Abstract
Mycobacterium tuberculosis (MTB) remains a significant global health burden despite the availability of antimicrobial chemotherapy. Increasing evidence indicates a critical role of the complement system in the development of host protection against the bacillus, but few studies have specifically explored the function of the terminal complement factors. Mice deficient in complement C7 and wild-type C57BL/6 mice were aerosol challenged with MTB Erdman and assessed for bacterial burden, histopathology, and lung cytokine responses at days 30 and 60 post-infection. Macrophages isolated from C7 −/− and wild-type mice were evaluated for MTB proliferation and cytokine production. C7 −/− mice had significantly less liver colony forming units (CFUs) at day 30; no differences were noted in lung CFUs. The C7 deficient mice had markedly reduced lung occlusion with significantly increased total lymphocytes, decreased macrophages, and increased numbers of CD4+ cells 60 days post-infection. Expression of lung IFN-γ and TNF-α was increased at day 60 compared to wild-type mice. There were no differences in MTB-proliferation in macrophages isolated from wild-type and knock-out mice. These results indicate a role for complement C7 in the development of MTB induced immunopathology which warrants further investigation.
1. Introduction
Tuberculosis (TB) remains a significant public health problem, causing nearly 2 million deaths each year [1]. Host protection against TB requires the development of a Th1 immune response [2]. Th1 responses are initiated by dendritic cell presentation of MTB antigen to naive CD4+ T-cells accompanied by synthesis of IL-12 [3]. These Th1 cells produce IFN-γ, which activates macrophages to cause phagosome acidification, phagolysosome fusion, and the generation of reactive nitrogen species that control MTB growth [2]. It is essential to understand the innate immune system functions that lead to protective T-cell responses to MTB in order to develop improved vaccines and therapies based on immune modulation.
Increasing evidence indicates that the complement system is essential for generating protective adaptive immune responses to mycobacterial infections [4–6], in addition to its clear role in both innate immunity and the initiation of adaptive immunity to a variety of other pathogens [7]. The three major pathways of complement are the classical, alternative, and lectin pathways. Each complement pathway leads to the cleavage of C3 generating C3a and C3b. C3b binding to pathogens promotes opsonization resulting in the enhanced elimination pathogens, as well as the formation of C5b-9, the membrane attack complex. Insertion of the complement component C7 into the cell membrane is the critical step in the formation of the membrane attack complex that leads to cell lysis.
The precise role of complement factors in immunity and pathology during mycobacterial infection is unclear. It was reported that mice C3 knockout mice infected with M. avium had no differences in granuloma formation or bacterial load in comparison to wild-type mice [8]. However, mice deficient in complement C5 have decreased survival, increased inflammation, and decreased macrophage cytokine production [4, 5, 9]. Little is known about the role of the terminal complement components in MTB infection. Thus, complement factor C7 knock-out (C7 −/−) mice were infected with MTB and evaluated for bacterial burden, lung immunopathology, and cytokine responses.
2. Methods
2.1. Animals
Six week-old C7 deficient mice (C7 −/−) and wild-type C57BL/6 mice were a kind gift from Michael C. Braun (Baylor College of Medicine, Houston, TX). Briefly, C57BL/6J ES cells with a targeted insertion of a gene trap vector in the intronic region between exons 6 and 7 of the C7 gene were obtained from the Texas Institute of Genomic Medicine (TIGM, Houston, Texas). Single insertion of the vector was confirmed by FISH analysis, and targeting to the intronic region between exons 6 and 7 of the C7 gene was confirmed by sequence analysis. Heterozygous chimeric mice with germ-line integration were then back-crossed with C57BL/6J mice. The successful gene trapping of C7 using the targeting vector was confirmed by RT-PCR, and hemolytic assay. Six to eight mice were used per group, per time points indicated, infected within biosafety level 3 facilities. All studies were performed under the approval of the Animal Welfare Committee at UTHSC, protocol AWC-11-020.
