Abstract
Hepatic fibrosis constitutes a serious insult to the liver, with a substantial negative impact on the quality of life of such patients worldwide. It is a consequence of severe liver damage and occurs as the result of several factors. Chronic alcoholism is the most common cause. Fibrosis also results from chronic viral hepatitis and autoimmune hepatitis. Prolonged exposure to environmental toxins such as carbon tetrachloride (CCl4) can also lead to fibrosis. In the present study, the hepato-protective effects of green tea extract (GTE) on hepatic fibrosis in a rat liver CCl4-induced fibrosis model were examined histologically, 3-dimensionally and biochemically. GTE was prepared from dried green tea leaves and lyophilized. Male albino rats (n=20) weighing 200–250 g were divided into four groups: GI, control; GII, administered 50 mg/kg GTE dissolved in physiological saline daily for four weeks; GIII, administered 40% CCl4 (1 ml/kg body weight) by subcutaneous injection daily for four weeks; and GIV, treated as GIII, followed by 50 mg/kg GTE dissolved in physiological saline daily for 4 weeks. Histology and 3-dimensional scanning electron microscopy showed hepatic fibrosis with intermingled fibers located between cells in the liver tissues of the CCl4-treated rats. Fibrotic lesions virtually disappeared after four weeks of treatment with GTE, returning the architecture of liver tissue back to its normal state. Also, the levels of the hepatic enzymes alanine aminotranferase and aspartate aminotransferase returned to their normal levels after treatment with GTE. The rats were found to regain their normal body weight and their fur color, which had faded due to weight loss. The autopsy results showed the animal liver returning to normal shape and color. Thus, green tea extract is a potent treatment for hepatic fibrosis caused by CCl4 in this animal model.
Keywords: green tea extract, alanine aminotranferase, aspartate aminotransferase, collagen fibers, extracellular matrix, carbon tetrachloride, scanning electron microscopy
Introduction
Fibrosis is the process of forming fibrous tissue, usually by degeneration. The process occurs normally in the formation of scar tissue to replace normal tissue lost through injury, infection or chronic liver insults (1–5). The cells responsible for extracellular matrix (ECM) fiber formation are hepatic stellate cells (HSCs), which enhance cell proliferation at the onset of liver injury (6). During our previous studies of the effect of green tea extract (GTE) on the liver, kidney, and stomach, we presented various observations on the role of GTE in altering the deleterious effects of drugs such as reserpine within 30 days of administration (7,8). This encouraged us to study the effect of GTE on the amelioration of hepatic fibrosis caused by carbon tetrachloride (CCl4) (9, Safer et al, Third Kuwait International Pharmacy Conference, Kuwait, 2011), which induces hepatic fibrosis through oxidative stress. This causes HSCs to become over-active (6) and trigger an increase in ECM synthesis; collagen fibers are then deposited in the extracellular spaces of the liver cells, causing them to lose their capacity for blood infusion and harden, leading to liver fibrosis (4,6).
The present study aimed to highlight the curative propensity of GTE towards hepatic fibrosis in rat liver through CCl4 administration.
Materials and methods
Preparation of GTE
Dried Japanese tea leaves (100 g) were powdered in a Waring blender and extracted with double-distilled water (1 liter), at 80°C for one hour. The extract was filtered through a nylon filter, and the filtrate was centrifuged at 3000 × g for 15 min. The clear supernatant was removed and the residual pellet was shaken with distilled water, warmed at 35°C, and centrifuged again. The supernatants were pooled and lyophilized, and the resulting material was stored at −20°C in a screw-capped bottle.
Animals
Male albino rats (n=20) weighing 200–250 g were used in this study. They were divided into four groups: GI, control; GII, administered 50 mg/kg GTE dissolved in pysiological saline daily for four weeks; GIII, administered subcutaneous injection of 40% CCl4 (1 ml/kg body weight) thrice weekly for four weeks; GIV, treated as GIII, followed by 50 mg/kg GTE dissolved in physiological saline daily for four weeks.
