEN | 13 | 9 | 4 | 5 | |||
| Control | 13 | 11 | 11 | 2 | |||
Table 2.
Correlation of angiogenesis-related biomarkers expression with pathological characteristics in BEN and control UTUC
| UTUC | N 110 | Altered expression of biomarker | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HIF 1α | p < | CD31 | p < | CD34 | p < | ||
| Low grade | N.S. | N.S. | N.S. | ||||
| BEN | 20 | 10 | 6.74±10.94 | 22.92±7.71 | |||
| Control | 24 | 14 | 3.40±8.42 | 22.81±11.92 | |||
| High grade | N.S. | N.S. | N.S. | ||||
| BEN | 30 | 15 | 4.60±7.21 | 21.77±10.32 | |||
| Control | 36 | 11 | 5.50±9.87 | 22.55±11.87 | |||
| Low stage | N.S. | N.S. | N.S. | ||||
| BEN | 22 | 13 | 5.97±9.31 | 21.63±7.01 | |||
| Control | 18 | 12 | 1.97±5.85 | 20.96±9.62 | |||
| High stage | N.S. | N.S. | N.S. | ||||
| BEN | 28 | 12 | 5.05±8.62 | 22.65±10.88 | |||
| Control | 42 | 13 | 5.81±10.28 | 23.38±12.64 | |||
| Papillary growth | N.S. | 0.05. | N.S. | ||||
| BEN | 16 | 12 | 8.63±11.70 | 22.44±5.68 | |||
| Control | 26 | 15 | 1.90 ±5.12 | 20.46 ±9.49 | |||
| Solid growth | N.S. | N.S. | N.S. | ||||
| BEN | 34 | 13 | 3.97±6.84 | 22.13±10.66 | |||
| Control | 34 | 10 | 6.77±11.14 | 24.33±13.18 | |||
| LV invasion-yes | N.S. | N.S. | N.S. | ||||
| BEN | 11 | 5 | 9.04±10.94 | 18.02±8.93 | |||
| Control | 20 | 8 | 6.21±9.12 | 19.53±7.51 | |||
| Necrosis - yes | N.S. | N.S. | N.S. | ||||
| BEN | 19 | 8 | 5.11±6.90 | 21.77±11.62 | |||
| Control | 25 | 11 | 3.48±7.46 | 25.16±13.08 | |||
| Nonmetaplastic | N.S. | N.S. | N.S. | ||||
| BEN | 37 | 19 | 4.78±8.38 | 22.37±9.35 | |||
| Control | 47 | 19 | 4.50±9.20 | 21.36±11.38 | |||
| Metaplastic | N.S. | N.S. | N.S. | ||||
| BEN | 13 | 6 | 7.38±10.18 | 21.81±9.52 | |||
| Control | 13 | 6 | 5.25±10.03 | 27.33±12.51 | |||
χ2 test was performed. *Mann Whitney U test was performed. N.S. - no significance
Comparison of angiogenesis-related biomarkers in BEN and control UTUC
Comparing angiogenesis-related biomarkers and group, the significant difference in VEGFR1 expression was detected (Table 3). BEN tumors had less frequent alteration of VEGFR1 than control tumors [25/25 (50%) vs. 47/13 (78.3%), χ2=9.68, p<0.005]. In contrast to this marker, the expression of VEGF, VEGFR2, HIF 1α, MVD CD31, and MVD CD34 was not significantly associated with the groups (Table 3).
Table 3.