2.2. Acute Tuberculosis Infection of Mice
MTB strain Erdman (TMC 107, American Type Cell Culture) was cultured in Middlebrook 7H9 broth, with 10% supplement, to log phase. Pelleted bacteria were resuspended in phosphate buffered saline (PBS) and diluted to 3 × 108 colony forming units (CFU) per mL using McFarland standard #1 (Thermo Fisher Scientific-Remel, Lenexa, KS). Bacteria were sonicated to disperse any clumps. C57BL/6 mice were infected using an aerosol inhalation exposure system (GLAS-COL Model #A4212 099c, Glas-Col, Terre Haute, IN) to achieve an average aerosol implantation of 100 CFUs, as described [10]. The inoculation dose was confirmed by sacrificing a subset of mice at day one after the-challenge and plating lung homogenates onto 7H11 agar plates (Remel, Lenexa, KS), which were incubated at 37°C for 3-4 weeks.
Mice were sacrificed at days 30 and 60 after the-MTB challenge. The lung, liver, and spleen were removed and sectioned. Portions used for determination of CFUs were placed into 5 mL PBS and homogenized. The organ homogenates were serial diluted and plated on Middlebrook 7H11 plates. Colonies were enumerated after a 3-4 week incubation period at 37°C.
2.3. Lung Histopathology Analysis
Lung tissue was fixed in 10% formalin and embedded in paraffin. Five μm thick sections were stained with hematoxylin and eosin by standard methods. Acid-fast staining was performed by the Ziehl-Neelsen method. Six lungs from each group at 30 and 60 days post-challenge were randomly selected for immunohistochemistry (IHC) for CD4 and CD8. Rat anti-mouse monoclonal antibodies (R&D Systems, Minneapolis, MN) were used to detect CD4+ and CD8+ cells using an anti-rat horseradish peroxidase 3,3′-diaminobenzidine cell and tissue staining kit according to the manufacturer's instructions (R&D Systems).
Histopathology images were obtained with the Nuance multispectral imaging system (CRI, Woburn, MA). This system allows the enumeration of cell types in defined areas of pathology. The entire section of the immunostained lungs was imaged using the 10x objective. Quantification of normal lung, area percentages of macrophages and lymphocytes, and total number of CD4+ and CD8+ cells was performed using the tissue and cell segmenting functions of Inform software (CRI) using the manufacturer's instructions.
2.4. Lung Cytokine Expression
Total lung RNA was isolated and used to produce cDNA as previously detailed [11, 12]. Analysis of cDNA was performed using qRT-PCR procedures, with previously detailed primers and probes [12]. The reaction mixture contained 5 μL cDNA, 1X PCR buffer (5Prime, Fisher Scientifics, Pittsburg, PA), 200 nM dNTPs (Invitrogen, Carlsbad, CA), 400 nM each for the forward and reverse primer (Integrated DNA Technologies, San Diego, CA), 100 nM probe (Biosearch Technologies, Novato, CA), 1x ROX reference dye (Invitrogen), and 1 U/50 μL Taq DNA polymerase (Fisher). The reaction was performed using the ABI Sequence Detection System (Applied Biosciences, Carlsbad, CA) by heating for 1 min at 95°C, and then 40 cycles of 95°C for 12 seconds and 60°C for 1 min. Data are expressed as fold change expression relative to naive controls after normalization to β-actin [13].
2.5. Preparation of Bone-Marrow-Derived Macrophages and Infection with MTB
Bone-marrow-derived macrophages from wild-type C57BL/6 and complement factor C7 −/− mice were generated by previously described methods [14]. Briefly, femurs were flushed with McCoy's media (Sigma, St. Louis, MO), and 2 × 106 cells were added to 24 well tissue culture plates (Corning Incorporated, Corning, NY). Cells were cultured in McCoy's media (Sigma) supplemented with 10% fetal bovine serum (FBS) (Sigma), 100 μg mL−1 gentamycin (Sigma), 100 U mL−1 penicillin (Sigma), and 10 ng mL−1 recombinant murine granulocyte/macrophage colony stimulating factor (GM-CSF; Chemicon, Billerica, MA). The cells were cultured at 37°C in 5% CO2 for seven days, with two media changes containing GM-CSF. Finally, cells were washed and suspended in antibiotic free Dulbecco's modified eagle's medium (DMEM; Sigma) supplemented with 10% FBS.
The cells were infected with log phage cultures of MTB Erdman at a multiplicity of infection (MOI) of 1 : 1. The supernatants were removed after 24 and 72 hours of incubation and analyzed for production of IL-6, TNF-α, IL-12p40, and TGF-β by ELISA according to the manufacturer's instructions (R&D Systems).