Histology
Liver tissues were fixed by immersion in 10% buffered neutral formalin for 18 h, then processed and stained with hematoxylin and eosin.
Semi-thin sections
Semi-thin sections (1-μm) were cut and stained with toluidine blue for light microscopic survey and photography.
Masson’s trichrome stain
Liver sections immersed in 10% buffered neutral formalin were processed for collagen fiber staining using Masson’s trichrome stain.
Three-dimensional architecture
Sample blocks from all groups were prepared and processed for scanning electron microscopy for 3-dimensional architectural observation. Blocks were fixed in 3% glutaraldehyde/cacodylate buffer, pH 7.2, using a tissue processor, dehydrated in ethanol, and solid-dried in CO2 and fractured inside-out of each block. Each half of the block was mounted on a stub with the newly exposed surface face-up. The latter was coated with platinum/gold using a sputter coater. A scanning electron microscope (Carryscope JCM 5700; JEOL, Japan) with a resolution of 5.0 nm was used for capturing the scanning electron microscopy (SEM) images.
Biochemical analysis
Alanine aminotranferase (ALT) and aspartate aminotransferase (AST) levels in rats treated with CCl4 and GTE were measured in serum samples using Randox kits. Sample results were expressed as U/I.
Results
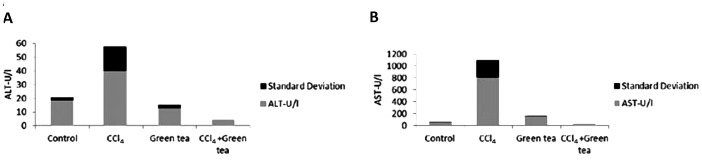
Rats treated with GTE only showed results similar to those in the control groups with normal AST and ALT levels (Fig. 1A and B). Serum AST and ALT levels were significantly elevated in rats treated with CCl4 only, indicating severe hepatic damage. The CCl4/GTE group showed a significant decrease in these enzyme levels.
Figure 1.
(A) Alanine aminotranferase (ALT) and (B) aspartate aminotransferase (AST) values in the rats treated with carbon tetrachloride (CCl4), green tea extract (GTE) or CCL4 and GTE.
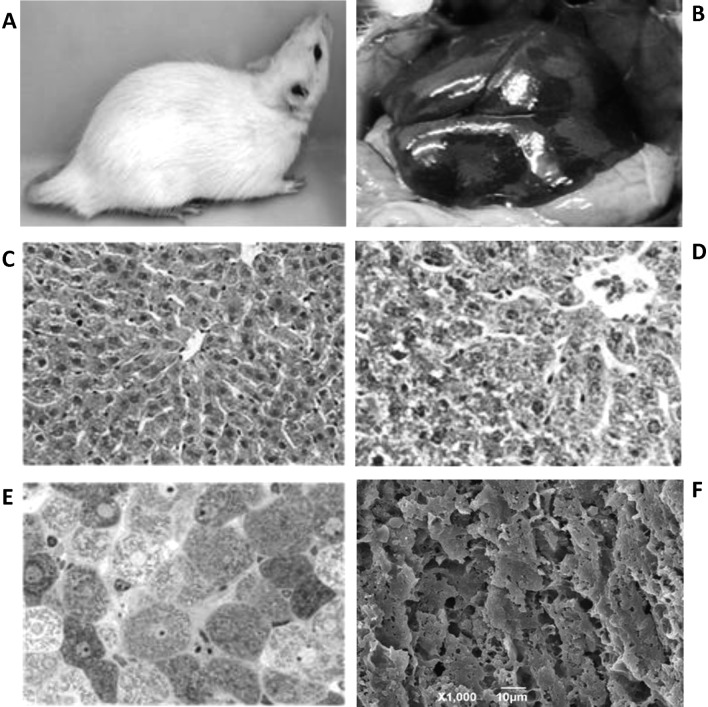
Hepatic fibrosis was evaluated by several criteria; namely, external features of the rats. In the control rats the fur color was bright white with a healthy-looking tail (Fig. 2A), and the gross anatomy at the onset of postmortem and prior to organ excision showed the liver with its normal brownish-red color with minimal loci of fat (Fig. 2B). In H&E-stained paraffin and toluidine blue-stained epon sections, the control group showed normal tissue and cell architecture (Fig. 2C and D). This was observed by 3-dimensional architecture at SEM levels (Fig. 2E).