Expression of angiogenesis-related biomarkers in UTUC associated with BEN and in control UTUC
| Molecular marker | N 110 | BEN (N = 50) | Control (N = 60) | p < |
|---|---|---|---|---|
| Expression N (%) | ||||
| VEGF | N.S. | |||
| Not altered | 32 (29.1) | 16 (32.0) | 16 (26.7) | |
| Altered | 78 (70.9) | 34 (68.0) | 44 (73.3) | |
| VEGFR1 | 0.005 | |||
| Not altered | 38 (34.5) | 25 (50.0) | 13 (21.7) | |
| Altered | 72 (65.5) | 25 (50.0) | 47 (78.3) | |
| VEGFR2 | NS | |||
| Not altered | 81 (73.6) | 35 (70.0) | 46 (76.7) | |
| Altered | 29 (26.4) | 15 (30.0) | 14 (23.3) | |
| MVD CD31* | N.S. | |||
| X± SD | 5.02±9.06 | 5.46±8.85 | 4.66±9.3 | |
| MVD CD34* | N.S. | |||
| X± SD | 22.46±10.68 | 22.23±9.30 | 22.65±11.79 | |
| HIF 1α | N.S. | |||
| Not altered | 60 (54.5) | 25 (50.0) | 35 (58.3) | |
| Altered | 50 (45.5) | 25 (50.0) | 25 (41.7) | |
χ2 test was performed.
Mann Whitney U test was performed.
N.S. - no significance
Discriminant analysis of the following angiogenic markers - VEGF, VEGFR1, VEGFR2, MVD CD31, MVD CD34, HIF 1α between BEN and control tumors, did not indicate statistically significant difference (Wilks’ Lambda=0.892, χ2 = 12.024, p= 0.061). Covariance matrices were not significantly different between them (Box’s M=20.446, F=0.915, p= 0.571) and coefficient of canonic correlation was low (0.329), indicating that those groups had similarity. The most important discriminant variable is the expression of VEGFR1, Wilks’ Lambda=0.912, F=10.424, p=0.002 (Table 4). Standardized canonical discriminant function coefficients showed that VEGFR1 (0.935) was characteristic that differ between groups (Table 4). Examined angiogenesis related variables better defined control tumors than BEN tumors which indicated group centroids (0.315 versus -0.378). Classification results showed that UTUC in 63.6%, correctly classified (Table 5).
Table 4.
Discriminant analysis of UTUC
| Parameters | Tests of Equality of Group Means | Standardized Canonical Discriminant Function Coefficients | ||
|---|---|---|---|---|
|
| ||||
| Wilks’Lambda | F | Sig. | ||
| VEGF | 0.997 | 0.370 | 0.544 | 0.133 |
| VEGFR1 | 0.912 | 10.424 | 0.002 | 0.935 |
| VEGFR2 | 0.994 | 0.617 | 0.434 | -0.415 |
| CD31 | 0.998 | 0.210 | 0.648 | -0.175 |
| CD34 | 1.000 | 0.043 | 0.836 | 0.004 |
| HIF 1α | 0.993 | 0.755 | 0.387 | -0.130 |
Table 5.
Classification results of discriminant analysis in UTUC
| GROUP | Predicted Group Membership | Total | |||
|---|---|---|---|---|---|
| 1 | 2 | ||||
| Original | Count | 1 | 44 | 16 | 60 |
| 2 | 24 | 26 | 50 | ||
| % | 1 | 73.3 | 26.7 | 100,0 | |
| 2 | 48.0 | 52.0 | 100.0 | ||
| Cross-validated | Count | 1 | 38 | 22 | 60 |
| 2 | 24 | 26 | 50 | ||
| % | 1 | 63.3 | 36.7 | 100.0 | |
| 2 | 48.0 | 52.0 | 100.0 | ||
a. Cross validation is done only for those cases in the analysis. In cross validation, each case is classified by the functions derived from all cases other than that case. b. 63.6% of original grouped cases correctly classified. c. 58.2% of cross-validated grouped cases correctly classified. Group 1 - Control tumor. Group 2 - BEN tumor
Correlation of HIF 1α and angiogenesis and apoptosis related markers in UTUC
Association of HIF 1α with expression of apoptosis (Survivin, Bcl-2, Bax, Fas and Caspase 3), and angiogenesis related markers showed that this marker is significantly connected with the expression of Bcl-2, Caspase-3 index, and MVD CD34 (χ2=7.19, p<0.01; Z= -2.89, p<0.005; Z= -2.61, p<0.01, respectively) (Table 6). Significant association of HIF 1α with other investigated markers - Survivin, Bax, Fas, VEGF, VEGFR1, VEGFR2 and CD31 were not found.