2.6. Statistics
The data are shown as the mean ± SD. Two-way ANOVA was used to determine the differences between groups by use of GraphPad Prism software. A P-value of less than 0.05 was defined as statistically significant.
3. Results
3.1. Decreased Bacterial Dissemination in C7 Deficient Mice
Bacterial CFUs in the lung, liver, and spleen are shown in Figure 1. C7 −/− mice had significantly reduced CFUs in the liver at day 30; specifically, liver log10 CFUs in wild-type mice were 3.82 ± 0.56 and 2.58 ± 0.40 at day 30 for the C7 −/− mice. No significant differences in bacterial load were observed in the lung and spleen at either time point.
Figure 1.
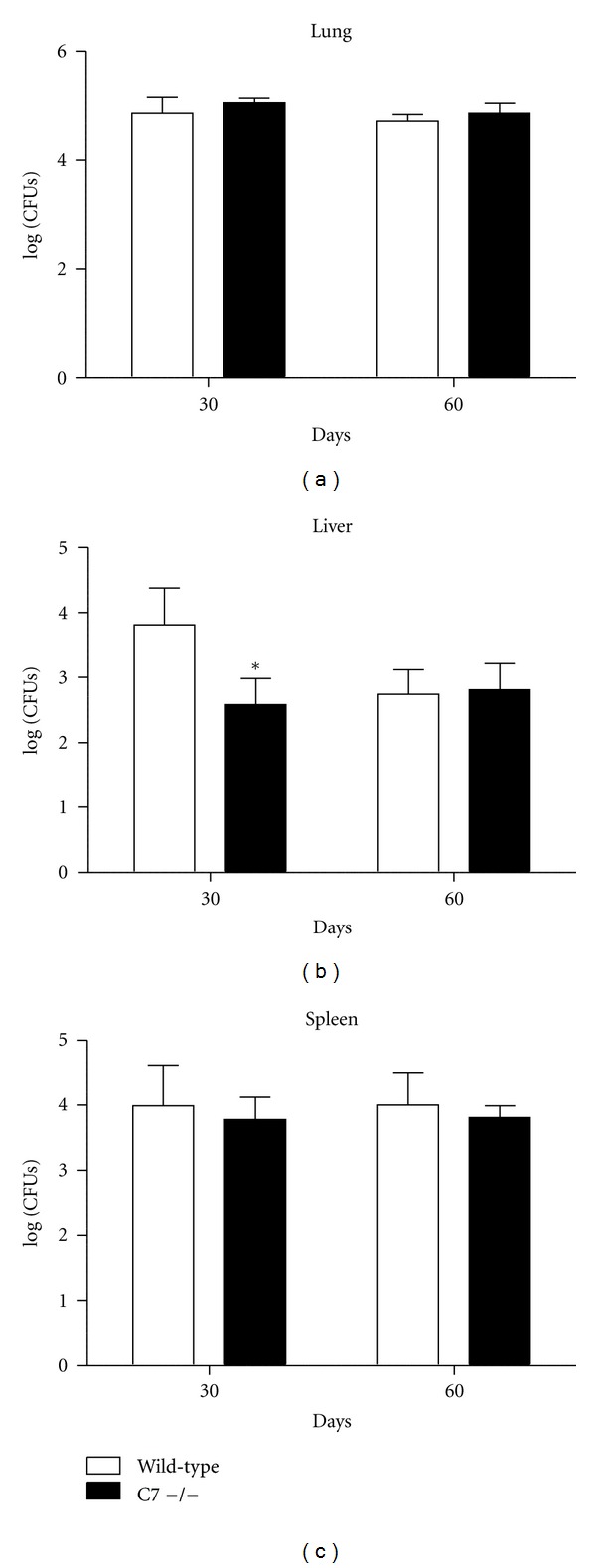
Decreased early bacterial dissemination to the liver in C7 −/− mice. C7 −/− mice had significantly reduced bacterial CFUs in the liver at day 30 after infection with MTB. There were no differences in bacterial burden in the lung or spleen. *P < 0.05 with comparison to wild-type C57BL/6 mice.
