Abstract
Overexpression of Cyclin D1 and Bcl-xL proteins has often been found in non-small-cell lung cancer (NSCLC). These two genes may play a significant role in tumorigenesis. However, the combined inhibition of the two genes in vitro is unclear in NSCLC. In this study, the effect of a combined intervention on Cyclin D1 and Bcl-xL in NSCLC is assessed and discussed. Three recombinant plasmids that expressed a cytomegalovirus (CMV) promoter-driven micro30 short hairpin RNA (shRNA) targeting the Cyclin D1 gene (Cyclin D1 shRNA), the Bcl-xL gene (Bcl-xL shRNA) and a combination of the two genes (Cyclin D1-Bcl-xL shRNA), based on the plasmid pcDNA6.2-GW/EmGFP-miR, were constructed. The cell lines A549 and NCI-H441 were divided into four groups; blank control (untreated cells), Cyclin D1 shRNA, Bcl-xL shRNA and Cyclin D1-Bcl-xL shRNA (transfected cells), respectively. The expression of mRNA and protein of Cyclin D1 or Bcl-xL was detected by reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting, respectively. The apoptosis and proliferation of the two cell lines were evaluated by dimethylthiazol-diphenyltetrazolium bromide (MTT), cell count and flow cytometry. The recombinant plasmid sufficiently mediated the RNA interference (RNAi) effects in A549 and NCI-H441 cells. The expression levels of mRNA and protein of Cyclin D1 or Bcl-xL in the three intervention groups were significantly reduced compared to the untreated cells (P<0.05). No statistical differences were found among the combined shRNAs and single shRNA regarding Cyclin D1 or Bcl-xL, respectively (P>0.05). In the assessment of proliferation and apoptosis, it was found that in all three intervention groups there was significant inhibition of cell proliferation and promotion of cell apoptosis compared with the untreated cells (P<0.05). Furthermore, the combined interference of the two genes was more effective than either single interference (P<0.05). Our results suggested that the combined targeting of Cyclin D1 and Bcl-xL genes has potential for NSCLC investigation, providing increased efficacy over Cyclin D1 or Bcl-xL inhibition alone.
Keywords: non-small-cell lung cancer, RNA interference, Bcl-xL, Cyclin D1, apoptosis
Introduction
Lung cancer is the most common cause of cancer-related mortality worldwide. The two major subtypes are small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) (1,2). Early stages of NSCLC are treated with curative surgical resection. However, over 60% of all NSCLC patients already have advanced or metastatic tumors at the time of diagnosis and are not suitable for surgery. The overall 5-year survival rate of patients with NSCLC remains extremely poor (3–5). Therefore, it is crucial to employ new therapeutic strategies in the treatment of NSCLC. With the constantly evolving knowledge of the molecular pathogenesis of lung cancer, targeted therapies have recently been introduced (6). Moreover, antisense gene therapies, such as small RNA interference (RNAi), have been evaluated in recent years (7–10).
Cyclin D1 is a pivotal cell cycle-regulatory protein that controls the cell cycle transition from G1 to S phase. It is also intricately involved in the regulation of apoptosis depending on the proliferative and differentiated state of the cell (11–14). Overexpression of the Cyclin D1 protein was reported in various types of cancer, such as breast, esophageal and lung cancer (15–17). Betticher et al reported that Cyclin D1 was associated with poor tumor differentiation and was known to be a negative indicator in NSCLC (17). When Cyclin D1 is overexpressed, it may increase the risk of tumor progression and early onset of cancer (19–22). Cyclin D1 overexpression enhances cell proliferation and cell cycle progression (19–22). Certain studies have reported targeted therapy aimed at Cyclin D1 by small RNAi (18). Down-regulation of the expression of Cyclin D1 inhibits tumor growth (18). In this regard, we hypothesized that the dysregulation of Cyclin D1 occurs relatively early in the process of tumorigenesis and may be promising for cancer therapy.
Bcl-xL is a critical member of the Bcl-2 family and is correlated to several malignancies, including NSCLC (23–26). As an anti-apoptotic protein, the overexpression of Bcl-xL may inhibit the mitochondrial cytochrome release, which is a mechanism by which cancer cells escape apoptosis and regulate the apoptosis of two signaling pathways, the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway (23). Previous studies have also reported targeted therapy aimed at Bcl-xL by small RNAi (25,26). Substantial research has shown that the down-regulation of anti-apoptotic gene expression is capable of sensitizing cancer cells to anticancer drugs and promoting cell apoptosis (25,26). Thus, Bcl-xL is a potential new therapeutic target in NSCLC.
As noted, a number of studies have reported RNAi aimed at Cyclin D1 or Bcl-xL. However, the effect of combining the two genes in vitro for an intervention study is unclear in NSCLC. Biliran et al have reported that the expression of Bcl-xL remained relatively high in the cells with overexpressed Cyclin D1 (27). Huang et al have reported that combined therapy with the two genes prolonged survival in mice with ovarian cancer (28). Thus, we formulated a hypothesis that combined interference of the two genes is a promising new strategy for improving lung cancer outcomes.