Figure 2.
Images of a control rat. (A) External features of a rat showing normal bright colored fur and healthy-appearing tail. (B) Gross morphology of healthy liver with normal reddish-brown color. (C) H&E section of liver tissue as in panel B; magnification, ×240. (D) Toluidine blue section of liver tissue as in panel B; magnification, ×300. (E) Masson’s trichrome staining of liver tissue; magnification, ×400. (F) SEM image showing fractured surface taken from within the liver parenchyma; magnification, ×1,000.
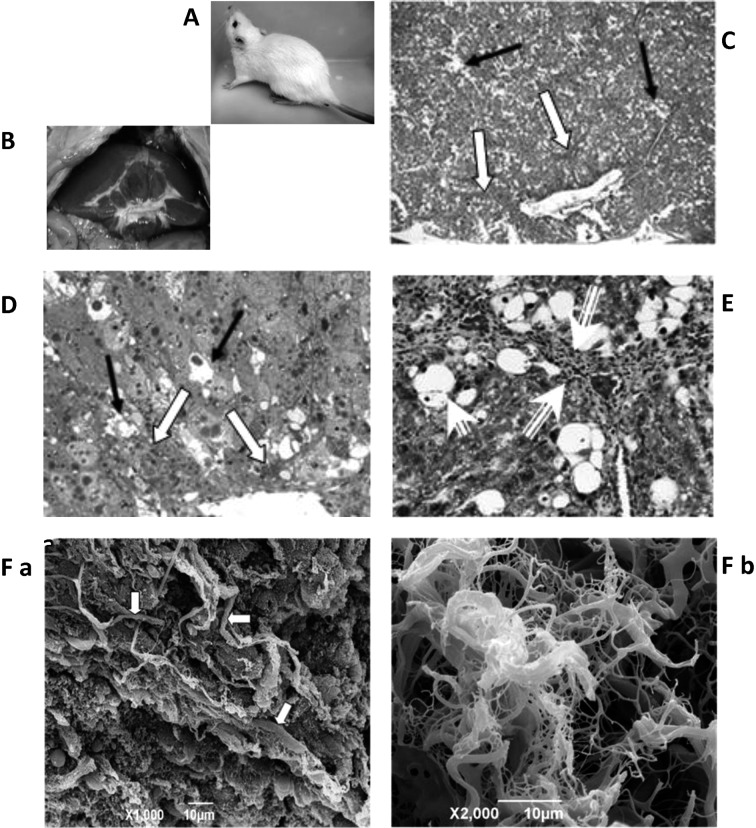
In the CCl4-treated group, the external features of the rat showed fur with off-white to brownish coloration with an abnormally dark-colored tail (Fig. 3A). At the onset of postmortem and prior to organ excision, the liver was fibrous-orange and topped with thick fat (Fig. 3B). H&E paraffin and toluidine blue epon sections exhibited pathological features, most notably the formation of an extensive amount of extracellular fibrous materials in the parenchyma of the liver (Fig. 3C and D). The fibrous materials (collagen fibers) were clearly noted in Masson’s trichrome-stained sections as shades of blue-green stained structures (Fig. 3E). Profuse collagen fiber deposits were found to fill numerous areas in the extracellular spaces of the liver parenchyma of CCl4-treated rats. The fibers varied in thickness from 250 to 1000 nm (Fig. 3F). Other pathological features observed were destruction of lobular architecture, inflammation, foamy vacuolated cytoplasm, necrosis, fatty cells, steatosis, nuclear shrinkage, abnormal tri-polar and tetra-polar divisions, nuclear karyorrhesis, nuclear karyolysis, nuclear hyperchromatism, dead cells, thickening of portal vein and portal triad, hypertension of arterioles, nuclear hyperchromatism, nuclear fragmentation, condensed eosinophilic protein, hyperactive Kuppfer cells and proliferation of HSCs.
