Abstract
Ovarian endometriosis has been associated with increased risk for ovarian clear cell carcinoma (CCC). Atypical endometriosis shares common molecular alterations with CCC and therefore, has been proposed as a precursor lesion of CCC, although it is unclear if benign endometriosis is pre-neoplastic. In this study, we examined some molecular alterations in ovarian benign endometriosis, atypical endometriosis, and CCC in comparison to papillary serous carcinoma (PSC). These included BAF250a (encoded by ARID1A), a recently identified major tumor suppressor in ovarian CCC, as well as hepatocyte nuclear factor (HNF)-1b, estrogen receptor (ER), progesterone receptor (PR), and P53. We confirmed that CCC but not PSC had loss of BAF250a expression, HNF-1b up-regulation, loss of ER expression and P53 expression. We further showed that both atypical endometriosis and adjacent CCC had loss of BAF250a expression (38.5% vs. 57.7%), HNF-1b up-regulation (53.8% vs. 92.3%), and loss of ER (84.6% vs. 92.3%) and PR (76.9% vs. 84.6%) expression. Importantly, about 20% of benign ovarian endometriosis had loss of BAF250a expression, 33% with HNF-1b up-regulation, 23% loss of ER expression and 50% loss of PR expression, respectively. The concurrent rate of loss of BAF250a expression, HNF-1b up-regulation, and loss of ER expression was not observed in any benign endometriosis, and was increased to 23.1% in atypical endometriosis, and was further increased to 42.3% in CCC. Therefore, the molecular alterations accumulate in a stepwise manner along the transformation process from benign endometriosis through atypical endometriosis to CCC. These data suggest that a portion of benign ovarian endometriosis has already undergone genetic alterations that lead to aberrant protein expression, possibly conferring a higher risk for malignant transformation.
Keywords: ARID1A/BAF250a, endometriosis, clear cell carcinoma, papillary serous carcinoma, hepatocyte nuclear factor-1b, atypical endometriosis, ovarian carcinoma
Introduction
Endometriosis is an estrogen-dependent, chronic inflammatory gynecological disorder affecting 5-15% of women of reproductive age [1,2]. It is classically defined as the presence of endometrial glands and stroma outside the uterine cavity and musculature. Although endometriosis is considered a benign condition, it shares some common features with malignant cells: uncontrolled growth, local invasion, and distant metastasis [3]. The suspected transformation of endometriosis into ovarian cancer was first reported in 1925 [4]. In recent years, data from large cohort and case-control studies demonstrate that women with ovarian endometriosis have an increased risk (2-13-fold) of ovarian clear cell carcinoma (CCC) [5-9]. Similarly, roughly 20-70% of patients with CCC that underwent surgery have simultaneous endometriosis, compared to 3-7% with papillary serous carcinoma (PSC) [10-12]. It is therefore conceivable that endometriosis might be a precursor lesion of CCC.
However, the frequency of malignant transformation of endometriosis has been estimated as low as 0.7-1.6% over an average of 8 years [5], suggesting that only a tiny proportion of endometriosis has the potential to progress into carcinoma. This has led investigators to seek morphological features and molecular/genetic markers that can identify endometriosis that might undergo malignant transformation. Atypical endometriosis has thus come to be considered the earliest stage of malignant transformation in ovarian endometriosis [13-15].
Atypical endometriosis, though controversial, as defined by the presence of either hyperplasia or cytological atypia, has been identified adjacent to concomitant CCC [13-15]. A direct continuous transition was noted from clearly benign endometriosis through atypical endometriosis to carcinoma [15]. Patients with previously biopsy-proven atypia within endometriosis developed CCC that arose in the same ovary a few years later [16], implicating a chronological association between these two conditions. Moreover, atypia is significantly more common in patients with CCC compared to patients with solitary endometriosis (61-100% versus 1-2%) [17]. At the molecular level, atypical endometriosis and CCC share common molecular/genetic alterations such as somatic PTEN mutations [18], PIK3CA mutations [19], hepatocyte nuclear factor (HNF)-1b up-regulation [20], loss of estrogen receptor (ER) and progesterone receptor (PR) [21], and rarely P53 mutations [22].