In the present study, we aimed to determine whether combined interference was superior to single interference.
Materials and methods
Construction of shRNA vectors
The pcDNA6.2-GW/EmGFP-miR vector was purchased from Invitrogen (Carlsbad, CA, USA) with a genetically engineered improved murine miR-155 skeleton structure containing a terminal loop and an internal loop. The recombinant plasmid pcDNA6.2-GW/EmGFP-miR that expressed a cytomegalovirus (CMV) promoter-driven micro30 short hairpin RNA (shRNA) targeting Cyclin D1 (Cyclin D1 shRNA), Bcl-xL (Bcl-xL shRNA) and a combination of the two genes (Cyclin D1-Bcl-xL shRNA) were constructed, respectively. The micro30 shRNA reverse sequencing primer site (C) occurred at bases 1607–1626. Green fluorescent protein (GFP) assays were implemented by co-transfection of cancer cells with plasmids encoding GFP and corresponding shRNA in order to observe the efficacy of transfection. The shRNAs were designed to target human Bcl-xL (accession no. NM_138578.1) and Cyclin D1 (accession no. NM_053056.2) mRNA. The sequences are shown in Table I. The micro30 shRNAs were synthesized by Invitrogen.
Table I.
The sequences of micro30 shRNA sense strands.
| Gene | Sequence |
|---|---|
| MR075-3-F | TGCTGTGTAGATGCACAGCTTCTCGGGTTTTGGCCACTGACTGACCCGAGAAGGTGCATCTACA |
| MR075-3-R | CCTGTGTAGATGCACCTTCTCGGGTCAGTCAGTGGCCAAAACCCGAGAAGCTGTGCATCTACAC |
| MR076-1-F | TGCTGAGAGAAAGTCAACCACCAGCTGTTTTGGCCACTGACTGACAGCTGGTGTGACTTTCTCT |
| MR076-1-R | CCTGAGAGAAAGTCACACCAGCTGTCAGTCAGTGGCCAAAACAGCTGGTGGTTGACTTTCTCTC |
The sequences that showed the most effectively inhibited rate in the pre-experiment were selected for use in combined Cyclin D1-Bcl-xL shRNA.
Cell culture
The human lung adenocarcinoma cell lines A549 and NCI-H441 were cultured in RPMI-1640 (Invitrogen) containing 10% fetal bovine serum (Invitrogen). The stock was maintained in a 5% CO2 incubator at 37°C in a humidified atmosphere. The cancer cells were divided into four groups, i.e., blank control (untreated cells), Cyclin D1 shRNA, Bcl-xL shRNA and Cyclin D1-Bcl-xL shRNA (transfected cells).
Plasmid transfection
The plasmids were transfected into A549 and NCI-H441 cells according to the manufacturer’s instructions for Lipofectamine™2000 (Invitrogen). Briefly, prior to transfection, 2–3×105 cells in 2 ml of growth medium without antibiotics were placed in 6-well plates. The 6-well plates were washed with 2 ml OptiMEM (Gibco, Invitrogen, USA) twice, and another 2 ml OptiMEM containing 4 μg pcDNA6.2-GW/EmGFP-miR mixed with 10 μl of lipofectamine was added to form liposomes. Following incubation at 37°C for 4–6 h, the 6-well plates were placed in 2 ml medium containing 10% FBS in a 5% CO2 incubator at 37°C in a humidified atmosphere overnight.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
The transfected cells were collected for real-time RT-PCR. Transfected and untreated cells were collected and washed with phosphate-buffered saline (PBS). Total RNA was extracted from the cells of the four groups using TRIzol reagent (Invitrogen) in a single-step method and cDNA was generated with a PrimeScript® RT reagent kit (Takara, Shiga, Japan) at a total volume of 20 μl according to the manufacturer’s instructions. Expression levels of target gene were normalized to the housekeeping gene β-actin (ΔCt). Gene expression values were then calculated based on the ΔΔCt method using the equation: RQ=2−ΔΔCt. PCR amplification was performed with SYBR® Premix Ex Taq™ (Takara), under the following PCR conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec, and a dissociation stage of 95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec. The real-time primer sequences were designed and synthesized by Takara Customer Services (Dalian, China). The primer sequences used were: human β-actin, sense: 5′-GCAAGCAGGAGTATGACGAG-3′ and antisense: 5′-CAAATAAAGCCATGCCAATC-3′ (144 bp); Cyclin D1, sense: 5′-ATGTTCGTGGCCTCTAAGATGA-3′ and antisense: 5′-CAGGTTCCACTTGAGCTTGTTC-3′ (138 bp); Bcl-xL, sense: 5′-AGCTTGGATGGCCACTTACCTG-3′ and antisense: 5′-TGCTGCATTGTTCCCATAGAGTTC-3′ (100 bp). Each assay was performed in triplicate and repeated three times.
