Abstract
The association between hepatocellular carcinoma (HCC) and the 61A>G polymorphism in the epidermal growth factor (EGF) gene has been analyzed in several studies, but results have been inconsistent. The aim of this study was to integrate previous findings and explore whether this polymorphism is associated with susceptibility to HCC. A meta-analysis was performed by searching PubMed, Web of Science, and Cochrane Library databases. Data were extracted using predefined form and pooled odds ratios (OR) with 95% confidence intervals (CI) and were calculated to evaluate the strength of this association. Five studies involving 690 cases, 514 healthy controls, and 1419 controls with cancer-free liver diseases were identified. On the basis of healthy controls, the significant main effects on HCC risk were observed in a heterozygote comparison (OR=1.76, 95% CI 1.07–2.90, p=0.02) and a dominant genetic model (OR=1.65, 95% CI 1.03–2.66, p=0.04). On the basis of the controls with cancer-free liver diseases, a significantly increased risk of HCC was found in all the genetic models. Subgroup analyses stratified by ethnicity and etiology of HCC also showed positive associations. The EGF 61G allele is a risk factor for developing HCC without the influence of ethnic and etiological diversity.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer worldwide and the third most frequent cause of cancer death. Half of these cases and deaths were estimated to occur in China (Jemal et al., 2011). Although hepatocarcinogenesis is usually attributed to chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, only a minority of those patients will develop HCC, suggesting that host genetic factors may play an important role in pathogenesis (Liu and Fan, 2007). Researchers argue that most population-attributable cancer heritability is related to polymorphic variations in the DNA sequence (Ponder, 2001).
Epidermal growth factor (EGF) results in cellular proliferation, differentiation, and tumorigenesis of epidermal and epithelial tissues by binding to its receptor (EGFR) and, hence, activating several signal pathways (Henson and Gibson, 2006). Mounting evidence supports a role for EGF in malignant transformation and tumor progression (Stoscheck and King, 1986; Harari et al., 2007; Limaye et al., 2008; Magnus et al., 2010; Morris et al., 2011; Miyake and Parsons, 2012). The EGF 61A>G polymorphism (rs4444903) is a commonly functional single-nucleotide polymorphism (SNP) in the 5′ untranslated region of the EGF gene, regulating EGF levels and effects on individual susceptibility to various carcinomas (Xu et al., 2010). The study by Shahbazi et al. (2002) first reported that this polymorphism was associated with increased EGF production and the risk of developing malignant melanoma. A recent meta-analysis by Zhang et al. (2010) was conducted to determine whether EGF 61A>G polymorphism alters cancer risk. Although they concluded that the EGF 61G allele is a risk factor of cancer, especially for gastric cancer and glioma, no study with regard to this polymorphism and HCC was included in their synthesis.
The association between EGF 61A>G polymorphism and the risk of HCC is still inconsistent and ambiguous, potentially due to the small number of relevant studies and relatively small sample size in single studies. Therefore, we performed a meta-analysis to derive a more precise estimation of the relationship between 61A>G polymorphism in EGF gene and HCC risk.
Materials and Methods
Study selection
We conducted an electronic search in the PubMed, Web of Science, and Cochrane Library, using the following terms “(epidermal growth factor OR EGF) AND (polymorphism OR genotype OR variant) AND (hepatocellular carcinoma OR liver cancer OR HCC)” for articles published from 1960 to October 2011. Reference lists of relevant publications were manually reviewed to identify additional studies.
Studies included in the current meta-analysis had to meet the following criteria: (1) evaluation of the correlation between HCC and EGF 61A>G polymorphism; (2) studies used a case-control design; (3) studies that are published as a full-text article in the English language. Major exclusion criteria were no control population, genotype frequency unavailable, and duplication of previous publications.
Data extraction
The bibliographic search and data extraction were independently conducted by two investigators (Z.Y. and Q.W.), and disagreements were resolved by consensus. Data abstracted from the studies included the first author's surname, year of publication, country of origin, ethnicity, genotyping methods, source of control, number of cases and controls, genotype frequencies in cases and controls, frequency of G allele, and Hardy–Weinberg equilibrium (HWE) of controls.