Although benign ovarian endometriosis shows no atypia morphologically, several studies have demonstrated molecular abnormalities in these benign-appearing lesions including monoclonality [23], loss of heterozygosity (LOH) [24,25], and PTEN mutations [18]. However, the results from these studies were somewhat inconsistent [26], perhaps in part due to the lack of specific molecular markers and the inability to clearly distinguish atypical endometriosis from benign ones. More recently, the loss of BAF250a expression caused by truncating mutations has been identified in both CCC and adjacent atypical endometriosis with high frequency, but rarely in PSC [27-29], suggesting that the loss of BAF250a expression is highly specific to endometriosis-associated ovarian cancer. Therefore, BAF250a might be a potentially useful marker to identify the initiation of malignant transformation of endometriosis. In this study, we examined whether benign endometriosis has already accumulated molecular alterations that are commonly observed in atypical endometriosis and CCC by analyzing the expression of BAF250a as well as the expression of other proteins such as HNF-1b, ER, PR, and P53.
Materials and methods
Sample collection
Hematoxylin- and eosin-stained sections retrieved from the files of the Department of Pathology, University Hospitals Case Medical Center, were reviewed. We selected 36 cases of solitary ovarian endometriosis, 26 of primary ovarian CCC, and 24 of primary ovarian PSC. Normal eutopic endometrium was chosen as a control. In ovarian endometriosis, patients with simultaneous ovarian cancer or any history of ovarian cancer were excluded. On the basis of the histopathological criteria described previously, of the 26 patients with CCC, 13 patients with synchronous endometriotic lesions were identified and all 13 patients had atypical endometriosis adjacent to the cancer [13]. All these patients had undergone surgical resection between 1995 and 2010. All specimens analyzed were formalin-fixed and paraffin-embedded tissue sections. Atypical endometriosis was diagnosed based on marked cytological atypia or hyperplastic changes of the epithelial cell component [13,19]. Institutional Review Board (IRB) of the University Hospitals Case Medical Center approved the research protocol.
Immunohistochemical (IHC) staining
Immunohistochemistry was performed by the diagnostic Immunohistochemist r y Laboratory of University Hospitals Case Medical Center. Briefly, unstained 4-μm sections were prepared from paraffin blocks and baked for 30 minutes at 60°C in a Boekel Lab oven. The slides were then processed using a Bond Automated Immunostainer (Leica)or a BenchMark XT (Ventana). The slides were deparaffinized, antigen retrieved, incubated in primary antibody and subsequently counterstained onboard the automated instruments (See Table 1).Histological images were obtained with the use of a ScanScope® XT digital scanning system (Aperio Technologies, Vista, CA, USA). For all the antibodies in this study, nuclear immunoreactivity was considered a positive expression.
Table 1.
Antibodies used in the study
| Antibody | Dilution | Source | Clone | Incubation | Instrumentation |
|---|---|---|---|---|---|
| p53+ | PDL* | Ventana;AZ | DO-7 | 24 min | BenchMark XT |
| PR+ | PDL* | Ventana;AZ | 1E2 | 20 min | BenchMark XT |
| ER+ | PDL* | Ventana;AZ | SP1 | 16 min | BenchMark XT |
| HNF-1b** | 1:100 | Santa Cruz Bio; CA | H-205 | 15 min | Leica Bond |
| BAF250a** | 1:100 | Sigma; MO | HPA005456 | 15 min | Leica Bond |
Denotes a pre-diluted antibody.
Antigen retrieval was performed with Bond Epitope Retrieval Solution 1 (Leica), a citrate buffer-based pH 6.0 solution for 20 minutes at 100 ° C.
Antigen retrieval was performed with Cell Conditioning 1 (Ventana), a tris-based buffer pH 8.3 solution for 30 minutes at 100°C.
Immunoreactivity was scored by two investigators independently based on the percentage and intensity of positive epithelial cells (percentage: 0: <1%, 1+: 1%-2-5%, 2+: 26-75%, 3+: 76%-100%; intensities: undetectable, weak, moderate and strong) [29,30]. Score 0 was considered negative. With respect to BAF250a expression outcome was considered to be the result of technical failure, when neither normal cells in the stroma nor tumor cells were immunoreactive. In addition, absence of immuno staining has previously been shown to correlate with ARID1A mutational status [27,28].
