Abstract
The aim of this study was to evaluate the efficacy and safety of polyethylene glycol 4000 (PEG 4000) for the treatment of constipation in children over 8 years of age. A total of 216 children from 7 hospitals were enrolled. A total of 105 patients received oral PEG 4000 (20 g/day) and 111 patients received oral lactulose (15 ml/day) for 2 weeks. The stool frequency, stool consistency and abdominal pain of the patients were monitored. In the PEG group, following one week and two weeks of treatment, the median weekly stool frequency improved from 2 times prior to treatment to 6 and 7 times, respectively, following treatment. The clinical remission rates of the PEG and lactulose groups following one week of treatment were 70.48 and 39.64%, respectively, and following two weeks of treatment were 72.38 and 41.44%, respectively. Abdominal pain disappeared in 74.6% of patients following two weeks of PEG 4000 treatment. No significant clinical adverse effects or abnormalities in the laboratory tests were observed in the two treatment groups. In conclusion, PEG 4000 is a safe and more effective drug compared to lactulose for the treatment of constipation in children.
Keywords: constipation, polyethylene glycol 4000, lactulose, children
Introduction
Constipation is a common digestive disease in children, which accounts for 3–5% of general pediatric visits and up to 25% of children with gastroenterological disorders (1). Constipation in children is presented as a decrease in stool frequency, firm or hard stools, difficult and painful defecation and voluntary stool retention. Constipation affects the emotion, appetite and quality of life of afflicted children. Conventional treatments for constipation include bowel habit training, intake of fiber-enriched food, increased drinking of water and physical exercise, and the use of various laxatives and stool softeners (2). Polyethylene glycol 4000 (PEG 4000, Forlax) is a non-toxic, hydrosoluble, high-molecular polymer, which is not absorbed in the gastrointestinal tract following oral administration. PEG 4000 acts as an osmotic agent that increases fecal water content. A number of clinical studies have demonstrated that PEG 4000 is effective in the treatment of constipation in adults and children (3–7). In order to investigate the application of PEG 4000 in Chinese children, we conducted a multicenter study involving 216 children (8–18 years of age) suffering from constipation from 7 hospitals in China. This study aimed to investigate the efficacy and safety of PEG 4000 treatment compared to that of lactulose treatment.
Patients and methods
Patients
Patients were selected from 227 children (8–18 years of age) who visited pediatric clinics with symptoms of constipation at the 7 participating hospitals in China from July 2004 to March 2005. Subjects were selected based on: i) symptoms which consisted of weekly stool frequency of 2 or less and stool consistency type 1–3 (Bristol Stool Scale) for at least 2 weeks; ii) no presence of organic or systemic disease; iii) no initiation of any treatment for constipation or administration of drugs affecting gastrointestinal movements; iv) obtainment of informed consent by patients and parents or guardians. The procedures were approved by the Ethics Committee for Research and Education of the Fourth Military Medical University, Xi’an, China. Among the 227 patients, 11 cases were excluded due to lack of medical records. A total of 216 subjects of either gender were randomly assigned into two groups. The PEG 4000 treatment group consisted of 105 cases and the control lactulose treatment group consisted of 111 cases.
Methods
Treatments
Patients in the PEG 4000 treatment group received 20 g of PEG 4000 dissolved in a glass of water or drink each morning before breakfast for two weeks. Patients in the lactulose treatment group received 15 ml of lactulose (10 g) oral solution (Duphlac) per day after breakfast for the first 3 days, and then 10 ml (6.7 g) per day for the following 11 days.
Study design
A blind randomized study design was adopted to reduce possible deviation in the statistical testing. First, a biostatistician constructed random digit tables using statistical software SAS v8.2. Then PEG 4000 and lactulose were digitally labeled. The researchers in each study center received corresponding drugs from drug administrators according to the patient sequence.
Assessment of the treatments
The efficacy and safety of the PEG 4000 treatment were compared with the lactulose treatment according to the following primary and secondary parameters.