Statistical analysis
Odds ratio (OR) with a 95% confidence interval (CI) was used to assess the strength of association between the EGF 61A>G polymorphism and the risk of HCC in allelic contrast (G-allele vs. A-allele), homozygote comparison (GG vs. AA), heterozygote comparison (GA vs. AA), dominant genetic model (GG+GA vs. AA), and recessive genetic model (GG vs. GA+AA) (Shaik et al., 2009, 2011). The pooled ORs were calculated first according to two kinds of controls: healthy group and controls with cancer-free liver diseases. Subgroup analyses were done by ethnicity and etiology of HCC. Heterogeneity (Ph) among studies was checked by the χ2-based Q test. A p-value>0.10 for the Q-test indicates a lack of heterogeneity; then, the pooled OR estimate of each study was calculated by the fixed-effects model (Mantel–Haenszel method). Otherwise, the random-effects model (DerSimonian and Laird method) was used. We also used the statistic I2 to test the heterogeneity, with I2<25%, 25%–75% and >75% to represent low, moderate, and high degrees of inconsistency, respectively. HWE was assessed by Pearson χ2 test for goodness of fit, and p-value<0.05 was considered significant. Then, we performed a sensitivity analysis by excluding the studies in which the controls deviated significantly from HWE. Publication bias was examined with the Begg's test and Egger's test. All the analyses just listed were conducted using the software Reviewer Manager (version 5.0; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) and Stata (version 10.0; Stata Corporation, College Station, TX).
Results
Study characteristics
Our search identified five eligible studies involving 690 cases, 514 healthy controls, and 1419 controls with cancer-free liver diseases (Tanabe et al., 2008; Qi et al., 2009; Li et al., 2010; Abu Dayyeh et al., 2011; Chen et al., 2011). Tanabe's study sorted the data in American and French; therefore, each cohort in their study was separately considered for pooling subgroup analyses. The study characteristics are presented in Table 1. All studies indicated that the distribution of genotypes in the controls were in agreement with HWE. The subjects in two American studies were mixed populations, but the majority of them were white (Tanabe et al., 2008; Abu Dayyeh et al., 2011). So, we considered both studies and the French study as in the Caucasian category. Another three studies from China were of Asians with the unique etiology of HBV infection (Qi et al., 2009; Li et al., 2010; Chen et al., 2011). The two populations involved in Tanabe's study differed in the etiology of cirrhosis—predominantly HCV in the American patients and solely alcohol in the French patients (Tanabe et al., 2008).
Table 1.
Study Characteristics
| |
|
|
|
|
|
|
|
Cases |
Controls |
G allele frequency |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Year | Country | Ethnicity | Genotyping methods | Source of control | Case no. | Control no. | GG | GA | AA | GG | GA | AA | In control | HWE |
| Tanabe | 2008 | United States | Mixed | PCR-RFLP | Cirrhosis | 59 | 148 | 23 | 27 | 9 | 32 | 65 | 51 | 0.44 | 0.19 |
| France | Caucasian | PCR-RFLP | Alcoholic cirrhosis | 44 | 77 | 15 | 17 | 12 | 12 | 37 | 28 | 0.40 | 0.97 | ||
| Qi | 2009 | China | Asian | PCR-RFLP | Healthy/HBV infection | 215 | 208/172 | 102 | 98 | 15 | 104/78 | 84/76 | 20/18 | 0.70/0.67 | 0.61/0.94 |
| Li | 2010 | China | Asian | PCR-RFLP | Healthy/HBV cirrhosis | 186 | 186/152 | 96 | 82 | 8 | 96/65 | 73/72 | 17/15 | 0.71/0.66 | 0.56/0.44 |
| Chen | 2011 | China | Asian | PCR-RFLP | Healthy/HBV cirrhosis | 120 | 120/120 | 62 | 51 | 7 | 61/45 | 49/61 | 10/14 | 0.71/0.63 | 0.97/0.33 |
| Abu Dayyeh | 2011 | United States | Mixed | Real-time PCR | HCV infection | 66 | 750 | 26 | 25 | 15 | 178 | 350 | 222 | 0.47 | 0.08 |
PCR-RFLP, polymerase chain reaction–restriction fragment length polymorphism; HWE, Hardy–Weinberg equilibrium; HBV, hepatitis B virus; HCV, hepatitis C virus.