Statistical analysis
Comparison of the ARID1A/BAF250a, HNF-1b, ER, PR, and P53 expression was done by using the Fisher’s exact test (two-tailed).
Results
Expression of BAF250a, HNF-1b, ER, PR, and P53 in CCC and PSC
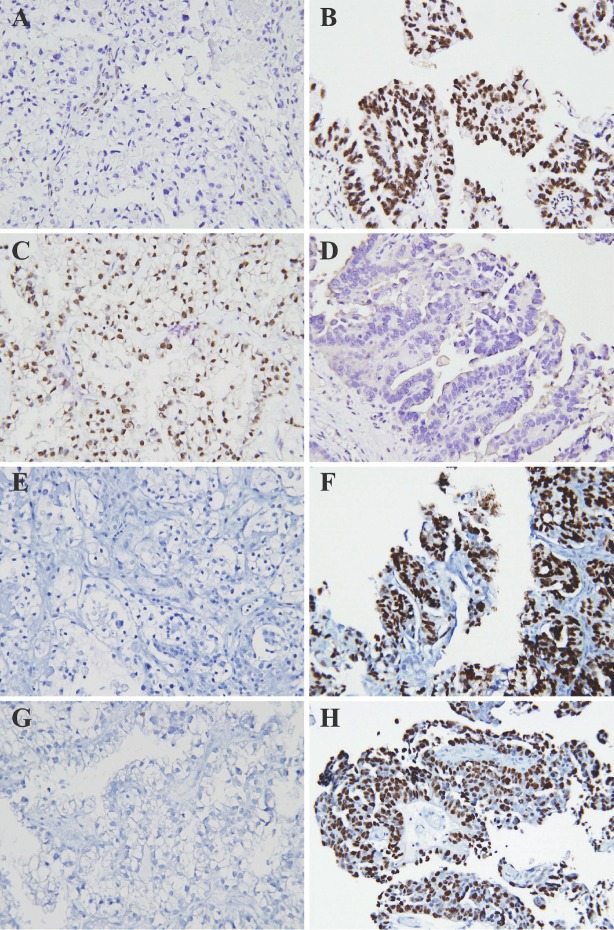
Of the 26 CCC, 15 (57.7%) had undetectable BAF250a undetectable by IHC (Table 2 and Figure 1). In contrast, none of the 24 PSC showed loss of BAF250a expression. The expression of HNF-1b was up-regulated in CCC, but not in PSC, while the expression of ER was detected in only 7.7 % (2/26) of CCCs and 91.8 % (22/24) of PSCs, respectively. P53 overexpression, a surrogate marker for P53 mutation, was found in 62.5 % (15/24) of PSCs, and only in 7.7 % ( 2/26) of CCCs. PR expression was not significantly different between CCCs and PSCs. These results demonstrate that BAF250a in combination with HNF-1b, ER and P53 can readily distinguish CCC from PSC in morphologically challenging cases.
Table 2.
Immunoprofiles of ovarian clear cell carcinoma (CCC) versus papillary serous carcinoma (PSC)
| BAF250a %(N) | HNF-1b %(N) | ER %(N) | P53 %(N) | PR %(N) | |
|---|---|---|---|---|---|
| CCC (N=26) | 42.3% (11/26) | 92.3% (24/26) | 7.7% (2/26) | 7.7% (2/26) | 15.4% (4/26) |
| PSC N=24) | 100% (24/24)* | 4.2% (1/24)* | 91.8% (22/24)* | 62.5% (15/24)* | 16.7% (4/24) |
p<0.001 by Fisher’s exact test
Figure 1.
Immunoprofiles of BAF250a (A,B), HNF-1b (C,D), ER (E,F), and P53 (G,H) in CCC and PSC by IHC. CCC showed undetectable expression of BAF250a(A), ER (E), and P53 (G), and expression of HNF-1b (C). In contrast, PSC showed expression of BAF250a (B), ER (F), and P53 (H), and undetectable expression of HNF-1b (D). Positive staining of all these markers is intranuclear. The expression of PR is not shown.