The examined primary efficacy parameters included weekly stool frequency and stool consistency. The stool consistency was classified into types according to the Bristol Stool Scale: 1, separate hard lumps; 2, sausage-shaped, but lumpy; 3, like a sausage but with cracks; 4, like sausage or snake, smooth and soft; 5, soft blobs with clear-cut edges; 6, fluffy pieces with ragged edges, a mushy stool; 7, watery without solid pieces.
The examined secondary efficacy parameters included the remission rate of abdominal pain and the rate of clinical remission. Clinical remission was achieved when the weekly stool frequency became >3 and stool consistency was normalized (Bristol type 4–6).
Safety of the treatments was evaluated by clinical observation and laboratory tests. Patients were monitored for adverse symptoms, including abdominal pain, diarrhea and blood in the stools. Laboratory tests, including full blood counts, urine tests, liver function tests (transaminases eg., AST and alkaline phosphatase), renal function (creatinine and blood urea nitrogen), blood glucose, and serum concentrations of sodium, potassium, calcium and phosphorus, were conducted. Blood pressure, pulse, height and weight were also monitored by physical examinations.
Statistical analysis
Data were analyzed by statistical software SAS v8.2. Differences in the stool frequencies between the two groups were examined by the Wilcoxon rank-sum test. The Cochran Mantel Haenszel χ2 test was used to compare the stool consistencies, the normal rate of stool frequency, and the normal rate of stool consistency. One-way ANOVA tests were used to compare the age, weight and height between the two groups, while the χ2 test was used to compare the gender proportion between the two groups.
The clinical remission rates and remission rates of abdominal pain were compared between the two group by using χ2 tests. Using descriptive statistics, a safety evaluation was made according to results from laboratory tests, physical examinations, vital signs and adverse events.
Results
Study population
As shown in Table I, there were no significant differences in the gender, age, weight and height of the patients between the PEG 4000 and the lactulose group.
Table I.
Comparison of the study population.
| Characteristics | PEG group (n=105) | Lactulose group (n=111) | Statistics | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 43 (40.95%) | 47 (42.34%) | χ2=0.043 | 0.8359 |
| Female | 62 (59.05%) | 64 (57.66%) | ||
| Age (years) | 11.29±2.80 | 11.20±2.75 | F=0.055 | 0.8149 |
| Weight (kg) | 36.23±11.65 | 35.74±10.99 | F=0.104 | 0.7477 |
| Height (cm) | 143.57±14.21 | 144.22±14.12 | F=0.115 | 0.7348 |
Effect of PEG 4000 and lactulose on stool frequency and stool consistency
Among the patients, the course of disease ranged from 2 to 887 weeks with a median of 104.36 weeks. Prior to treatment, the patients in the two groups had a median stool frequency of 2 per week. Upon PEG 4000 treatment, the median stool frequency significantly increased to 6 times per week following one week of treatment, and 7 times per week following two weeks of treatment. In the lactulose group, the median stool frequency following one week of treatment increased to 5 times per week, and following two weeks of treatment increased to 6 times per week. Statistical analyses demonstrated that the median stool frequency both one and two weeks following treatment in the PEG group was significantly higher than that in the lactulose group (Fig. 1).
Figure 1.
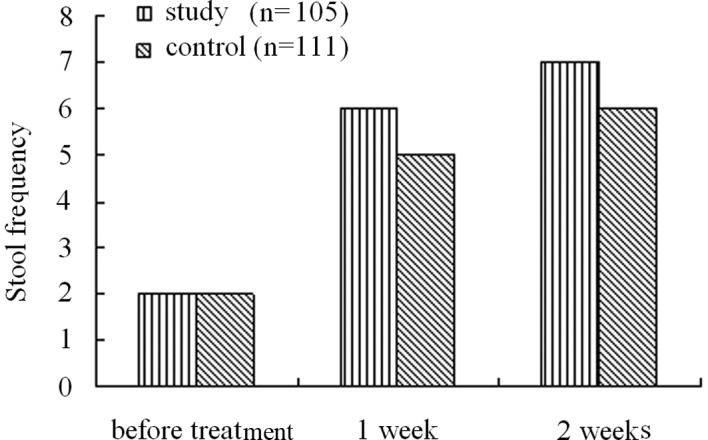
Effect of PEG 4000 and lactulose on stool frequency.