Quantitative synthesis
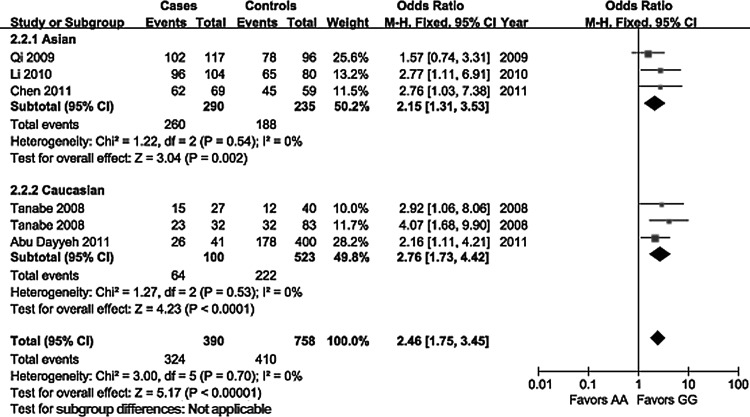
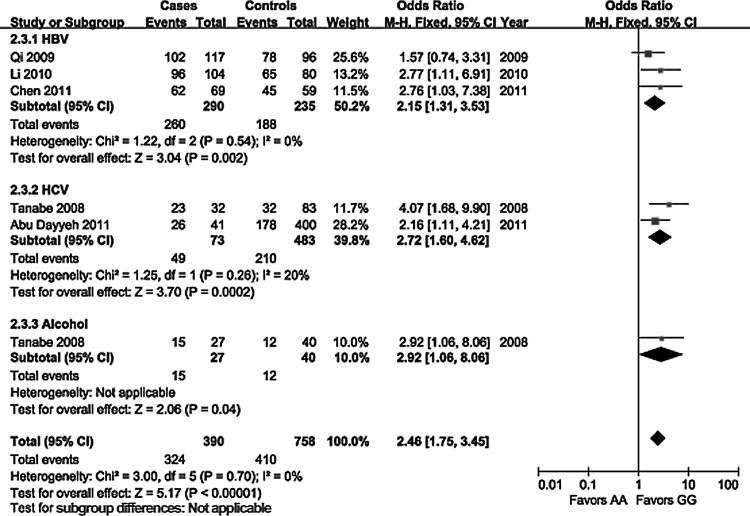
The evaluation of association between the EGF 61A>G polymorphism and the risk of HCC is displayed in Table 2. On the basis of healthy controls, significant main effects on HCC risk were observed in a heterozygote comparison (OR=1.76, 95% CI 1.07–2.90, p=0.02) and a dominant genetic model (OR=1.65, 95% CI 1.03–2.66, p=0.04), but no significant association was found in allelic contrast (OR=1.06, 95% CI 0.88–1.29, p=0.52), a homozygote comparison (OR=1.56, 95% CI 0.96–2.55, p=0.07), and a recessive genetic model (OR=0.97, 95% CI 0.76–1.23, p=0.78). On the basis of controls with cancer-free liver diseases, a significantly increased risk of HCC was found in all genetic models. Subgroup analyses stratified by ethnicity showed a consistently significant association without ethnic differences in all genetic models (Fig. 1), except for a heterozygote comparison in the Caucasian population (OR=1.35, 95% CI 0.87–2.11, p=0.18). In the subgroup analyses by etiology of HCC (Fig. 2), a significant association in patients with HBV infection was observed in all genetic models. No significant relationships were observed in patients with HCV infection under a heterozygote comparison, and in patients with alcoholic cirrhosis under a heterozygote comparison and a dominant genetic model. Our meta-analysis did not detect any significant heterogeneity among the studies according to the Q test.
Table 2.