Expression of BAF250a, HNF-1b, ER and PR in ovarian endometriosis
We also identified atypical endometriosis concomitant with CCC in 13 cases (13/26, 50%). None of the PSC had endometriosis (0/24). Of the 13 cases with atypical endometriosis, 5 showed loss of BAF250a expression (5/13, 38.5%, p=0.6 compared to 57.7% in CCC) that was observed in the concomitant CCC as well. In addition, atypical endometriosis had up-regulation of HNF-1b, and loss of ER and PR expression; these observations are not significantly different from those for CCC (Table 3 and Figure 2). Interestingly, of the 2 CCCs with P53 over-expression, 1 had P53 over-expression in the concomitant atypical endometriosis (data not shown). In summary, atypical endometriosis had a similar immunostaining profile to its nearby CCC, supporting the notion that atypical endometriosis is probably a precursor lesion of CCC [3].
Table 3.
Immunoprofiles of ovarian endometriosis
| BAF250a %(N) | HNF-1b %(N) | ER %(N) | PR %(N) | |
|---|---|---|---|---|
| Normal endometrium (N=5) | 100% (5/5) | 0 (0/5) | 100% (5/5) | 100% (5/5) |
| Benign endometriosis (N=36) | 80.6% (29/36) | 33.3% (12/36) | 77.8% (28/36) | 50% (18/36) |
| Atypical endometriosis (N=13) | 61.5% (8/13) | 53.8% (7/13) | 15.4%* (2/13) | 23.1% (3/13) |
p<0.01 vs. benign endometriosis (Fisher’s exact test).
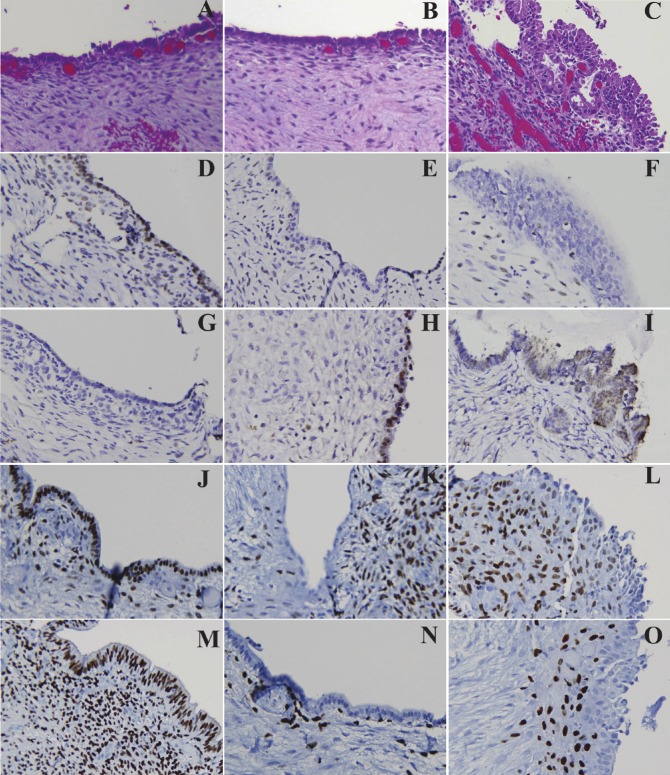
Figure 2.
Comparison of Immunoprofiles of BAF250a, HNF-1b, ER, and PR in benign and atypical endometriosis by IHC. Panels A-C, show the H&E sections of normal endometrium (A), benign endometriosis (B) and atypical endometriosis (C); panels D-F, expression patterns of BAF250a; panels G-I, HNF1-b; panels J-L, ER; panels M-O, PR. Normal endometrium (A, D ,G, J, M) showed expression of BAF250a (D), ER (J), and PR (M), and undetectable expression of HNF-1b (G). A small portion of benign ovarian benign endometriosis (B, E, H, K, N) showed undetectable expression of BAF250a (E), ER (K), and PR (N), and expression of HNF-1b (H), similar to atypical endometriosis (C, F, I, L, O) and CCC (see Figure 1).