Western blot analysis
Transfected cells were collected for Western blot analysis. Following transfection with shRNAs 72 h later, the cells were lysed in EBC buffer with protease inhibitor on ice and centrifuged at 10,000 x g for 10 min at 4°C. Lysates were separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then transferred electrophoretically onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in PBS-T containing 5% non-fat dry milk and 0.1% Tween-20 for 2 h at room temperature. Subsequently, the membranes were washed with PBS-T containing 0.1% Tween-20 and incubated with rabbit anti-human Bcl-xL antibody (Cell Signaling, Beverly, MA, USA) at a dilution of 1:1000, and mouse anti-human Cyclin D1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:500 overnight at 4°C. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The peroxidase activity was detected using the enhanced chemiluminescence system (Pierce, Rockford, IL, USA), according to the manufacturer’s instructions.
Cell proliferation analysis
To determine the effect of Cyclin D1 and Bcl-xL shRNAs on cell proliferation, cells were seeded in 12-well plates at a density of 20×104 cells/well and cultivated with RPMI-1640 in the CO2 incubator at 37°C. Following transfection with Cyclin D1 and Bcl-xL shRNAs 48 h later, untreated cells and the transfected cells were collected. The total cell number was determined with a hematocytometer under an inverted microscope. Each assay was performed in triplicate and repeated three times.
Dimethylthiazol-diphenyltetrazolium bromide (MTT) assay
Cell proliferation was assessed by the MTT assay. A549 and NCI-H441 cells were seeded in 96-well plates and transfected with shRNAs for 48 h. Then, 10 μl of 5 mg/ml MTT (in PBS) was added to each well and continually incubated for 4 h at 37°C. The formazan granules obtained from cells were dissolved in 150 μl dimethyl sulfoxide (DMSO) for 10 min. Cell viability was then measured in terms of optical density (OD) at a wavelength of 490 nm. Each cell viability assay was performed in quadruplicate and repeated three times.
Annexin V/propidium iodide (PI) staining
To determine the effect of Cyclin D1 and Bcl-xL shRNAs on cell apoptosis, cells were seeded in 6-well plates at a final concentration of 3×105/ml and transfected with those plasmids, respectively. Cells were collected, washed with PBS and successively resuspended in 500 μl binding buffer. Cells were incubated with 5 μl FITC-conjugated human Annexin V (KeyGen Biotech, Nanjing, China) in the dark for 15 min at 4°C and then stained with 5 μl PI (KeyGen Biotech). After 10 min, samples were immediately analyzed with a FACSCalibur (BD Biosciences, San Jose, USA) flow cytometer. Each assay was performed in triplicate and repeated three times.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Differences in the data were analyzed by ANOVA. P<0.05 was considered to be statistically significant. Statistical analysis was carried out using SPSS 13.0 software.
Results
Effects of three shRNAs on Cyclin D1 and Bcl-xL expression in cancer cells
The plasmids were transfected into A549 and NCI-H441 cells. The efficacy of transfection was detected by measuring the percentage of the fluorescent cells, which were transfected by the plasmids containing the GFP gene. Results showed the efficacy of transfection of these plasmids in the A549 and NCI-H441 cells to be >70%.
The silencing effects of RNAi were evaluated using real-time RT-PCR analysis and Western blot analysis to detect the levels of mRNA and protein, respectively. We observed the expression of mRNA and the proteins in NSCLC A549 and NCI-H441 cells. Cyclin D1 and Bcl-xL shRNAs are capable of down-regulating the expression levels of mRNA (Fig. 1) and protein (Fig. 2) (P<0.05), while the blank control showed no effects (P>0.05). Additionally, no significant differences were observed between the combined and single interference groups regarding Cyclin D1 or Bcl-xL, respectively (P>0.05).
Figure 1.