3.2. C7 −/− Mice Have Decreased Lung Immunopathology
Representative images of lung histopathology for wild-type and C7 −/− mice at 60 days post-infection are shown in Figure 2. Wild-type mice demonstrate significant lung inflammatory infiltrates, which were predominantly macrophages (Figures 2(a) and 2(b)). In contrast, C7 −/− mice have reduced destructive lung pathology with smaller granulomas and decreased parenchymal inflammation (Figure 2(c) and Table 1). Abundant clusters of lymphocytes were observed in the C7 −/− mice, primarily near the vasculature and adjacent to granulomas (Figure 2(d)). Quantitative IHC demonstrated increased numbers of CD4+ lymphocytes and reduced areas of macrophages in the C7 −/− mice (Table 1). Rare multinucleated giant cells were noted in the C7 −/− mice (Figure 2(e)).
Figure 2.
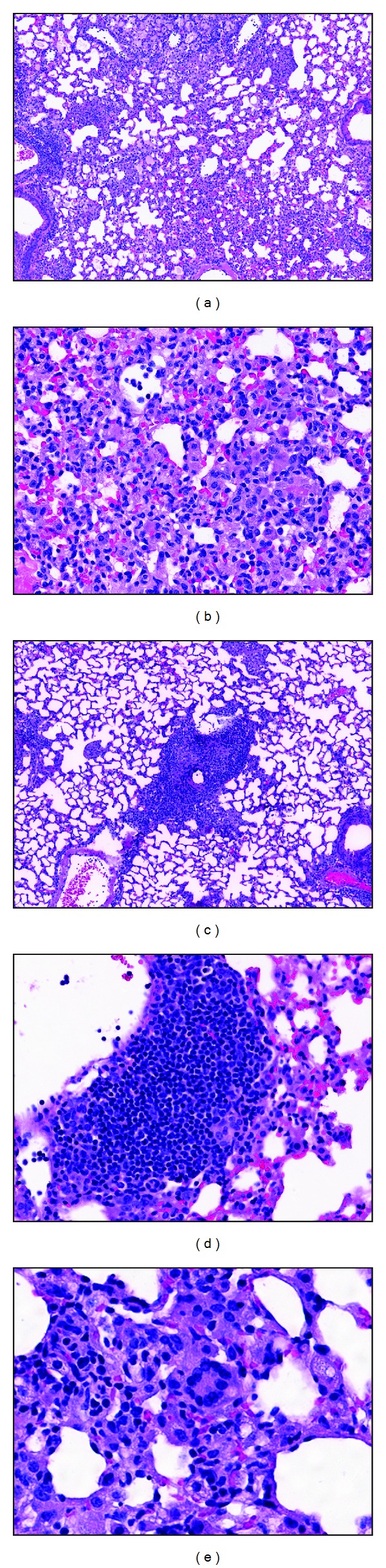
Lung histopathology of wild-type C57BL/6 and C7 −/− mice after the-challenge with MTB Erdman. (a) C57BL/6 mice demonstrate extensive granulomatous inflammation at 60 days post infection, 100x. (b) High power images of wild-type mice show that infiltrates are largely macrophages, 400x. (c) C7 −/− mice have reduced lung immunopathology at day 60 after the infection, 100x. (d) Abundant areas with clusters of lymphocytes are observed in C7 −/− mice, 400x. (e) Rare multinucleated giant cell in C7 −/− mice, 400x.
Table 1.
Quantitative IHC of wild-type C57BL/6 mice and C7 deficient mice infected with MTB.
| Control | C7 −/− | |
|---|---|---|
| % lung occlusion | ||
| Day 30 | 50.6 ± 12.1 | 47.8 ± 12.3 |
| Day 60 | 59.1 ± 12.8 | 40.8 ± 7.5* |
| % macrophages | ||
| Day 30 | 50.8 ± 12.0 | 39.9 ± 8.1 |
| Day 60 | 49.2 ± 13.1 | 30.3 ± 10.1* |
| % lymphocytes | ||
| Day 30 | 4.3 ± 2.8 | 6.7 ± 3.7 |
| Day 60 | 9.1 ± 3.1 | 17.0 ± 8.7* |
| CD4+ lymphocytes | ||
| Day 30 | 277.2 ± 122.9 | 348.9 ± 239.5 |
| Day 60 | 291.1 ± 155.9 | 973.9 ± 449.2* |
| CD8+ lymphocytes | ||
| Day 30 | 321.3 ± 177.1 | 417.3 ± 169.8 |
| Day 60 | 422.3 ± 284.8 | 440.3 ± 216.4 |
3.3. Absence of Complement Factor C7 Modulates Lung Cytokine Expression
Lung cytokine expression of TNF-α, IL-6, IL-12p40, TGF-β, IL-10, IFN-γ, and IL-17 was measured by quantitative PCR (Figure 3). Expression of all cytokines increased in challenged wild-type mice relative to naive mice. C7 −/− mice had significantly increased expression of TNF-α and IFN-γ at day 60 after the-MTB challenge, compared to wild type mice. There were no significant differences in lung expression of IL-6, IL-12p40, TGF-β, or IL-17 (data not shown).