To avoid possible confounding factors, we selected 36 benign solitary endometriosis involving the ovary, and none of these showed morphological atypia histologically. The low frequency of atypia in solitary ovarian endometriosis is consistent with published data [17]. Even though no morphologic atypia was present, some of the endometriosis already showed loss of BAF250a expression (7/36, 19.4%). The expression pattern of other proteins, such as up-regulation of HNF-1b, and loss of expression of ER and PR, was also similar to that of atypical endometriosis and CCC, albeit to a lesser degree. Therefore, a small portion of typical ovarian endometriosis harbors the molecular genetic alterations that are commonly present in atypical endometriosis and CCC before morphological atypia is identifiable.
Co-expression patterns of biomarkers in ovarian endometriosis and CCC
We then compared the co-expression pattern of these biomarkers in ovarian endometriosis and CCC (Table 4). Of benign endometriosis, 3 were BAF250a negative/HNF-1b positive, and 3 were BAF250a negative/ER positive, suggesting that loss of BAF250a, functioning as an early transformation event, could occur independently of or together with HNF-1b up-regulation and loss of ER. On the other hand, the HNF-1b up-regulation and the loss of ER didn’t occur in benign endometriosis. Nevertheless, the combined altered expression pattern of BAF250a negative/HNF-1b positive/ER negative has been detected in 23.1% of atypical endometriosis and 42.3% of CCC, respectively, but not in benign endometriosis. The findings support the notion that atypical endometriosis should be categorized as a separate entity, which harbors a genetic alteration similar to that of CCC. This also indicates that in atypical endometriosis and CCC, the loss of BAF250a expression is most often accompanied by HNF-1b up-regulation and loss of ER expression.
Table 4.
Co-expression patterns of biomarkers in ovarian endometriosis and CCC
| BAF250a-/HNF-1b+ %(N) | BAF250a-/ER- %(N) | HNF-1b+/ER- %(N) | BAF250a-/HNF-1b+/ER- %(N) | |
|---|---|---|---|---|
| Benign endometriosis (N=36) | 8.3% (3/36) | 8.3% (3/36) | 0 (0/36) | 0 (0/36) |
| Atypical endometriosis (N=13) | 23.1% (3/13) | 23.1% (3/13) | 46.2% (6/13) | 23.1% (3/13) |
| CCC (N=26) | 53.8%* (14/26) | 46.2%* (12/26) | 76.9%* (20/26) | 42.3%* (11/26) |
p<0.01 vs. benign endometriosis (Fisher’s exact test).
Discussion
Mutations of ARID1A are a frequent event in CCC, with 46%-57% showing mutations in the ARID1A gene [27,28]. ARID1A encodes BAF250a protein, a key component of the multi-protein SWI/SNF chromatin-remodeling complex [31]. Mutations of ARID1A identified in CCC correlate with the loss of BAF250a expression [27,28], suggesting that ARID1A functions as a major tumor suppressor gene in CCC. Interestingly, based on their distinctive clinicopathologic and molecular features, CCC has been classified as type 1 tumors as opposed to type 2 tumors such as papillary serous carcinoma [32]. It has, therefore, been proposed that ARID1A mutation plays an important role in the development of type 1 tumors. In line with this, loss of BAF250a expression has also been noted in other type 1 tumors such as ovarian endometrioid carcinoma [27,28], uterine endometrioid carcinoma (about 30%) and uterine CCC (about 30%) [33,34]. Since loss of BAF250a expression is specific to CCC compared to PSC, it can be potentially useful as a biomarker to differentiate them, as shown in current and previous studies. In addition to BAF250a, the expression pattern of HNF-1b, ER and P53 is also helpful to differentiate CCC from PSC.
In the above study, nearly all ARID1A mutations are truncation mutations, which result in the loss of BAF250a protein expression, and the study also showed a strong relationship of ARID1A truncation mutation and loss of BAF250a protein expression by immunohistochemisty. However, if there is other non-truncated mutation present in ARID1A, the immunohistochemical staining of BAF250a would not be able to differentiate the mutated protein from the normal protein. Fortunately, based on the current available study, that possibility is minimum [27].
Loss of BAF250a expression has also been noted in atypical endometriosis adjacent to CCC [27,29]. Wiegnand et al. also reported that loss of BAF250a expression in atypical endometriosis in the cul-de-sac area and development of a frank endometrioid carcinoma at this site two years later [33]. A recent study from Yamamoto et al further showed loss of BAF250a expression concomitant with PIK3CA mutations in atypical endometriosis adjacent to CCC, but not in endometriosis distant from CCC or any solitary endometriosis [29]. These studies collectively suggest that loss of BAF250a expression, similar to PIK3CA mutations, occurs in atypical endometriosis before the development of carcinoma and is an early event during malignant transformation of ovarian endometriosis.