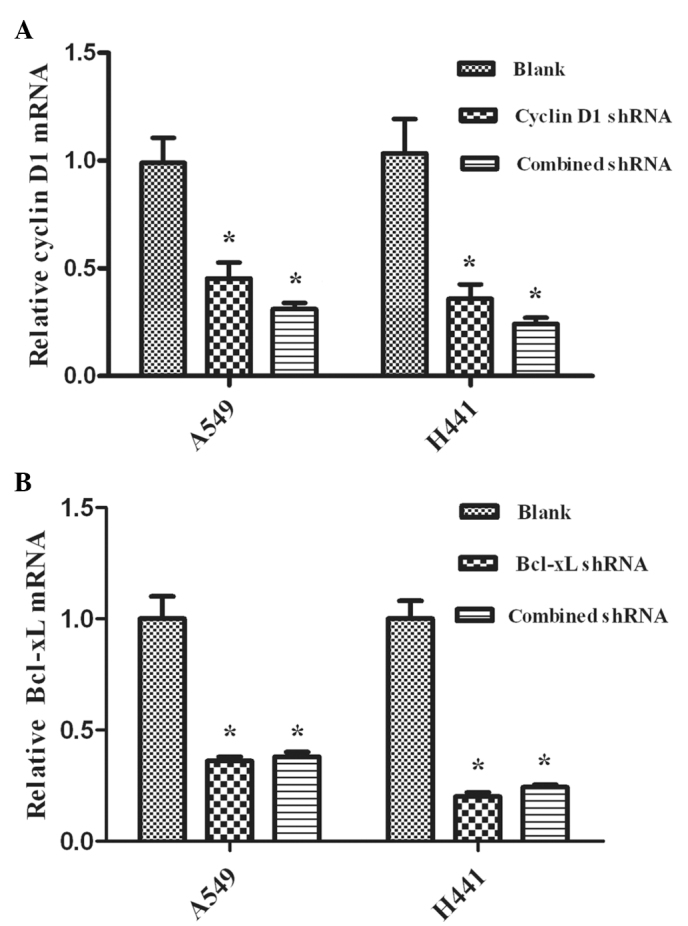
Effect of micro30 shRNA on the mRNA of Cyclin D1 and Bcl-xL (mean ± SD). The plasmids were transfected into A549 and NCI-H441 cells. Gene expression values were then calculated. (A) Cyclin D1 mRNA expression was significantly reduced in A549 and NCI-H441 lung cancer cells transfected with Cyclin D1 shRNA and Cyclin D1-Bcl-xL shRNA (combined shRNA) compared with the blank control group, *P<0.05. (B) Bcl-xL mRNA expression was significantly reduced in A549 and NCI-H441 lung cancer cells transfected with Bcl-xL shRNA and Cyclin D1-Bcl-xL shRNA compared with the blank control, *P<0.05. No significant difference was found among the combined shRNAs and single shRNA on Cyclin D1 or Bcl-xL, respectively, in the transfected cells, P>0.05. SD, standard deviation; shRNA, short hairpin RNA; RT-PCR, reverse transcripion-polymerase chain reaction.
Figure 2.
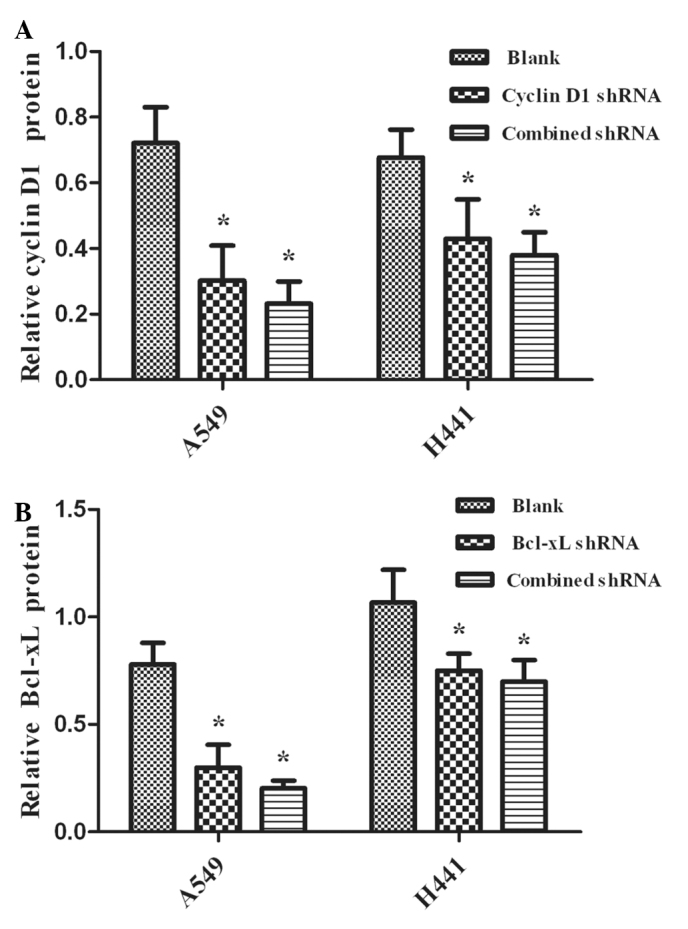
Quantification of (A) Cyclin D1 and (B) Bcl-xL protein expression level in A549 and NCI-H441 cells (mean ± SD). The plasmids were transfected into A549 and NCI-H441 cells. The transfected cells were collected for Western blot analysis. The intensity of the Bcl-xL and Cyclin D1 bands upon each treatment was quantified for 3 independent experiments and the mean values were obtained. (A and B) The protein expression levels of Bcl-xL and Cyclin D1 were significantly reduced by Cyclin D1 shRNA, Bcl-xL shRNA and Cyclin D1-Bcl-xL shRNA (combined shRNA) in the groups of A549 and NCI-H441 cells compared with the blank group, *P<0.05. No significant difference was found among the combined shRNAs and single shRNA on Cyclin D1 or Bcl-xL, respectively, in the transfected cells, P>0.05. SD, standard deviation; shRNA, short hairpin RNA.