Prior to treatment, the stool consistencies of all enrolled patients ranged from type 1 to 3 on the Bristol Stool Scale. A total of 49.52% patients in the PEG group and 40.54% patients in the lactulose group were classified as Bristol type 2. There was no significant difference in the stool frequencies between the two groups prior to treatment. Treatment of PEG 4000 markedly increased the stool consistency to Bristol type 4.34±1.11 following one week of treatment and 4.26±0.89 following two weeks of treatment. Lactulose treatment also improved the stool consistency to 3.64±1.33 following one week of treatment and 3.63±1.33 following two weeks. However, the increase in stool consistency by lactulose was significantly less than that by the PEG 4000 treatment (Fig. 2).
Figure 2.
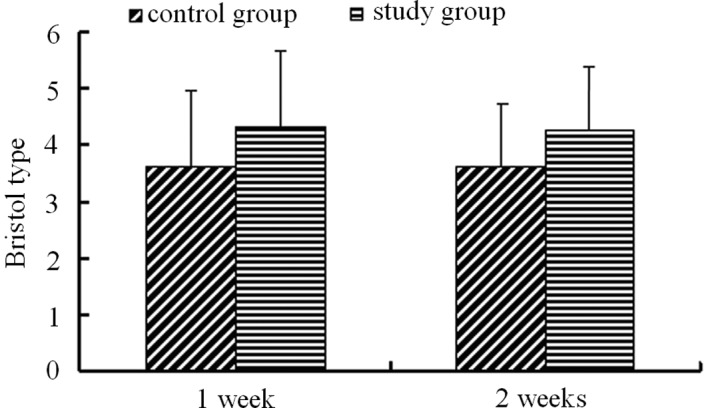
Effect of PEG 4000 and lactulose on stool consistency.
Effect of PEG 4000 and lactulose on secondary efficacy parameters
Clinical remission rate
Following one week of treatment, the clinical remission rate was 70.48% in the PEG group and 39.64% in the lactulose group. Following two weeks of treatment, the remission rate was 72.38% in the PEG group and 41.44% in the lactulose group. PEG 4000 treatment demonstrated a significantly higher remission rate than that of lactulose treatment (Fig. 3).
Figure 3.
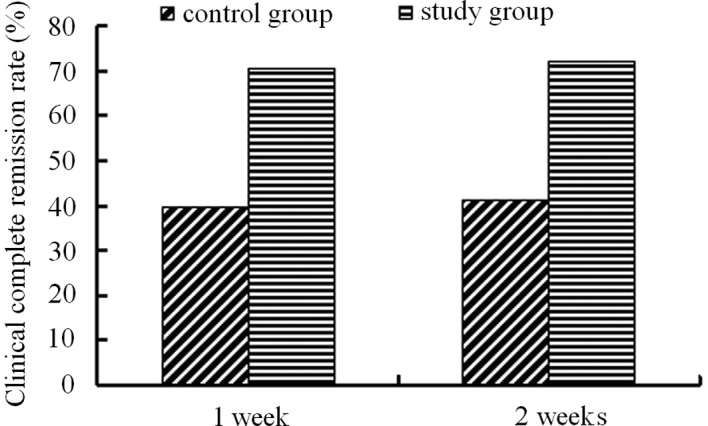
Clinical remission rates of children with constipation following PEG 4000 and lactulose treatment.