Figure 3.
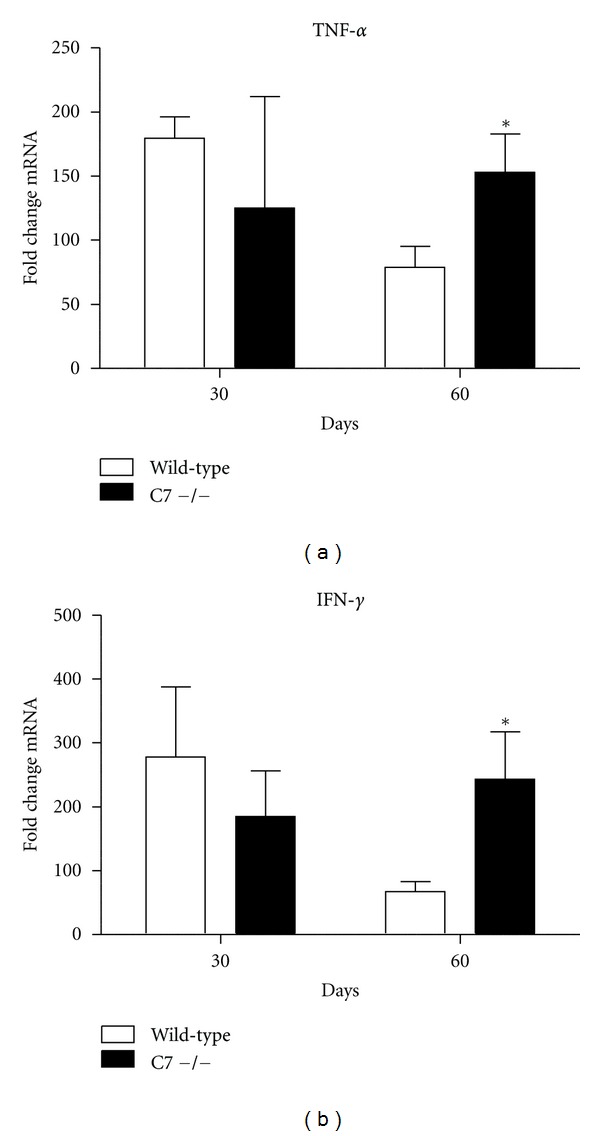
Lung expression of proinflammatory mediators TNF-α and IFN-γ in wild-type and C7 −/− mice. Lung cytokine expression of TNF-α, and IFN-γ as measured by quantitative PCR in wild type (solid bars) and C7 −/− (open bars) mice at 60 days post challenge with virulent MTB. Data are expressed as fold change relative to naïve mice after normalization to β-actin. Data are presented as the mean with SD, n = 6 mice per group, per time point. *P < 0.05, comparisons are made to control mice. There were no significant differences in lung expression of IL-6, IL-12p40, TGF-β, or IL-17 (data not shown).
3.4. Modulation of Macrophage Cytokine Production by Complement C7
Proinflammatory cytokine production was measured from MTB-infected bone-marrow-derived macrophages isolated from wild-type and C7 −/− mice (Figure 4). The macrophages from the C7 −/− mice had slight, but significant, decreased synthesis of IL-12p40 and increased TGF-β at 72 hours post-infection. There were no differences in synthesis of TNF-α and IL-6 (not shown). Additionally, the absence of complement factor C7 did not affect MTB proliferation in macrophages (not shown).
Figure 4.