Pooled Analyses of the Epidermal Growth Factor 61A>G Polymorphism on Hepatocellular Carcinoma Risk
| |
|
|
G-allele vs. A-allele |
GG vs. AA |
GA vs. AA |
GG+GA vs. AA |
GG vs. GA+AA |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Cases/Controls | OR (95% CI) | Ph | I2,% | p-Value | OR (95% CI) | Ph | I2,% | p-Value | OR (95% CI) | Ph | I2,% | p-Value | OR (95% CI) | Ph | I2,% | p-Value | OR (95% CI) | Ph | I2,% | p-Value | |
| Healthy controls | 3 | 521/514 | 1.06 (0.88–1.29) | 0.86 | 0 | 0.52 | 1.56 (0.96–2.55) | 0.70 | 0 | 0.07 | 1.76 (1.07–2.90) | 0.72 | 0 | 0.02 | 1.65 (1.03–2.66) | 0.70 | 0 | 0.04 | 0.97 (0.76–1.23) | 0.90 | 0 | 0.78 |
| Controls with liver diseases | 6 | 690/1419 | 1.49 (1.28–1.73) | 0.31 | 16 | <0.001 | 2.46 (1.75–3.45) | 0.70 | 0 | <0.001 | 1.51 (1.09–2.11) | 0.64 | 0 | 0.01 | 1.85 (1.35–2.52) | 0.71 | 0 | <0.001 | 1.59 (1.29–1.96) | 0.17 | 36 | <0.001 |
| Ethnicities | ||||||||||||||||||||||
| Asian | 3 | 521/444 | 1.33 (1.10–1.62) | 0.38 | 0 | 0.004 | 2.15 (1.31–3.53) | 0.54 | 0 | 0.002 | 1.74 (1.06–2.86) | 0.86 | 0 | 0.03 | 1.93 (1.20–3.12) | 0.72 | 0 | 0.007 | 1.35 (1.05–1.74) | 0.32 | 13 | 0.02 |
| Caucasian | 3 | 169/975 | 1.76 (1.38–2.26) | 0.61 | 0 | <0.001 | 2.76 (1.73–4.42) | 0.53 | 0 | <0.001 | 1.35 (0.87–2.11) | 0.29 | 19 | 0.18 | 1.79 (1.19–2.69) | 0.34 | 9 | 0.005 | 2.28 (1.58–3.30) | 0.85 | 0 | <0.001 |
| Etiologies | ||||||||||||||||||||||
| HBV | 3 | 521/444 | 1.33 (1.10–1.62) | 0.38 | 0 | 0.004 | 2.15 (1.31–3.53) | 0.54 | 0 | 0.002 | 1.74 (1.06–2.86) | 0.86 | 0 | 0.03 | 1.93 (1.20–3.12) | 0.72 | 0 | 0.007 | 1.35 (1.05–1.74) | 0.32 | 13 | 0.02 |
| HCV | 2 | 125/898 | 1.77 (1.34–2.34) | 0.32 | 0 | <0.001 | 2.72 (1.60–4.62) | 0.26 | 20 | <0.001 | 1.46 (0.87–2.44) | 0.14 | 54 | 0.15 | 1.88 (1.17–3.02) | 0.16 | 50 | 0.009 | 2.18 (1.45–3.27) | 0.81 | 0 | <0.001 |
| Alcohol | 1 | 44/77 | 1.75 (1.03–2.97) | NA | NA | 0.04 | 2.92 (1.06–8.06) | NA | NA | 0.04 | 1.07 (0.44–2.60) | NA | NA | 0.88 | 1.52 (0.68–3.42) | NA | NA | 0.31 | 2.80 (1.17–6.73) | NA | NA | 0.02 |
Ph, p-value of Q-test for heterogeneity test; NA, data not available; OR, odds ratio; CI, confidence interval.
FIG. 1.
Forest plot of hepatocellular carcinoma (HCC) risk associated with the epidermal growth factor (EGF) 61A>G polymorphism (GG vs. AA) stratified by ethnicity.
FIG. 2.
Forest plot of HCC risk associated with the EGF 61A>G polymorphism (GG vs. AA) stratified by etiology of HCC.
Sensitivity analysis and publication bias
We did not perform a sensitivity analysis due to the small number of included studies and no deviation from HWE in all the studies. The Begg's test and Egger's test were performed to access the publication bias of the literatures (Table 3). The results suggested that publication bias was not evident on the basis of healthy controls. However, in controls with liver diseases, an obvious evidence of publication bias was found for a recessive genetic model (Begg's test p=0.009, Egger's test p=0.023).
Table 3.
Publication Bias Tests
| Genetic model | Begg's test p-value | Egger's test p-value |
|---|---|---|
| Healthy controls | ||
| G-allele vs. A-allele | 1.000 | 0.676 |
| GG vs. AA | 1.000 | 0.725 |
| GA vs. AA | 1.000 | 0.902 |
| GG+GA vs. AA | 1.000 | 0.792 |
| GG vs. GA+AA | 0.296 | 0.415 |
| Controls with liver diseases | ||
| G-allele vs. A-allele | 0.024 | 0.055 |
| GG vs. AA | 0.452 | 0.175 |
| GA vs. AA | 0.452 | 0.279 |
| GG+GA vs. AA | 0.260 | 0.168 |
| GG vs. GA+AA | 0.009 | 0.023 |
Discussion
In this study, we investigated the association between the EGF 61A>G polymorphism and the risk of HCC. We found that the SNP involving an A-to-G mutation at position 61 of the 5′ untranslated region of the EGF gene could alter the expression of EGF and subsequently increase the risk of HCC, making it a potential predictive marker for clinical outcomes. This result may be biologically plausible. The overexpression of EGF is associated with the growth and invasion of malignant tumors via autocrine and paracrine pathways (Stoscheck and King, 1986; Normanno et al., 2001). Activation of the EGFR by EGF generates a cascade of intracellular signaling molecules, which is important for proliferation, differentiation, apoptosis, or anti-apoptosis (Grunwald and Hidalgo, 2003). It has been demonstrated that the EGFR system plays a critical role in the linkage of chronic liver injury to cancer under inflammatory stimulation (Berasain et al., 2009). Inhibitions of EGFR have become one of the logical strategies of anti-cancer (Grunwald and Hidalgo, 2003; Berasain et al., 2009).