However, in our study we found that the loss of BAF250a expression was also observed in about 20% of benign ovarian endometriosis. Similarly, Sato et al. previously reported PTEN mutation in 15% of benign endometriosis [18]. A later study from Samartzis et al showed that loss of BAF250a expression in 15% of ovarian endometriosis [30], although in this study, loss of BAF250a expression was observed in both the epithelium and stromal cells, raising concern about the staining quality. Here we showed normal BAF250a expression in stromal cells (Figure 2), which serves as an internal positive control. Regardless, both studies suggest that loss of BAF250a expression already occurs in benign ovarian endometriosis before the development of atypia, indicating that a small portion of benign endometriosis has undergone the transformation process. This notion is further strengthened by HNF-1b up-regulation and the loss of ER and PR expression in benign endometriosis as shown in this study, all of which are commonly seen in atypical endometriosis and CCC. Interestingly, concurrence of these molecular alterations (i.e., loss of BAF-250a expression, HNF-1b up-regulation, and loss of ER expression) is extremely rare in benign endometriosis, increases to around 23.1% in atypical endometriosis, and peaks at 42.3% in CCC in our study, clearly demonstrating that the accumulation of these molecular alterations escalates along with the process of malignant transformation in a stepwise manner.
That atypical endometriosis has similar genetic alterations to CCC, suggests that the former is an early malignant lesion, and should be documented. More importantly, a small percentage of benign ovarian endometriosis has already undergone genetic alterations that lead to aberrant protein expression, including loss of BAF250a expression, HNF-1b up-regulation, and loss of ER and PR expression, before showing morphological atypia. These molecules might be potentially useful in screening high-risk endometriosis. Our findings provide evidence that aberrant expression of these biomarkers can be viewed as indication of high risk for malignant transformation and suggest that routine screening for expression of these biomarkers after resection might be helpful in identifying high-risk patients who may develop cancer. The development of this paradigm to screen high-risk patients with endometriosis and to intervene appropriately should be further studied.
Acknowledgments
We would like to thank Dr. Dave Carrino for editorial help.
Conflict of interest statement
The authors have no relevant financial interest in the products or companies described in this article.
Financial support
This work was supported in part by a small research grant from the Department of Pathology, University Hospital Case Medical Center, Case Western Reserve University (to W. Xiao and W. Xin) and startup funds from Department of Pathology of Case Western Reserve University (to W. Xin).
References
- 1.Bulun SE. Endometriosis. The New England journal of medicine. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecologic oncology. 2012;124:164–169. doi: 10.1016/j.ygyno.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 5.Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, Morioka E, Arisawa K, Terao T. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2007;17:37–43. doi: 10.1111/j.1525-1438.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 6.Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. American journal of epidemiology. 2002;155:217–224. doi: 10.1093/aje/155.3.217. [DOI] [PubMed] [Google Scholar]
- 7.Ness RB, Grisso JA, Klapper J, Schlesselman JJ, Silberzweig S, Vergona R, Morgan M, Wheeler JE. Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. SHARE Study Group. Steroid Hormones and Reproductions. American journal of epidemiology. 2000;152:233–241. doi: 10.1093/aje/152.3.233. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. American journal of obstetrics and gynecology. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 9.Melin A, Sparen P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Human reproduction. 2006;21:1237–1242. doi: 10.1093/humrep/dei462. [DOI] [PubMed] [Google Scholar]
- 10.Vercellini P, Parazzini F, Bolis G, Carinelli S, Dindelli M, Vendola N, Luchini L, Crosignani PG. Endometriosis and ovarian cancer. American journal of obstetrics and gynecology. 1993;169:181–182. doi: 10.1016/0002-9378(93)90159-g. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Kaku T, Amada S, Kobayashi H, Hirakawa T, Ariyoshi K, Kamura T, Nakano H. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecologic oncology. 2000;77:298–304. doi: 10.1006/gyno.2000.5765. [DOI] [PubMed] [Google Scholar]