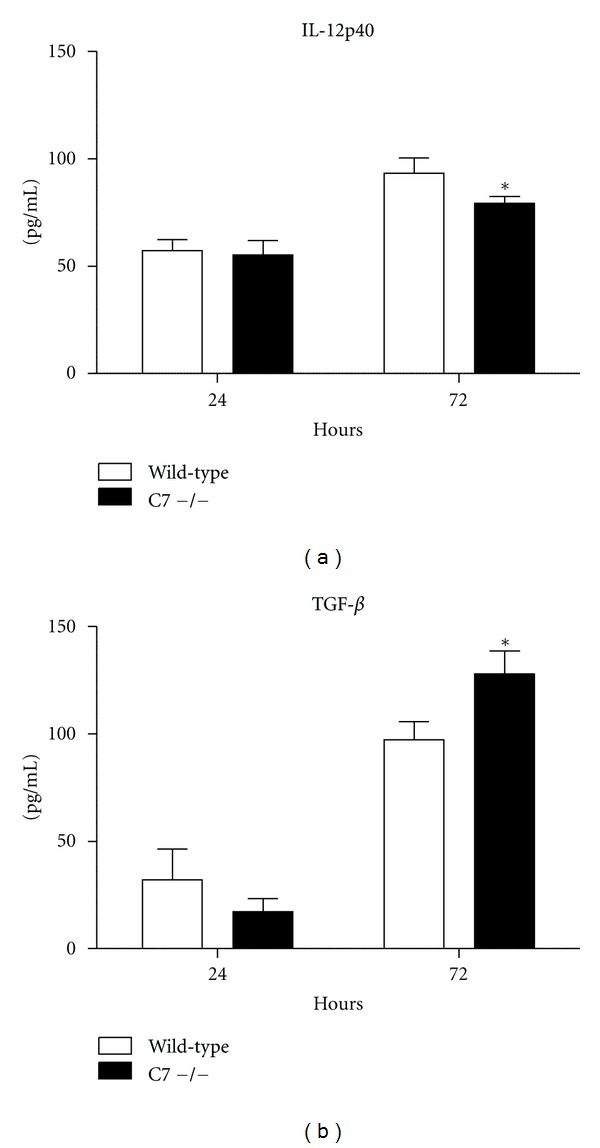
Cytokine production from infected bone-marrow-derived macrophages. Macrophages were isolated from wild-type and C7 −/− mice and infected with MTB at a MOI of 1 : 1. Cytokines production was measured 24 and 72 hours after infection by ELISA. *P < 0.05, comparisons are made to control mice.
4. Discussion
While deficiencies in the terminal complement factors such as C7 are associated with Neisseria meningitidis infections [15], their function in mycobacterial infections is unknown. These studies indicate a role for complement C7 in the development of lung immunopathology caused by infection with MTB. Mycobacteria are known to activate all three complement pathways [16], but the results of specific pathway activation on MTB pathogenesis are mixed. While complement activation likely promotes the uptake of mycobacteria into macrophages [8, 17], deficiency of C5 resulted in enhanced susceptibility to TB and its associated trehalose 6,6′-dimycolate glycolipid; this was evident by a reduced IL-12 production by macrophages and reduced IFN-γ synthesis and production [6, 9]. Prior investigations demonstrate that macrophages and dendritic cells produce complement C7 [18, 19]; we therefore determined the impact of complement C7 deficiency on MTB-infected macrophage cytokine secretion. A slight reduction in IL-12p40 and an increase in TGF-β were noted, possibly indicating that C7 deficiency may impact induction of T-cell responses.
The complement C7 deficient mice had increased expression of IFN-γ and TNF-α at 60 days post-challenge with MTB accompanied by increased numbers of CD4+ lymphocytes, despite a decrease in IL-12p40 by infected macrophages. Numerous investigations link the early complement cascade components to the development of Th1 responses [20]. For example, the binding of complement factors C3a and C5a to their receptors promotes the migration of antigen presenting cells to areas of infection, where they present antigen to T-cells and modulate IL-12 production that is essential for induction of IFN-γ synthesis [21–23]. Binding of immune complexes to the C1q receptor on T-cells promotes T-cell activation with production of TNF-α and IFN-γ [24]. Both CD4+ and CD8+ T-cells responses in C3 deficient mice are decreased in viral infection models [25–27]. The precise role of complement C7 in induction of IFN-γ synthesis is unclear; however, there are reports of CD59 modulation of T-cell responses. These data suggest that the crosslinking of CD59, an inhibitor of MAC formation at the level of C9, resulted in augmented IL-2 production and cell proliferation [28]. Furthermore, CD59a deficient mice have enhanced specific CD4+ T-cell responses after challenge with recombinant vaccinia virus [29]. It should be noted that the increase in lung expression of IFN-γ in the C7 −/− mice did not result in a decrease in lung bacterial burden; only an early reduction in liver CFUs was observed. Some investigators report little correlation between the levels of specific IFN-γ producing CD4+ and CD8+ cells and protection against MTB [30].