Table 6.
Expression of HIF 1α in UTUC with relation to angiogenesis and apoptosis related biomarkers
| Molecular marker | N 104 | HIF 1α normal (N = 60) | HIF 1α altered (N = 50) | p < |
|---|---|---|---|---|
| Expression N (%) | ||||
| Bcl-2 | 0.01 | |||
| 1 | 88 (84.6) | 54 (93.1) | 34 (73.9) | |
| 2 | 16 (15.4) | 4 (6.9) | 12 (26.1) | |
| Caspase-3 index* | ||||
| X± SD | 2.55±7.42 | 1.64±7.61 | 3.72±7.09 | 0.005 |
| MVD CD34* | ||||
| X± SD | 22.46±10.68 | 21.18±12.12 | 23.99±8.53 | 0.01 |
χ2 test was performed.
Mann Whitney U test was performed
Discussion
BEN is associated with dramatic kidney fibrosis and severe hypoxia [4,22]. It is well known that hypoxia promotes formation of new blood vessels, especially in cancer [23]. According to the morphological characteristics of BEN, it is to be expected that tumor cells in UTUC arising in BEN starve for oxygen thus producing large amounts of VEGF. In fact, this assumption is strongly confirmed by significantly higher expression of VEGF in UTUC with prominent necrosis, however, statistically significant difference in the expression of the VEGF between BEN and control tumors was not detected in this study.
It has been reported that VEGF is produced by malignant cells that have undergone genetic changes, irrespective of hypoxia status [24,25]. Tumors that undergo neovascularization can enter a phase of rapid cell growth and may have increased metastatic potential. In the absence of neovascularization, tumors become necrotic and/or apoptotic. Recently, Shariat with colleagues showed a strong association of VEGF expression and established clinicopathological features of aggressiveness in urothelial carcinoma of the bladder [26]. High VEGF expression in superficial bladder cancers is associated with early recurrence and progression to a more invasive phenotype and thereby can predict progression in Ta bladder tumors at first presentation [27]. Over expression of VEGF in high grade tumors may be an indicator of genetic instability and prone to early metastasis [28].
Present investigation has revealed that VEGF-R1 expression was more frequently altered in control tumors than in BEN tumors, and it was associated with grade, stage, growth pattern and concomitant metaplastic changes in UTUC. Discriminant analysis of groups and angiogenic markers indicate that BEN and control tumors share similarity. The most important investigated discriminant variable was VEGFR1. In the current study, expression of VEGFR1 in malignant cells defined control tumors better than BEN tumors.
Higher expression of VEGFR1 in control tumors was demonstrated in the present study, and BEN UTUC with papillary growth had significantly higher MVD CD31 than control tumors. Therefore, during angiogenesis, high levels of pro-angiogenic factors, such as VEGFA and VEGFC, stimulate VEGFR2, principal receptor for VEGF signaling. These signals are essential to select ‘tip cells’ for sprouting [29]. Hence, VEGFA signaling induces the motility and invasiveness of the ‘tip cells’ and activates the angiogenic switch [30]. Expression of VEGFR1 and activation of Notch pathway repress ‘tip cells’ behavior to maintain the hierarchical organization of sprouting endothelial cells [31]. By binding VEGF, soluble VEGFR1 reduce the angiogenic activity of VEGF, acting like indispensable decoy receptor for VEGFA that limits its availability to activate VEGFR2. Finally, molecules, that initially induce angiogenesis, become angiogenic inhibitors, thereby providing a negative feedback [32]. In tumor tissue, failure of potential inhibitors to block angiogenesis allows transformed cells further nourishment and spreading.