Induction of proliferation in A549 and NCI-H441 cells by treatment with RNAi
Proliferation was assessed by the cell count (Fig. 3A) and MTT assay (Fig. 3B). shRNA-treated cells exhibited a significant decrease compared with the untreated cells (P<0.05). Furthermore, the proliferation efficacy of the group with combined interference had a significant decrease compared with the groups of Cyclin D1 shRNA- and Bcl-xL shRNA-treated cells (P<0.05).
Figure 3.
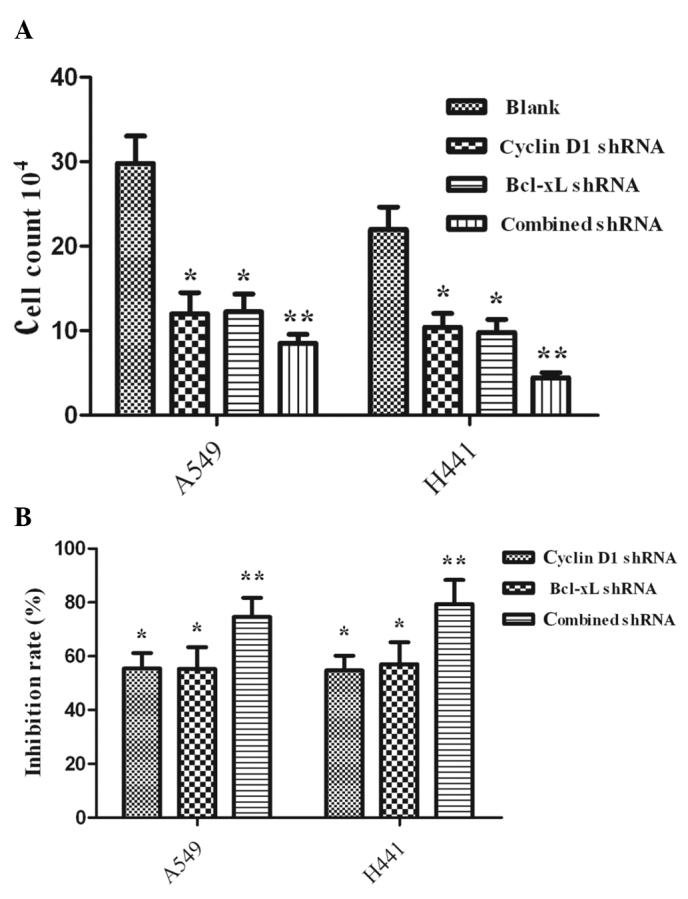
Effect of three shRNAs on tumor cell proliferation (mean ± SD). The plasmids were transfected into A549 and NCI-H441 cells. Proliferation was assessed by (A) the cell count and (B) MTT assay. Values are the mean ± SD. (A) The number of Cyclin D1 shRNA and Bcl-xL shRNA cells showed that the three different interventions inhibited cell proliferation compared with the blank group in A549 and NCI-H441 cells, P<0.05. A549 and NCI-H441 cells showed a more significant decrease in Cyclin D1-Bcl-xL shRNA (combined shRNA) compared with either of the single interventions, **P<0.05. (B) The inhibition rate of the three intervention groups was significantly more marked in the A549 and NCI-H441 cells compared with the blank group, *P<0.05. A significant decrease was found for the combined shRNA group compared with the Cyclin D1 shRNA or Bcl-xL shRNA groups, **P<0.05. SD, standard deviation; MTT, dimethylthiazol-diphenyltetrazolium bromide; shRNA, short hairpin RNA.
Induction of apoptosis in A549 and NCI-H441 cells by treatment with RNAi
Apoptosis was assessed by annexin V/PI nuclear staining. As shown in Fig. 4, combined targeted interference significantly promoted cell apoptosis compared with single interference (P<0.05). The three interference groups also had statistically significant promotion of cell apoptosis compared with the blank control (P<0.05).
Figure 4.