Of potential significance, the C7 deficient mice had markedly reduced lung immunopathology in this mouse model of MTB infection. The histopathology generated by the host in response to MTB possibly contributes to the persistence and dissemination of the organism [31]. For example, MTB in granulomas convert to a state of non-replicating persistence that is characterized by alterations in biochemical pathways and reduced bacterial proliferation, changes that make the bacteria resistant to antibiotics [32, 33]. The altered cytokines in the infected C7 −/− mice are clearly important in granuloma induction and maintenance of structure [12]. MTB contained with granulomas are physically sequestered from cytotoxic T-cells capable of eliminating infected cells [31]. Reduction of lung immune-mediated pathology may additionally enhance the penetration of antibiotics into infected tissue, possibly enabling a more rapid response to antimicrobials [34]. Indeed, modulation of this pathology has been proposed as a novel therapeutic strategy to shorten the treatment of TB, an approach that has had some success in human clinical trials [35, 36]. Future studies are needed to explore the therapeutic potential of C7 on MTB-induced pathology.
The data presented in these studies indicate a role for complement C7 in the development of lung histopathology in MTB infection, with a potential role for dysregulation in innate interaction with lymphocytes and their subsequent production of cytokines. Future studies are needed to further investigate the role of C7, to specifically define the direct link between the regulation of response affecting MTB-induced immunopathology. In this manner, we can further understand the relationship of pathological dysregulation directly attributed to C7 compared to its contribution as related to other terminal complement components.
References
- 1.World Health Organization. WHO Report 2009. Geneva, Switzerland: World Health Organization; 2009. Global tuberculosis control: epidemiology, strategy, financing. [Google Scholar]
- 2.Co DO, Hogan LH, Kim SI, Sandor M. Mycobacterial granulomas: keys to a long-lasting host-pathogen relationship. Clinical Immunology. 2004;113(2):130–136. doi: 10.1016/j.clim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Rao A, Avni O. Molecular aspects of T-cell differentiation. British Medical Bulletin. 2000;56(4):969–984. doi: 10.1258/0007142001903634. [DOI] [PubMed] [Google Scholar]
- 4.Actor JK, Breij E, Wetsel RA, Hoffmann H, Hunter RL, Jagannath C. A role for complement C5 in organism containment and granulomatous response during murine tuberculosis. Scandinavian Journal of Immunology. 2001;53(5):464–474. doi: 10.1046/j.1365-3083.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 5.Jagannath C, Hoffmann H, Sepulveda E, Actor JK, Wetsel RA, Hunter RL. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component (C5) Scandinavian Journal of Immunology. 2000;52(4):369–379. doi: 10.1046/j.1365-3083.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- 6.Mashruwala MA, Smith AK, Lindsey DR, et al. A defect in the synthesis of interferon-gamma by the T cells of complement-C5 deficient mice leads to enhanced susceptibility for tuberculosis. Tuberculosis. 2011;91(Supplement 1):S82–S89. doi: 10.1016/j.tube.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollmann TJ, Mueller-Ortiz SL, Braun MC, Wetsel RA. Disruption of the C5a receptor gene increases resistance to acute Gram-negative bacteremia and endotoxic shock: opposing roles of C3a and C5a. Molecular Immunology. 2008;45(7):1907–1915. doi: 10.1016/j.molimm.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohlson SS, Strasser JA, Bower JJ, Schorey JS. Role of complement in Mycobacterium avium pathogenesis: in vivo and in vitro analyses of the host response to infection in the absence of complement component C3. Infection and Immunity. 2001;69(12):7729–7735. doi: 10.1128/IAI.69.12.7729-7735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borders CW, Courtney A, Ronen K, et al. Requisite role for complement C5 and the C5a receptor in granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate. Scandinavian Journal of Immunology. 2005;62(2):123–130. doi: 10.1111/j.1365-3083.2005.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SA, Welsh KJ, Boyd S, Kruzel ML, Actor JK. Comparing efficacy of BCG/lactoferrin primary vaccination versus booster regimen. Tuberculosis (Edinb) 2011;91(supplement 1):S90–S95. doi: 10.1016/j.tube.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh KJ, Hwang SA, Hunter RL, Kruzel ML, Actor JK. Lactoferrin modulation of mycobacterial cord factor trehalose 6-6′-dimycolate induced granulomatous response. Translational Research. 2010;156(4):207–215. doi: 10.1016/j.trsl.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh KJ, Abbott AN, Hwang SA, et al. A role for tumour necrosis factor-α, complement C5 and interleukin-6 in the initiation and development of the mycobacterial cord factor trehalose 6,6′-dimycolate induced granulomatous response. Microbiology. 2008;154(6):1813–1824. doi: 10.1099/mic.0.2008/016923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Indrigo J, Hunter RL, Actor JK. Influence of trehalose 6,6′-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology. 2002;148(7):1991–1998. doi: 10.1099/00221287-148-7-1991. [DOI] [PubMed] [Google Scholar]