Birkhahn et al [33], demonstrated that over expression of VEGFR2, mediator for most of VEGF responses, was a determinant of nodal metastasis in bladder cancer, and predictor of progression in Ta tumors. In accordance with previous studies, in the present study UTUC with the high stage showed significantly higher immunoreactivity for VEGFR2, however, without significant difference between BEN and control tumors.
Hypoxia has a decisive impact in different molecular pathways, modulating several cellular functions, like proliferation, apoptosis, angiogenesis, pH balance and anaerobic glycolysis. The HIF-1 pathway, with its numerous downstream targets, is the key controller in this reaction [25,34,35]. Our study of HIF 1α in UTUC showed significant association with the low stage and papillary growth, but without difference between BEN and control tumors. In superficial bladder cancer overexpression of HIF 1α significantly correlated with recurrence, survival, and poor prognosis especially when combined with altered p53 expression [36].
The present research explored potential correlation between nuclear accumulation of HIF 1α and apoptosis and angiogenesis in UTUC. Apoptotic markers were explored in our previous study [12], and in this research we found that cleaved caspase 3 index was higher in UTUC with altered expression of HIF 1α. In addition, HIF 1α expression inversely correlated with Bcl-2, main antiapoptotic protein, that controls mitochondrial membrane permeability and inhibits caspase activation [37,38]. Moreover, altered expression of HIF 1α strongly correlated with higher MVD CD34 in investigated UTUC. These indicators closely relate to prognostic parameters in bladder urothelial carcinoma and may be helpful tool for providing reliable prognosis and the appropriate treatment planning [39].
In conclusion, present study demonstrates resemblance in expression of angiogenic markers among BEN and control groups, highlighting the VEGFR1 as the best discriminant characteristic of control UTUC. Accumulation of HIF-1α, together with apoptotic markers and MVD, could be used in the evaluation of UTUC, especially in the low stage, selecting the tumors that could require an intensive follow-up, or more aggressive therapy.
Acknowledgements
This work was supported by a grant No 175092 from the Ministry of Education and Science of the Republic of Serbia.
Conflict of interest statement
Authors declare none.
References
- 1.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998 Oct;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 2.Petkovic SD. Epidemiology and treatment of renal pelvic and ureteral tumors. J Urol. 1975 Dec;114:858–865. doi: 10.1016/s0022-5347(17)67160-x. [DOI] [PubMed] [Google Scholar]
- 3.Stefanovic V, Radovanovic Z. Balkan endemic nephropathy and associated urothelial cancer. Nat Clin Pract Urol. 2008 Feb;5:105–112. doi: 10.1038/ncpuro1019. [DOI] [PubMed] [Google Scholar]
- 4.Polenaković M, Stefanović V. Balkan Nephropathy. In: Cameron JS, Davison AM, Grunfeld JP, Kerr D, Ritz E, editors. Oxford Textbook of Clinical Nephrology. Oxford, UK: Oxford University Press; 1992. pp. 857–866. [Google Scholar]
- 5.Stefanović V, Polenaković M. Fifty years of research in Balkan endemic nephropathy: where are we now? Nephron Clin Pract. 2009;112:c51–56. doi: 10.1159/000213081. [DOI] [PubMed] [Google Scholar]
- 6.Ivić M. Etiology of endemic nephropathy. Lijec Vjesn. 1969;91:1273–1281. [PubMed] [Google Scholar]
- 7.Cosyns JP, Jadoul M, Squifflet JP, De Plaen JF, Ferluga D, Van Ypersele de Strihou C. Chinese herbs nephropathy: a clue to Balkan endemic nephropathy? Kidney Int. 1994 Jun;45:1680–1688. doi: 10.1038/ki.1994.220. [DOI] [PubMed] [Google Scholar]