Since Shahbazi et al. (2002) first identified the EGF 61A>G polymorphism and discovered that the GG genotype was associated with an increased risk of malignant melanoma compared with the AA genotype, many studies have been published that deal with the association between this SNP and different types of cancer, such as glioma (Tan et al., 2010), esophageal cancer (Lanuti et al., 2008), gastric cancer (Goto et al., 2005), breast cancer (Wang et al., 2008), lung cancer (Kang et al., 2007), and so on. Two meta-analyses published in the last year assessed the overall cancer risk influenced by the EGF 61A>G polymorphism (Xu et al., 2010; Zhang et al., 2010). However, one article did not include a study about HCC (Zhang et al., 2010), the other only contained the Tanabe study and another study in the Chinese language (Xu et al., 2010). The results of our meta-analysis strongly supported the conclusion that the EGF 61G allele is a risk factor for developing HCC according to the large sample size and the significant associations without any heterogeneity among the studies for all genetic models in controls with cancer-free liver diseases, for the dominant genetic model, and for a heterozygote comparison in healthy controls. Moreover, the associations were not affected by the ethnicity and etiology of HCC; whereas there are contradictory findings about ethnicity in other cancer types using this polymorphism (Tan et al., 2010).
There are still some limitations in this meta-analysis. First, the current results were based on unadjusted estimates, while a more precise analysis should be conducted if individual data were available for the adjustment by other covariates, including age, sex, family history, environmental factors, cancer stage, and lifestyle. Second, the interactions between gene-gene, gene-environment, and even different polymorphic loci of the same gene may modulate HCC risk (Tan et al., 2010). Third, our previous study design intended to pool the data about EGF serum level, which could not be accessed in most individual studies. Fourth, publication bias may exist in this meta-analysis, because non-significant or negative findings may be unpublished.
In conclusion, our meta-analysis suggests that the EGF 61A>G polymorphism is associated with an increased risk of HCC without the influence of ethnic and etiological diversity. Further well-designed large studies, particularly those assessing gene-gene and gene-environment interaction, are needed to confirm these findings. Such research may eventually lead to our better and comprehensive understanding of the association mechanism between the EGF 61A>G polymorphism and HCC risk.
Acknowledgments
This study was funded in part by the National Natural Science Foundation of China (grant number 81172062 and 81000988). The funding source had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation or review of the article; or the decision to submit the article for publication.
Author Disclosure Statement
No competing financial interests exist.
References
- Abu Dayyeh BK. Yang M. Fuchs BC, et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology. 2011;141:141–149. doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasain C. Perugorria MJ. Latasa MU, et al. The epidermal growth factor receptor: a link between inflammation and liver cancer. Exp Biol Med (Maywood) 2009;234:713–725. doi: 10.3181/0901-MR-12. [DOI] [PubMed] [Google Scholar]
- Chen K. Wei YG. Yang HT, et al. Epidermal growth factor +61 G/A polymorphism and the risk of hepatocellular carcinoma in a Chinese population. Genet Test Mol Biomarkers. 2011;15:251–255. doi: 10.1089/gtmb.2010.0208. [DOI] [PubMed] [Google Scholar]
- Goto Y. Ando T. Goto H, et al. No association between EGF gene polymorphism and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2454–2456. doi: 10.1158/1055-9965.EPI-05-0401. [DOI] [PubMed] [Google Scholar]
- Grunwald V. Hidalgo M. Developing inhibitors of the epidermal growth factor receptor for cancer treatment. J Natl Cancer Inst. 2003;95:851–867. doi: 10.1093/jnci/95.12.851. [DOI] [PubMed] [Google Scholar]