- 12.Lim MC, Chun KC, Shin SJ, Lee IH, Lim KT, Cho CH, Park SY, Nam JH. Clinical presentation of endometrioid epithelial ovarian cancer with concurrent endometriosis: a multicenter retrospective study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:398–404. doi: 10.1158/1055-9965.EPI-09-0750. [DOI] [PubMed] [Google Scholar]
- 13.Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Advances in anatomic pathology. 2007;14:241–260. doi: 10.1097/PAP.0b013e3180ca7d7b. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 15.LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Human pathology. 1988;19:1080–1084. doi: 10.1016/s0046-8177(88)80090-x. [DOI] [PubMed] [Google Scholar]
- 16.Moll UM, Chumas JC, Chalas E, Mann WJ. Ovarian carcinoma arising in atypical endometriosis. Obstetrics and gynecology. 1990;75:537–539. [PubMed] [Google Scholar]
- 17.Prefumo F, Todeschini F, Fulcheri E, Venturini PL. Epithelial abnormalities in cystic ovarian endometriosis. Gynecologic oncology. 2002;84:280–284. doi: 10.1006/gyno.2001.6529. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer research. 2000;60:7052–7056. [PubMed] [Google Scholar]
- 19.Yamamoto S, Tsuda H, Takano M, Iwaya K, Tamai S, Matsubara O. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. The Journal of pathology. 2011;225:189–194. doi: 10.1002/path.2940. [DOI] [PubMed] [Google Scholar]
- 20.Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19:83–89. doi: 10.1038/modpathol.3800492. [DOI] [PubMed] [Google Scholar]
- 21.Fujimura M, Hidaka T, Kataoka K, Yamakawa Y, Akada S, Teranishi A, Saito S. Absence of estrogen receptor-alpha expression in human ovarian clear cell adenocarcinoma compared with ovarian serous, endometrioid, and mucinous adenocarcinoma. The American journal of surgical pathology. 2001;25:667–672. doi: 10.1097/00000478-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Akahane T, Sekizawa A, Purwosunu Y, Nagatsuka M, Okai T. The role of p53 mutation in the carcinomas arising from endometriosis. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2007;26:345–351. doi: 10.1097/pgp.0b013e31802b41a8. [DOI] [PubMed] [Google Scholar]
- 23.Nilbert M, Pejovic T, Mandahl N, Iosif S, Willen H, Mitelman F. Monoclonal origin of endometriotic cysts. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 1995;5:61–63. doi: 10.1046/j.1525-1438.1995.05010061.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X, Hitchcock A, Bryan EJ, Watson RH, Englefield P, Thomas EJ, Campbell IG. Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumor suppressor gene loci. Cancer research. 1996;56:3534–3539. [PubMed] [Google Scholar]
- 25.Prowse AH, Manek S, Varma R, Liu J, Godwin AK, Maher ER, Tomlinson IP, Kennedy SH. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. International journal of cancer. Journal international du cancer. 2006;119:556–562. doi: 10.1002/ijc.21845. [DOI] [PubMed] [Google Scholar]
- 26.Mayr D, Amann G, Siefert C, Diebold J, Anderegg B. Does endometriosis really have premalignant potential? A clonal analysis of laser-microdissected tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:693–695. doi: 10.1096/fj.02-0562fje. [DOI] [PubMed] [Google Scholar]
- 27.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih Ie M, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. The New England journal of medicine. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:615–24. doi: 10.1038/modpathol.2011.189. [DOI] [PubMed] [Google Scholar]
- 30.Samartzis EP, Samartzis N, Noske A, Fedier A, Caduff R, Dedes KJ, Fink D, Imesch P. Loss of ARID1A/BAF250a-expression in endometriosis: a biomarker for risk of carcinogenic transformation? Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:885–92. doi: 10.1038/modpathol.2011.217. [DOI] [PubMed] [Google Scholar]
- 31.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nature reviews Cancer. 2011;11:481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 32.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Human pathology. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, Steidl C, Wiseman SM, Gascoyne RD, Gilks B, Huntsman DG. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. The Journal of pathology. 2011;224:328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 34.Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL, Shih Ie M. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. The American journal of surgical pathology. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]