- 8.Petkova-Bocharova T, Castegnaro M. Ochratoxin A contamination of cereals in an area of high incidence of Balkan endemic nephropathy in Bulgaria. Food Addit Contam. 1985;2:267–270. doi: 10.1080/02652038509373555. [DOI] [PubMed] [Google Scholar]
- 9.Toncheva D, Dimitrov T, Stojanova S. Etiology of Balkan endemic nephropathy: a multifactorial disease? Eur J Epidemiol. 1998 Jun;14:389–394. doi: 10.1023/a:1007445120729. [DOI] [PubMed] [Google Scholar]
- 10.Stefanovic V, Toncheva D, Atanasova S, Polenakovic M. Etiology of balkan endemic nephropathy and associated urothelial cancer. Am J Nephrol. 2006;26:1–11. doi: 10.1159/000090705. [DOI] [PubMed] [Google Scholar]
- 11.Velickovic LJ, Hattori T, Stefanovic V. Molecular markers in upper urothelial carcinoma associated to Balkan endemic nephropathy. Aristolochic acid as the major risk factor of the worldwide disease. Scientific World Journal. 2009 Dec;9:1360–1373. doi: 10.1100/tsw.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankovic-Velickovic L, Stojnev S, Ristic-Petrovic A, Dolicanin Z, Hattori T, Mukaisho K, Stojanovic M, Stefanovic V. Pro- and antiapoptotic markers in upper tract urothelial carcinoma associated with Balkan endemic nephropathy. Scientific World Journal. 2011;11:1699–1711. doi: 10.1100/2011/752790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quek ML, Quinn DI, Daneshmand S, Stein JP. Molecular progression in bladder cancer - a current perspective. Eur J Cancer. 2003 Jul;39:1501–1510. doi: 10.1016/s0959-8049(03)00300-9. [DOI] [PubMed] [Google Scholar]
- 14.Gontero P, Banisadr S, Frea B, Brausi M. Metastatic markers in bladder cancer: a review of the literature and clinical considerations. Eur Urol. 2004 Sep;46:296–311. doi: 10.1016/j.eururo.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Mitra AP, Bartsch CC, Cote RJ. Strategies for molecular expression profiling in bladder cancer. Cancer Metastasis Rev. 2009 Dec;28:317–326. doi: 10.1007/s10555-009-9196-5. [DOI] [PubMed] [Google Scholar]
- 16.Iordache S, Saftoiu A, Georgescu CV, Ramboiu S, Gheonea DI, Filip M, Schenker M, Ciurea T. Vascular endothelial growth factor expression and microvessel density - two useful tools for the assessment of prognosis and survival in gastric cancer patients. J Gastrointestin Liver Dis. 2010 Jun;19:135–139. [PubMed] [Google Scholar]
- 17.Lopez-Beltran A, Sauter G, Gasser T. Tumors of the urinary system. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Infiltrating urothelial carcinoma. World Health Organization Classification of Tumors. Pathology and Genetics. Tumors of the Urinary System and Male Genital Organs. Lyon, France: IARC; 2004. pp. 93–109. [Google Scholar]
- 18.Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. 6th edition. New York, USA: John Wiley & Son; 2002. [Google Scholar]
- 19.Genega EM, Kapali M, Torres-Quinones M, Huang WC, Knauss JS, Wang LP, Raghunath PN, Kozlowski C, Malkowicz SB, Tomaszewski JE. Impact of the 1998 World Health Organization/International Society of Urological Pathology classification system for urothelial neoplasms of the kidney. Mod Pathol. 2005 Jan;18:11–18. doi: 10.1038/modpathol.3800268. [DOI] [PubMed] [Google Scholar]