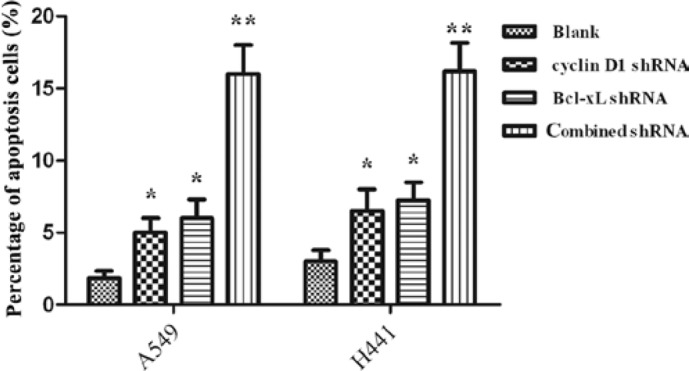
Apoptosis of lung cancer cells A549 and NCI-H441 induced by shRNAs (mean ± SD). The plasmids were transfected into A549 and NCI-H441 cells. Apoptosis was assessed by Annexin V/PI nuclear staining. Three different interventions induced cell apoptosis compared with the blank group in A549 and NCI-H441 cells, P<0.05. Apoptosis of the A549 and NCI-H441 cells was more effectively induced by Cyclin D1-Bcl-xL shRNA (combined shRNA) compared with either of the single interventions, **P<0.05. SD, standard deviation; shRNA, short hairpin RNA; PI, propidium iodide.
Discussion
Findings of the present study showed that the cell cycle-regulatory molecule Cyclin D1 and the anti-apoptotic protein Bcl-xL were overexpressed in A549 and NCI-H441 lung cancer cells. Thus, Cyclin D1 and Bcl-xL may be potential therapeutic targets in NSCLC. The down-regulation of Cyclin D1 and Bcl-xL in NSCLC by single interference has been reported (18,25,26). We performed a combined intervention of the two genes, and the results demonstrated that the combined intervention was more effective in promoting cell apoptosis and reducing cell proliferation in the NSCLC A549 and NCI-H441 cell lines than single intervention.
Previous studies have confirmed that combinational interference with shRNA and chemo/radiation therapy may increase the efficacy of individual therapy (28,29). To increase the efficacy of shRNA therapy, combinational therapy (double or triple therapy) may be a novel strategy for cancer treatment (30,31).
In the present study, we first successfully constructed pcDNA6.2-GW/EmGFP-miR vectors expressing Bcl-xL shRNA, Cyclin D1 shRNA and Cyclin D1-Bcl-xL shRNA, respectively. The results indicated that Bcl-xL shRNA, Cyclin D1 shRNA and Cyclin D1-Bcl-xL shRNA efficiently inhibited the expression of the target genes, as demonstrated by the mRNA and protein levels shown in Figs. 2 and 3. The protein expression levels of Bcl-xL and Cyclin D1 were significantly reduced in the groups of Cyclin D1 shRNA, Bcl-xL shRNA and Cyclin D1-Bcl-xL in A549 and NCI-H441 cells compared with the blank group. The results of quantitative RT-PCR analysis of Bcl-xL and Cyclin D1 revealed mRNA variations that generally correlated with the Western blotting data in our study. RT-PCR and Western blot analysis further indicated that shRNA-transfected cells had successfully silenced the target gene.
Cyclin D1 shRNA transfection resulted in marked changes in the levels of proliferation and apoptosis in A549 and NCI-H441 cells. A number of gene therapy strategies targeting Cyclin D1 have been used in vitro and in vivo, and have been shown to suppress tumor growth and promote tumor apoptosis in lung cancer (32,33). Driscoll et al have reported that Cyclin D1 antisense oligonucleotide-transfected A549 and NCI-H441 cells exhibited a reduced growth rate with a range of 40–60% at 0–8 day growth curves, which was consistent with our results (32). However, Huang et al have reported cell proliferation assay analysis performed at 1-, 3-, 5- and 7-day time points (18). These authors found that cells transfected with Cyclin D1-targeted shRNA exhibited a significant decrease in cell proliferation only at the 7-day time point (P<0.05), and there was no significant difference at the 1-, 3- and 5-day time points (P>0.05). We analyzed the possible reasons for these differences. Firstly, the intervention methods utilized are different. Oligonucleotide transfection efficacy was higher than plasmid transfection efficacy. Secondly, cell proliferation and apoptosis were related to the time of interference. We detected the proliferation and apoptosis of lung cancer cells within 48 h following transfection.
Bcl-xL has been found to be overexpressed in various types of cancer, such as lung and prostate cancer (25,34). Kim et al have proven that Bcl-xL as an anti-apoptotic protein may result in a multiple drug-resistant phenotype (35). Antisense oligonucleotide-directed Bcl-xL has been shown to cause sensitization to chemotherapy and significant apoptosis in NSCLC (36,37). Lei et al have reported that inhibition of Bcl-xL small interfering RNA (shRNA) on the cisplatin (DDP)-resistant human lung adenocarcinoma cell line A549/DDP was 12.65–58.75% (25). It is thus further confirmed that Bcl-xL may be a potent intervention target in NSCLC.