- Harari PM. Allen GW. Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- Henson ES. Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell Signal. 2006;18:2089–2097. doi: 10.1016/j.cellsig.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Jemal A. Bray F. Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kang HG. Choi JE. Lee WK, et al. +61A>G polymorphism in the EGF gene does not increase the risk of lung cancer. Respirology. 2007;12:902–905. doi: 10.1111/j.1440-1843.2007.01152.x. [DOI] [PubMed] [Google Scholar]
- Lanuti M. Liu G. Goodwin JM, et al. A functional epidermal growth factor (EGF) polymorphism, EGF serum levels, and esophageal adenocarcinoma risk and outcome. Clin Cancer Res. 2008;14:3216–3222. doi: 10.1158/1078-0432.CCR-07-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Xie Q. Lu F, et al. Association between epidermal growth factor 61A/G polymorphism and hepatocellular carcinoma susceptibility in Chinese patients. Liver Int. 2010;30:112–118. doi: 10.1111/j.1478-3231.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- Limaye PB. Bowen WC. Orr AV, et al. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Fan D. Hepatitis B in China. Lancet. 2007;369:1582–1583. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- Magnus N. Garnier D. Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010;116:815–818. doi: 10.1182/blood-2009-10-250639. [DOI] [PubMed] [Google Scholar]
- Miyake T. Parsons SJ. Functional interactions between Choline kinase α, epidermal growth factor receptor and c-Src in breast cancer cell proliferation. Oncogene. 2012;31:1431–1441. doi: 10.1038/onc.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LG. Taylor BS. Bivona TG, et al. Genomic dissection of the epidermal growth factor receptor (EGFR)/PI3K pathway reveals frequent deletion of the EGFR phosphatase PTPRS in head and neck cancers. Proc Natl Acad Sci U S A. 2011;108:19024–19029. doi: 10.1073/pnas.1111963108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N. Bianco C. De Luca A, et al. The role of EGF-related peptides in tumor growth. Front Biosci. 2001;6:685–707. doi: 10.2741/normano. [DOI] [PubMed] [Google Scholar]
- Ponder BA. Cancer genetics. Nature. 2001;411:336–341. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- Qi P. Wang H. Chen YM, et al. No association of EGF 5′UTR variant A61G and hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. Pathology. 2009;41:555–560. doi: 10.1080/00313020903071603. [DOI] [PubMed] [Google Scholar]
- Shahbazi M. Pravica V. Nasreen N, et al. Association between functional polymorphism in EGF gene and malignant melanoma. Lancet. 2002;359:397–401. doi: 10.1016/S0140-6736(02)07600-6. [DOI] [PubMed] [Google Scholar]
- Shaik AP. Jamil K. Das P. CYP1A1 polymorphisms and risk of prostate cancer: a meta-analysis. Urol J. 2009;6:78–86. [PubMed] [Google Scholar]
- Shaik AP. Sultana A. Bammidi VK, et al. A meta-analysis of eNOS and ACE gene polymorphisms and risk of pre-eclampsia in women. J Obstet Gynaecol. 2011;31:603–607. doi: 10.3109/01443615.2011.598971. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM. King LE., Jr. Role of epidermal growth factor in carcinogenesis. Cancer Res. 1986;46:1030–1037. [PubMed] [Google Scholar]
- Tan D. Xu J. Li Y, et al. Association between +61G polymorphism of the EGF gene and glioma risk in different ethnicities: a meta-analysis. Tohoku J Exp Med. 2010;222:229–235. doi: 10.1620/tjem.222.229. [DOI] [PubMed] [Google Scholar]
- Tanabe KK. Lemoine A. Finkelstein DM, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA. 2008;299:53–60. doi: 10.1001/jama.2007.65. [DOI] [PubMed] [Google Scholar]
- Wang Y. Tian T. Hu Z, et al. EGF promoter SNPs, plasma EGF levels and risk of breast cancer in Chinese women. Breast Cancer Res Treat. 2008;111:321–327. doi: 10.1007/s10549-007-9784-4. [DOI] [PubMed] [Google Scholar]
- Xu W. Li Y. Wang X, et al. Association between EGF promoter polymorphisms and cancer risk: a meta-analysis. Med Oncol. 2010;27:1389–1397. doi: 10.1007/s12032-009-9392-8. [DOI] [PubMed] [Google Scholar]
- Zhang YM. Cao C. Liang K. Genetic polymorphism of epidermal growth factor 61A>G and cancer risk: A meta-analysis. Cancer Epidemiol. 2010;34:150–156. doi: 10.1016/j.canep.2010.02.004. [DOI] [PubMed] [Google Scholar]