- 15.Kuijpers TW, Nguyen M, Hopman CTP, et al. Complement factor 7 gene mutations in relation to meningococcal infection and clinical recurrence of meningococcal disease. Molecular Immunology. 2010;47(4):671–677. doi: 10.1016/j.molimm.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Carroll MV, Lack N, Sim E, Krarup A, Sim RB. Multiple routes of complement activation by Mycobacterium bovis BCG. Molecular Immunology. 2009;46(16):3367–3378. doi: 10.1016/j.molimm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. Journal of Immunology. 1990;144(7):2771–2780. [PubMed] [Google Scholar]
- 18.Hetland G, Johnson E, Aasebo U. Human alveolar macrophages synthesize the functional alternative pathway of complement and active C5 and C9 in vitro. Scandinavian Journal of Immunology. 1986;24(5):603–608. doi: 10.1111/j.1365-3083.1986.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 19.Reis ES, Barbuto JAM, Isaac L. Complement components, regulators and receptors are produced by human monocyte-derived dendritic cells. Immunobiology. 2007;212(3):151–157. doi: 10.1016/j.imbio.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nature Reviews Immunology. 2007;7(1):9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 21.Drouin SM, Corry DB, Kildsgaard J, Wetsel RA. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. Journal of Immunology. 2001;167(8):4141–4145. doi: 10.4049/jimmunol.167.8.4141. [DOI] [PubMed] [Google Scholar]
- 22.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. Journal of Immunology. 2002;169(10):5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 23.Hawlisch H, Wills-Karp M, Karp CL, Köhl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Molecular Immunology. 2004;41(2-3):123–131. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Chen A, Gaddipati S, Hong Y, Volkman DJ, Peerschke EIB, Ghebrehiwet B. Human T cells express specific binding sites for C1q: role in T cell activation and proliferation. Journal of Immunology. 1994;153(4):1430–1440. [PubMed] [Google Scholar]
- 25.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nature Medicine. 2002;8(4):373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 26.Suresh M, Molina H, Salvato MS, Mastellos D, Lambris JD, Sandor M. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. Journal of Immunology. 2003;170(2):788–794. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 27.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. Journal of Immunology. 2003;171(10):5363–5371. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 28.Korty PE, Brando C, Shevach EM. CD59 functions as a signal-transducing molecule for human T cell activation. Journal of Immunology. 1991;146(12):4092–4098. [PubMed] [Google Scholar]
- 29.Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD4+ T cell activity in a complement-independent manner. Journal of Immunology. 2005;175(11):7098–7102. doi: 10.4049/jimmunol.175.11.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittrücker HW, Steinhoff U, Köhler A, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(30):12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe’s point of view. Cellular Microbiology. 2010;12(3):301–309. doi: 10.1111/j.1462-5822.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 32.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clinics in Chest Medicine. 2009;30(4):769–782. doi: 10.1016/j.ccm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis . Annual Review of Microbiology. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 34.Roy E, Lowrie DB, Jolles SR. Current strategies in TB immunotherapy. Current Molecular Medicine. 2007;7(4):373–386. doi: 10.2174/156652407780831557. [DOI] [PubMed] [Google Scholar]
- 35.Mayanja-Kizza H, Jones-Lopez E, Okwera A, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. Journal of Infectious Diseases. 2005;191(6):856–865. doi: 10.1086/427995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis RS, Kyambadde P, Johnson JL, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18(2):257–264. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]