Remission rate of abdominal pain
Prior to treatment, among the 105 cases in the PEG group (60.0%), there were 63 patients associated with abdominal pain. While among the 111 patients in the lactulose group, 60 patients were associated with abdominal pain (54.1%). Following one week of treatment, the abdominal pain in 45 patients of the PEG group disappeared (71.43%), while 34 patients in the lactulose group described the disappearance of abdominal pain (56.67%). Following two weeks of treatment, the disappearance of abdominal pain was 74.60% (47 patients) in the PEG group and 56.67% (34 patients) in the lactulose group. Treatment with PEG 4000 resulted in increased abdominal pain remission than that of lactulose treatment (Fig. 4).
Figure 4.
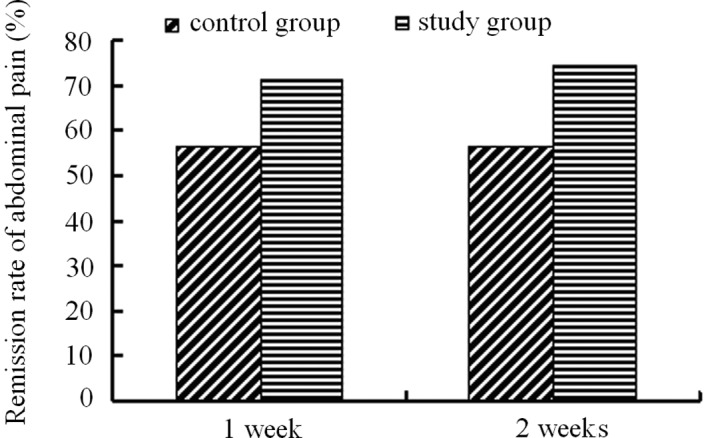
Remission rates of abdominal pain in children treated with PEG 4000 and lactulose.
Safety evaluation
Adverse effects
There were no significant adverse effects following the treatments of PEG 4000 and lactulose. In the PEG group, one patient developed diarrhea and another developed abdominal pain. Diarrhea may have been related to PEG 4000 treatment since it resolved following withdrawal of PEG 4000. There were no adverse effects in the lactulose group.
Laboratory tests
Serum concentrations of sodium, potassium, calcium and glucose were measured, and full blood counts, urine tests and blood tests for renal and liver function were performed before and after treatment. All patients in the study demonstrated normal results in the above tests before and after treatment, except one patient in the lactulose group who had a slightly increased level of AST (58 units/l). However, due to loss of contact following the two weeks of treatment, the liver function test on this patient was not followed up.
Physical examinations
No evident changes in blood pressure, pulse, height and weight or abnormalities following physical examinations were observed.
Discussion
Children with chronic constipation often receive long-term laxative treatment. Thus, a safe, effective and non-stimulant laxative is preferred. PEG 4000 is tasteless, non-toxic, hydrosoluble, non-absorbable and is not metabolized by colonic bacteria. It acts as a pure osmotic agent that adds moisture to stools to produce ‘hypocatharsis’. With the addition of favorable flavors, it is palatable and increases children compliance with the treatment. Lactulose is a synthetic disaccharide, which is non-absorbable, but is metabolized by colonic bacteria to produce gas, resulting in abdominal discomfort. Lactulose is well known as an effective laxative for the treatment of constipation in adults and children (8). PEG 4000 was first registered for clinical trials in China in 2003. The present study aimed to evaluate the safety and efficacy of PEG 4000 treatment compared to lactulose for the constipation of Chinese children over 8 years of age.
This randomized, controlled study was conducted in 7 hospitals in China. A total of 216 children with constipation were investigated, with 105 cases in the PEG group and 111 in the lactulose group. PEG 4000 and lactulose treatments significantly increased the weekly stool frequency and stool consistency of the patients. Following one week of treatment, the weekly stool frequencies in the PEG 4000 treatment group and lactulose treatment group achieved 6 and 5 times, respectively. Following two weeks of treatment, the stool frequencies further increased to 7 and 6 times, respectively. At the end of the treatment, the rate of normal stool consistency (Bristol type >3) reached 83.70% in the PEG group and 60.76% in the lactulose group. The clinical remission rates achieved 72.38% in the PEG group and 41.44% in the lactulose group. PEG 4000 and lactulose treatment were effective in the treatment of constipation in children, with significantly improved results for the PEG 4000 treatment.