Figure 3.
Images from a CCl4-treated rat. (A) External features of rat showing brownish fur and dark-colored tail. (B) Abnormal fatty liver with orange patches. (C) H&E section of liver tissue. Note the intermingled fibrous materials among the hepatocytes (white arrows). Also note vacuolations and lipid droplets (black arrows); magnification, ×50. (D) Toluidine blue section of liver tissue. Note the intermingled fibrous materials among the hepatocytes (white arrows). Also note vacuolations and lipid droplets (black arrows); magnification, ×400. (E) Masson’s trichrome-stained rat liver showing accumulation of collagen fibers in the ECM (long arrows). Many areas of lipid droplets and inflammation (short arrows) are noted; magnification, ×400. (F) a: SEM image of rat liver. Note thick collagen fibers formation in the ECM (arrows); magnification, ×1,000. b: Enlarged SEM image of rat liver ECM. Note condensed collagen fibers formation; magnification, ×2,000.
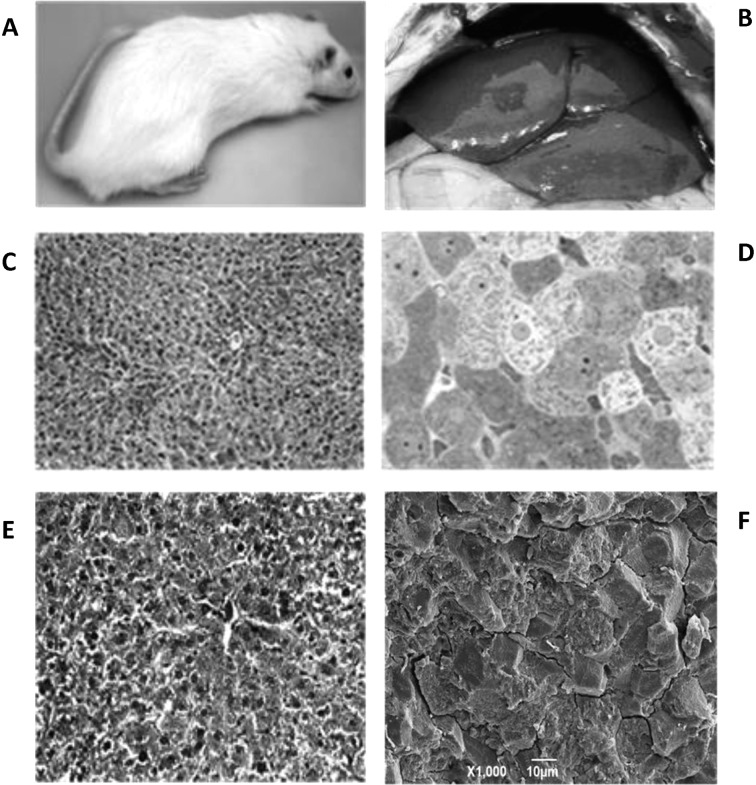
Rats treated with GTE after a month of CCl4 treatment exhibited prominent restoration of liver function. Externally the animals’ healthy, bright color, including the appearance of the tail, was restored (Fig. 4A). In gross morphology, the liver looked fairly normal: bright red color, and no fat present (Fig. 4B). The fibrous materials and lipid droplets were not present whether observed by H&E paraffin or toludine blue epon sections (Fig. 4C and D). Masson’s trichrome-stained liver tissue sections exhibited prominent restoration of liver function. The fibrous materials were entirely absent from the ECM and there were no signs of lipid droplets (Fig. 4E). This feature was also evident in SEM images (Fig. 4F).
Figure 4.