Cyclin D1-Bcl-xL shRNA transfection was more effective in promoting cell apoptosis and reducing cell proliferation compared with single shRNA transfection. The inhibition rate of the combined intervention was less than the sum of the individual Cyclin D1 shRNA and Bcl-xL shRNA groups. However, apoptosis of the Cyclin D1-Bcl-xL shRNA group was 14.3% more than the sum of the Cyclin D1 shRNA (4.835%) and Bcl-xL shRNA (5.41%) in the A549 cell groups. A similar result was achieved in the NCI-H441 cells with Cyclin D1-Bcl-xL shRNA (15.5%), Bcl-xL shRNA (6.2%) and Cyclin D1 shRNA (5.7%). The change in apoptosis and growth suppression in the Cyclin D1-Bcl-xL shRNA group was not consistent. This inconsistency may be due to the fact that Cyclin D1 and Bcl-xL play roles in carcinogenesis through different molecular mechanisms with apoptosis inhibitors and cell-cycle regulators, respectively (7,23). A number of studies have reported that activation of the signal transducer and activator of transcription (STAT) 3, a potent transcription factor, is capable of suppressing apoptosis by mediating survival gene products including Bcl-xL and may lead to cell proliferation through its ability to induce the expression of Cyclin D1 in NSCLC (38–40). Combined interference using Cyclin D1 and Bcl-xL may affect STAT3 signaling, resulting in growth suppression that is less than the sum of single interference. Additionally, besides its role in cell cycle regulation, Cyclin D1 is intricately involved in the regulation of apoptosis. The effect of Cyclin D1 may be proapoptotic or antiapoptotic, depending on the different state of the cell (17,27). That may account for the combined interference using Cyclin D1 and Bcl-xL promoting apoptosis more effectively than the effect of the sum of single interference. Whether the two genes are capable of acting synergistically requires further investigation. Biliran et al have reported that the expression of cell survival proteins, such as bcl-xL, remained relatively high in Cyclin D1-overexpressing cells (27). Therefore, combined interference using Cyclin D1 and Bcl-xL may be more effective than targeted single interference.
In conclusion, we have shown that Cyclin D1 and Bcl-xL were overexpressed in NSCLC A549 and NCI-H441 cells, and that the molecular-targeted repression of the combined Cyclin D1 and Bcl-xL genes was a more effective therapeutic strategy for NSCLC than the down-regulation of either single gene in promoting cell apoptosis and reducing cell proliferation. Combined interference on Cyclin D1 and Bcl-xL may therefore be a potent target strategy in NSCLC therapy. Although our findings in vitro were only from two cell lines, these results may have significant implications. Further in vivo studies are required to elucidate whether combined interference of Cyclin D1 and Bcl-xL may also more effectively inhibit tumors. In future, for effective in vivo delivery, tumor specificity, TNM staging and non-specific immune responses should be overcome before this technology may be successfully used in clinical research.
Acknowledgments
This work was supported by the Ministry of Education Scientific Research Foundation for Returned Overseas Students (no. 20071108) and the Natural Science Foundation of Hubei Province (no. 2010CDB09304).
References
- 1.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review 1975–2007. Available at: http://seer.cancer.Gov/csr/1975-2007. [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Stinchcombe TE, Socinski MA. Current treatments for advanced stage none small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. doi: 10.1513/pats.200809-110LC. [DOI] [PubMed] [Google Scholar]
- 5.Smythe WR. Treatment of stage I non-small cell lung carcinoma. Chest. 2003;123:181S–187S. [PubMed] [Google Scholar]
- 6.Hirsh V. Systemic therapies in metastatic non-small-cell lung cancer with emphasis on targeted therapies: the rational approach. Curr Oncol. 2010;17:13–23. doi: 10.3747/co.v17i2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg AS, Weinberg RA. Control of the cell cycle and apoptosis. Eur J Cancer. 1999;35:531–539. [PubMed] [Google Scholar]
- 8.Buckley MF, Sweeney KJ, Hamilton JA, et al. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 9.Ramalingam S, Belani CP. Recent advances in targeted therapy for non-small cell lung cancer. Expert Opin Ther Targets. 2007;11:245–57. doi: 10.1517/14728222.11.2.245. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson M. Therapeutic potential of RNA interference. N Engl J Med. 2004;351:1772–1777. doi: 10.1056/NEJMra045004. [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 12.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 13.Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han EK, Ng SC, Arber N, Begemann M, Weinstein IB. Roles of cyclinD1 and related genes in growth inhibition, senescence and apoptosis. Apoptosis. 1999;4:213–219. doi: 10.1023/a:1009618824145. [DOI] [PubMed] [Google Scholar]