- 20.Theodoropoulos VE, Lazaris ACh, Sofras F, Gerzelis I, Tsoukala V, Ghikonti I, Manikas K, Kastriotis I. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004 Aug;46:200–208. doi: 10.1016/j.eururo.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Shirotake S, Miyajima A, Kosaka T, Tanaka N, Maeda T, Kikuchi E, Oya M. Angiotensin II type 1 receptor expression and microvessel density in human bladder cancer. Urology. 2011 Apr;77:1009. doi: 10.1016/j.urology.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Slade N, Moll UM, Brdar B, Zorić A, Jelaković B. p53 mutations as fingerprints for aristolochic acid - an environmental carcinogen in endemic (Balkan) nephropathy. Mutat Res. 2009 Apr;663:1–6. doi: 10.1016/j.mrfmmm.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003 Jun;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 24.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 25.Rak J, Yu JL, Klement G, Kerbel RS. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J Investig Dermatol Symp Proc. 2000 Dec;5:24–33. doi: 10.1046/j.1087-0024.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 26.Shariat SF, Youssef RF, Gupta A, Chade DC, Karakiewicz PI, Isbarn H, Jeldres C, Sagalowsky AI, Ashfaq R, Lotan Y. Association of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J Urol. 2010 May;183:1744–1750. doi: 10.1016/j.juro.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Palit V, Phillips RM, Puri R, Shah T, Bibby MC. Expression of HIF-1alpha and Glut-1 in human bladder cancer. Oncol Rep. 2005 Oct;14:909–913. doi: 10.3892/or.14.4.909. [DOI] [PubMed] [Google Scholar]
- 28.Rahmani A, Alzohairy M, Khadri H, Mandal AK, Rizvi MA. Expressional evaluation of vascular endothelial growth factor (VEGF) protein in urinary bladder carcinoma patients exposed to cigarette smoke. Int J Clin Exp Pathol. 2012;5:195–202. [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert SP, Stainier DY. Molecular control of endothelial cell behavior during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011 Aug;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003 Jun;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001 Nov;2:667–673. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000 Sep;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 33.Birkhahn M, Mitra AP, Williams AJ, Lam G, Ye W, Datar RH, Balic M, Groshen S, Steven KE, Cote RJ. Predicting recurrence and progression of noninvasive papillary bladder cancer at initial presentation based on quantitative gene expression profiles. Eur Urol. 2010 Jan;57:12–20. doi: 10.1016/j.eururo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rademakers SE, Span PN, Kaanders JH, Sweep FC, van der Kogel AJ, Bussink J. Molecular aspects of tumour hypoxia. Mol Oncol. 2008 Jun;2:41–53. doi: 10.1016/j.molonc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivridis E, Giatromanolaki A, Gatter KC, Harris AL, Koukourakis MI. Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer. 2002 Sep;95:1055–1063. doi: 10.1002/cncr.10774. [DOI] [PubMed] [Google Scholar]
- 36.Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, Patsouris E, Sofras F. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005 Feb;95:425–431. doi: 10.1111/j.1464-410X.2005.05314.x. [DOI] [PubMed] [Google Scholar]
- 37.Mitra AP, Lin H, Datar RH, Cote RJ. Molecular biology of bladder cancer: prognostic and clinical implications. Clin Genitourin Cancer. 2006 Jun;5:67–77. doi: 10.3816/CGC.2006.n.020. [DOI] [PubMed] [Google Scholar]
- 38.Kadhim HS, Abdulamir AS, Hafidh RR, Abubaker F, Abbas KA. Investigations in the molecular events of transitional cell carcinoma of the bladder. Am J Biochem Biotechnol. 2008;4:408–415. [Google Scholar]
- 39.Deniz H, Karakök M, Yagci F, Güldür ME. Evaluation of relationship between HIF-1α immunoreactivity and stage, grade, angiogenic profile and proliferative index in bladder urothelial carcinomas. Int Urol Nephrol. 2010 Mar;42:103–107. doi: 10.1007/s11255-009-9590-5. [DOI] [PubMed] [Google Scholar]