Images of a rat treated with CCl4 (4 weeks) followed by GTE treatment (4 weeks). (A) External features of the rat returned to normal with bright fur and healthy-looking tail. (B) Gross morphology of a healthy liver with normal reddish-brown color. (C) H&E section of liver tissue from panel B. Note that the image is devoid of fibers and lipid droplets; magnification, ×200. (D) Toluidine blue section of liver tissue from panel B. Note that the image is devoid of fibers and lipid droplets; magnification, ×900. (E) Masson’s trichrome staining in rat liver with no collagen fibers in the ECM. This image also lacks lipid droplets; magnification, ×400. (F) SEM image from the same group as in panel B, showing liver ECM devoid of extracellular fiber; magnification, ×1,000.
Discussion
Studies involving experimental animals and humans have suggested that green tea and GTE may be beneficial in the treatment of numerous health conditions including atherosclerosis, high cholesterol and cancer of the bladder, breast, ovary, colorectum, esophagus, lung, pancreas, prostate, skin and stomach. Consumption of GTE has been reported to have a therapeutic effect on inflammatory bowel disease, diabetes, weight loss, dental caries, arthritis, cartilage breakdown and genital warts, and a preventative effect on symptoms of colds and influenza and liver disease (10–24; Safer et al, International Conference on Free Radicals in Biosystems, Kuwait, 2007).
Hepatic fibrosis is a serious insult to the liver due to accumulation of extracellular matrix (ECM) proteins, specifically several types of collagen fibers (2,25). The main source of hepatofibrosis occurs when hepatic stellate cells proliferate to formulate ECM (6). In the present study, hepatofibrosis was successfully induced by subcutaneous injection of 40% CCl4 for a period of 4 weeks. Histological observation of liver tissues in H&E and toluidine blue- and Masson’s trichrome-stained sections all coincide with the external state of the animals and the autopsy features as explained above. Histopathological changes, such as destruction of lobular architecture and extracellular fibrous materials scattered across the extracellular matrix of liver parenchyma, were clear in H&E and toluidine blue liver sections. Masson’s trichrome-stained liver tissues clearly revealed the intermingled fibrous materials in the liver of the GIII group (CCl4) as blue-green fibrous structures among the cells and around the blood vessels. Such fibers were not present in the GTE-treated groups (GIV), which resembled the control group (GI). A more detailed cellular pathology will be observed in our forthcoming publication. 3D-architecture and surface topography of the fractured surface of the liver blocks was observed under SEM. This technique showed that certain types of fibers of various thicknesses and directions intermingled in the liver parenchyma. A number of studies have stated that during hepatic fibrosis, collagen types I and III mainly proliferate (26). A recent western blot analysis revealed various fiber types present in the liver, particularly type I, III, V, and VI collagens. However, types I and III are the most profuse ECM components. When hepatic fibrosis occurs, expression of type I and III collagen was found to increase to account for 90–95% of total collagen, and the overall balance of collagen types was disrupted (9).
Administration of GTE either simultaneously or following CCl4 administration prevented or demolished hepatic fibrosis. The rats were found to regain their normal body weight and their fur color, which had earlier faded due to weight loss. The autopsy results also showed the animal liver returning to normal shape and color, and fibrotic lesions virtually disappeared following 4 weeks of treatment with GTE, thus returning the architecture of the liver tissue to its normal state. This indicates that GTE inhibits the proliferation of HSCs (4,27) as in the case of the CCl4/GTE-treated mice, or remained in a state of ‘stand-by’ for the potential toxin attack, as in the case of GTE treatment alone. Scanning electron microscopy techniques for capturing detailed 3D-architecture of the liver samples fractured-face inside-out was the optimal choice for observing the morphological features, distribution and depth of collagen fibers in hepatic fibrosis. The CCl4/GTE-treated group exhibited marked efficacy in removing almost all fibers observed in an area comparable to that of the CCl4 group. A group of rats treated with CCl4 and left untreated for a month (unpublished data) exhibited minimal effects; connective tissue fibers were still present. However, there was no marked difference in terms of fiber formation between CCl4-damaged liver and that of CCl4-treated rats who had subsequently been left untreated for a month. Although this is a preliminary study, we found GTE inhibited proliferation of HSCs (4) and several studies demonstrated a causal relationship between green tea and reversal of damage caused by oxidants, possibly through blocking the expression of TGF-B1 (27).