PEG 4000 and lactulose treatment markedly reduced abdominal pain in children with constipation. Among the 216 cases, there were 123 patients with abdominal pain prior to treatment; 63 cases in the PEG group and 60 in the lactulose group. Following two weeks of treatment, the remission rates of the abdominal pain reached 74.60% in the PEG group and 56.67% in the lactulose group. The resolution of abdominal pain was markedly higher with PEG 4000 treatment than with lactulose treatment.
No significant clinical adverse effects or abnormalities in the laboratory tests were observed in the two treatment groups. The main adverse effects of PEG 4000 and lactulose were diarrhea, abdominal pain and abdominal distention. As PEG 4000 is not absorbed in the gastrointestinal tract it may not have systematic toxicity following oral administration. Thus, PEG 4000 is safe for the treatment of constipation in children (9,10).
In this study, we observed that each gram of PEG 4000 could retain 2.7 g of water (by means of hydrogen bonding). This feature of PEG 4000 leads to an increase in fecal water content, thus it acts as a stool softener facilitating defecation. Soft feces could be formed 24–48 h following treatment. A large number of children with constipation returned to normal defecation following 5 days of treatment. The majority of patients recovered from constipation following 2 to 3 weeks of therapy.
In conclusion, following two weeks of treatment, PEG 4000 and lactulose were effective and safe for the treatment of constipation in children. PEG 4000 therapy demonstrated a higher rate of success compared to lactulose treatment, thus suggesting that PEG 4000 is a promising new therapy for constipation in Chinese children.
Acknowledgments
The authors express their gratitude to Dr Michael Nowicki (University of Mississippi Medical Center, MS, USA) for his critiques, valuable suggestions and help in preparing the manuscript. This work was supported by the National Natural Science Foundation of China (30800417, 81170331).
References
- 1.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang MG, Wang BX. Initial therapy of constipation in children. J Appl Clin Pediatr. 2006;21:446–448. [Google Scholar]
- 3.Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in treatment of chronic nonorganic constipation. Dig Dis Sci. 1996;41:1636–1642. doi: 10.1007/BF02087913. [DOI] [PubMed] [Google Scholar]
- 4.Attar A, Lémann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999;44:226–230. doi: 10.1136/gut.44.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95:446–450. doi: 10.1111/j.1572-0241.2000.01765.x. [DOI] [PubMed] [Google Scholar]
- 6.Loening-Baucke V. Polyethylene glycol without electrolytes for children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 2002;34:372–377. doi: 10.1097/00005176-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Pashankar DS, Bishop WP. Efficacy and optimal dose of daily polyethylene glycol 3350 for treatment of constipation and encopresis in children. J Pediatr. 2001;139:428–432. doi: 10.1067/mpd.2001.117002. [DOI] [PubMed] [Google Scholar]
- 8.Freedman MD, Schwartz HJ, Roby R, Fleisher S. Tolerance and efficacy of polyethylene glycol 3350/electrolyte solution versus lactulose in relieving opiate induced constipation: a double-blinded placebo-controlled trial. J Clin Pharmacol. 1997;37:904–907. doi: 10.1002/j.1552-4604.1997.tb04264.x. [DOI] [PubMed] [Google Scholar]
- 9.Kinservik MA, Friedhoff MM. The efficacy and safety of polyethylene glycol 3350 in the treatment of constipation in children. Pediatr Nurs. 2004;30:232–237. [PubMed] [Google Scholar]
- 10.Pashankar DS, Loening-Baucke V, Bishop WP. Safety of polyethylene glycol 330 for the treatment of chronic constipation in children. Arch Pediatr Adolesc Med. 2003;155:661–664. doi: 10.1001/archpedi.157.7.661. [DOI] [PubMed] [Google Scholar]