- 15.Gillett C, Fantl V, Smith R, et al. Amplification and overexpression of cyclinD1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- 16.Jiang W, Kahn SM, Tomita N, et al. Amplification and expression of the human cyclinD gene in esophageal cancer. Cancer Res. 1992;52:2980–2983. [PubMed] [Google Scholar]
- 17.Betticher DC, Heighway J, Hasleton PS, et al. Prognostic significance of CCND1 (cyclinD1) overexpression in primary resected non-small-cell lung cancer. Br J Cancer. 1996;73:294–300. doi: 10.1038/bjc.1996.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Hu YD, Li N, Zhu Y. Inhibition of tumor growth and metastasis by non-small cell lung cancer cells transfected with cyclin D1-targeted siRNA. Oligonucleotides. 2009;19:151–162. doi: 10.1089/oli.2008.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes DM, Gillett CE. Cyclin D1 in breast cancer. Breast Cancer Res Treat. 1998;52:1–15. doi: 10.1023/a:1006103831990. [DOI] [PubMed] [Google Scholar]
- 20.Bartkova J, Lukas J, Müller H, Lützhøft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- 21.Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res. 1997;57:1634–1637. [PubMed] [Google Scholar]
- 22.Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15:158–163. doi: 10.1016/s0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 23.Yin XM. Signal transduction mediated by Bid, apro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- 24.Karczmarek-Borowska B, Filip A, Wojcierowski J, Smolen A, Korobowicz E, Korszen-Pilecka I, Zdunek M. Estimation of prognostic value of Bcl-xL gene expression in non-small cell lung cancer. Lung Cancer. 2006;51:61–69. doi: 10.1016/j.lungcan.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Lei X, Huang Z, Zhong M, Zhu B, Tang S, Liao D. Bcl-xL small interfering RNA sensitizes cisplatin-resistant human lung adenocarcinoma cells. Acta Biochim Biophys Sin (Shanghai) 2007;39:344–350. doi: 10.1111/j.1745-7270.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 26.Sasazawa Y, Futamura Y, Tashiro E, Imoto M. Vacuolar H+-ATPase inhibitors overcome Bcl-xL-mediated chemoresistance through restoration of a caspase-independent apoptotic pathway. Cancer Sci. 2009;100:1460–1467. doi: 10.1111/j.1349-7006.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biliran H, Jr, Wang Y, Banerjee S, Xu H, Heng H, Thakur A, Bollig A, Sarkar FH, Liao JD. Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin Cancer Res. 2005;11:6075–6086. doi: 10.1158/1078-0432.CCR-04-2419. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Lin T, Gu J, Zhang L, Roth JA, Stephens LC, Yu Y, Liu J, Fang B. Combined TRAIL and Bax gene therapy prolonged survival in mice with ovarian cancer xenograft. Gene Ther. 2002;9:1379–1386. doi: 10.1038/sj.gt.3301810. [DOI] [PubMed] [Google Scholar]
- 29.Sledz CA, Williams BR. RNA interference and double stranded-RNA-activated pathways. Biochem Soc Trans. 2004;32:952–956. doi: 10.1042/BST0320952. [DOI] [PubMed] [Google Scholar]
- 30.Bueno MJ, Pérez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7:3143–3148. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 31.Jovanovic M, Hengartner MO. miRNA and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 32.Driscoll B, Wu L, Buckley S, Hall FL, Anderson KD, Warburton D. Cyclin D1 antisense RNA destabilizes pRb and retards lung cancer cell growth. Am J Physiol. 1997;273:L941–L949. doi: 10.1152/ajplung.1997.273.5.L941. [DOI] [PubMed] [Google Scholar]
- 33.Sauter ER, Herlyn M, Liu SC, Litwin S, Ridge JA. Prolonged response to antisense cyclin D1 in a human squamous cancer xenograft model. Clin Cancer Res. 2000;6:654–660. [PubMed] [Google Scholar]
- 34.Bruckheimer EM, Gjertsen BT, McDonnell TJ. Implications of cell death regulation in the pathogenesis and treatment of prostate cancer. Semin Oncol. 1999;26:382–398. [PubMed] [Google Scholar]
- 35.Kim IK, Jung YK, Noh DY, Song YS, Choi CH, Oh BH, Masuda ES, Jung YK. Functional screening of genes suppressing TRAIL-induced apoptosis: distinctin hibitory activities of Bcl-XL and Bcl-2. Br J Cancer. 2003;88:910–917. doi: 10.1038/sj.bjc.6600795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leech SH, Olie RA, Gautschi O, et al. Induction of apoptosis in lung cancer cells following bcl-xl antisense treatment. Int J Cancer. 2000;86:570–576. doi: 10.1002/(sici)1097-0215(20000515)86:4<570::aid-ijc20>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Yang D, Lippman ME. Targeting Bcl-2 and Bcl-XL with nonpeptidic small-molecule antagonists. Semin Oncol. 2003;30:133–142. doi: 10.1053/j.seminoncol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Zhang J, Wang L, Wei H, Tian Z. Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer. 2007;7:149. doi: 10.1186/1471-2407-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weerasinghe P, Garcia GE, Zhu Q, Yuan P, Feng L, Mao L, Jing N. Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. Int J Oncol. 2007;31:129–136. [PubMed] [Google Scholar]
- 40.Zhang X, Zhang J, Wei H, Tian Z. STAT3-decoy oligodeoxynucleotide inhibits the growth of human lung cancer via down-regulating its target genes. Oncol Rep. 2007;17:1377–1382. [PubMed] [Google Scholar]