The hepatoprotective effects of green tea are mainly attributed to its antioxidant properties and the ability of its polyphenolic catechins to scavenge reactive oxygen species (28), which were generated in the present study by CCl4 (29). These properties are due to the presence of the phenolic hydroxy groups on the B-ring in ungalloylated catechins (epicatechin and epigallocatechin) and in the B- and D-rings of the galloylated catechins (epigallocatechin-3-gallate and epicatechin-3-gallate) (30). This possesses the ability to inhibit several growth factor signaling cascades, either by direct blockade of growth factor receptors or through downstream effects (31). In addition to its antioxidant effects, green tea exhibits effects on several cellular and molecular targets in signal transduction pathways associated with cell death and cell survival (32).
The low levels of ALT and AST in the GTE-treated group (GIV) beyond the control (GI) may indicate that the hepatic parenchyma was at its recovery stage from the trauma caused by CCl4 and the marked loss of hepatocytes and their organelles, as observed at the ultrastructural level (Safer et al, Third Kuwait International Pharmacy Conference, Kuwait, 2011).
This study provides a clear indication, for the first time, that Japanese GTE is a potent anti-fibrotic agent against hepatic damage caused by CCl4. With these histopathological studies, we hope to further advance and establish the impact of GTE in providing a protective shield against the deleterious effect of chemicals such as CCl4, and possible other reactive oxygen species (ROS), on rat liver cells. We anticipate that this will confirm GTE as a strong therapeutic candidate and preventive measure against hepatic fibrosis.
This study demonstrates that GTE has an anti-fibrotic effect in CCl4-induced fibrotic rats and may be used as a therapeutic and preventive measure against hepatic fibrosis. However, the anti-fibrotic mechanism of GTE requires further investigation.
Acknowledgments
Our thanks to Mr Mahmoud El-Sayed, the Nanoscopy Science Center Group, Department of Biological Sciences, Faculty of Science, Kuwait University, and The Pharmaceutical Research Institute at Albany College of Pharmacy and Health Sciences, Albany, NY, USA for all the efforts carried out to make this research possible.
References
- 1.Paz Z, Shoenfeld Y. Anti-fibrosis: to reverse the irreversible. Clin Rev Allergy Immunol. 2010;38:276–286. doi: 10.1007/s12016-009-8157-7. [DOI] [PubMed] [Google Scholar]
- 2.Bateller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissell DM. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998;33:295–302. doi: 10.1007/s005350050087. [DOI] [PubMed] [Google Scholar]
- 4.Kim HK, Yang TH, Cho HY. Antifibrotic effects of green tea on in vitro and in vivo models of liver fibrosis. World J Gastroenterol. 2009;15:5200–5205. doi: 10.3748/wjg.15.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CH, Piao DM, Xu WX, Yin ZR, Jin JS, Shen ZS. Morphological and serum hyaluronic acid, laminin and type IV collagen changes in dimethylnitrosamine-induced hepatic fibrosis of rats. World J Gastroenterol. 2005;28:7620–7624. doi: 10.3748/wjg.v11.i48.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 7.Al-Bloushi S, Safer AM, Afzal M, Shaker M. Green tea modulates reserpine toxicity in animal model. J Toxicol Sci. 2009;34:77–87. doi: 10.2131/jts.34.77. [DOI] [PubMed] [Google Scholar]
- 8.Safer AM, Afzal M, Al-Bloushi S, Rafique M, Mousa SA. Inhibition property of green tea extract in relation to reserpine-induced ribosomal strips of rough endoplasmic reticulum (rER) of the rat kidney proximal tubule cells. J Toxicol Sci. 2009;34:637–645. doi: 10.2131/jts.34.637. [DOI] [PubMed] [Google Scholar]
- 9.Wang XH, Zhao J, Zhang W, Zhang L, Ma R, Wang L, Zhang S, Tian L. Scanning electron microscopic observation: three-dimensional architecture of the collagen in hepatic fibrosis rats. Chin Med J. 2007;120:308–312. [PubMed] [Google Scholar]
- 10.Borrelli F, Capasso R, Russo A, Ernst E. Systematic review: green tea and gastrointestinal cancer risk. Aliment Pharmacol Ther. 2004;19:497–510. doi: 10.1111/j.1365-2036.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: part I. Review of non-cancer health benefits. J Altern Complement Med. 2005;11:521–528. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 12.Fujita H, Yamagami T. Anti-hypercholesterolemia effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia. Nutr Res. 2008;28:450–456. doi: 10.1016/j.nutres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Gross G, Meyer KG, Pres H, Thielert C, Tawfik H, Mescheder A. A randomized, double-blind, four-arm parallel-group, placebo-controlled Phase II/III study to investigate the clinical efficacy of two galenic formulations of Polyphenon E in the treatment of external genital warts. J Eur Acad Dermatol Venereol. 2007;2:1404–1412. doi: 10.1111/j.1468-3083.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Tajima K, Mizutani M. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167:175–182. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- 15.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Zheng RH, Li YM. Green tea consumption and liver disease: a systematic review. Liver Int. 2008;28:990–996. doi: 10.1111/j.1478-3231.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 17.Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem. 2008;56:8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs EM, Lejeune MP, Nijs I, Westerterp-Plantenga MS. Effects of green tea on weight maintenance after body-weight loss. Br J Nutr. 2004;91:431–437. doi: 10.1079/BJN20041061. [DOI] [PubMed] [Google Scholar]
- 19.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 20.Low Dog T, Riley D, Carter T. Traditional and alternative therapies for breast cancer. Altern Ther Health Med. 2001;7:36–47. [PubMed] [Google Scholar]
- 21.Lyn-Cook BD, Rogers T, Yan Y, Blann EB, Kadlubar FF, Hammons GJ. Chemopreventive effects of tea extracts and various components on human pancreatic and prostate tumor cells in vitro. Nutr Cancer. 1999;35:80–86. doi: 10.1207/S1532791480-86. [DOI] [PubMed] [Google Scholar]
- 22.Inoue M, Sasazuki S, Wakai K, Suzuki T, Matsuo K, Shimazu T, Tsuji I, Tanaka K, Mizoue T, Nagata C, et al. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut. 2009;58:1323–1332. doi: 10.1136/gut.2008.166710. [DOI] [PubMed] [Google Scholar]
- 23.Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umeqaki K, Hara Y, Ikeda M, Tomita T. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27–32. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 25.Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis molecular mechanisms and drug targets (Review) Annu Rev Pharmacol Toxicol. 2005;45:605–628. doi: 10.1146/annurev.pharmtox.45.120403.095906. [DOI] [PubMed] [Google Scholar]
- 26.Gressner AM. The cell biology of liver fibrogenesis – an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447–452. doi: 10.1007/s004410051073. [DOI] [PubMed] [Google Scholar]
- 27.Oh SW, Kim DH, Ha JR, Kim DY. Anti-fibrotic effects of a methylenedioxybenzene compound, CW209292 on dimethylnitrosamine-induced hepatic fibrosis in rats. Biol Pharm Bull. 2009;32:1364–1370. doi: 10.1248/bpb.32.1364. [DOI] [PubMed] [Google Scholar]
- 28.Yang CS. Tea and health. Nutrition. 1999;15:946–949. doi: 10.1016/s0899-9007(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 29.Ohta Y, Kongo M, Sasaki E, Nishida K, Ishiguro I. Therapeutic effect of melatonin on carbon tetrachloride-induced acute liver injury in rats. J Pineal Res. 2000;28:119–126. doi: 10.1034/j.1600-079x.2001.280208.x. [DOI] [PubMed] [Google Scholar]
- 30.Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 31.Gouni-Berthold I, Sachinidis A. Molecular mechanisms explaining the preventive effects of catechins on the development of proliferative diseases. Curr Pharm Des. 2004;10:1261–1271. doi: 10.2174/1381612043452578. [DOI] [PubMed] [Google Scholar]
- 32.Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med. 2004;37:304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]