Abstract
In order to identify the antitumor effect of TAT-Apoptin on the human bladder cancer EJ cell line and study its impact on the expression of the apoptosis-related genes bax, bcl-2, caspase-3 and survivin, the MTT assay, real-time quantitative PCR and western blot analysis were used in this study. The results of the MTT assay indicated that TAT-Apoptin was able to inhibit the proliferation of EJ cells in a dose-dependent manner. The expression of Bax, Bcl-2, Caspase-3 and Survivin mRNA and protein following the treatment of the EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 24, 48 and 72 h was analyzed. Cell proliferation was significantly different after treatment with the various concentrations of TAT-Apoptin and the durations of treatment. The level of expression of Bcl-2 and Survivin in EJ cells decreased significantly, while that of Bax and Caspase-3 increased significantly at the mRNA and protein levels.
Keywords: TAT-Apoptin, EJ cell line, inhibition, apoptosis, expression
Introduction
Apoptin is a type of viral protein from the chicken anemia virus and is able to induce apoptosis in a variety of tumor cells, but not in normal cells. This makes it a candidate for the development of novel therapeutic strategies. Studies have revealed that Apoptin is able to kill numerous types of tumor cells, including hepatoma carcinoma cells (1–3), oral cancer cells (4) and ovarian cancer cells (5). However, the effect of this protein on bladder cancer cells remains unknown. This study attempts to identify the antitumor effect of Apoptin on the human bladder cancer EJ cell line and study its effect on the expression of the apoptosis-related genes bax, bcl-2, caspase-3 and survivin.
Materials and methods
Cell lines and TAT-Apoptin
The human bladder cancer EJ cell line was stored at the Yunnan Institute of Urology, Kunming, China. EJ cells were cultured in DMEM supplemented with 10% fetal bovine serum and incubated in 5% CO2/95% air at 37°C. The TAT-Apoptin protein was purified by Wuhan Huamei Biotech Co. (Wuhan, China).
Detection of the inhibition of EJ cell growth by the MTT method
The improved MTT method was used to study the effect of TAT-Apoptin on the growth of EJ cells (6). The cell concentration was adjusted to 5×104 cells/ml and the cells were added to a 96-well plate, at 90 μl/well. There were six concentrations of TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) and each concentration was set in three parallel wells with 10 μl/well. Control groups had 10 μl 10% (volume) serum-free medium 1640 added to them in place of TAT-Apoptin, while blank control groups were made by adding 100 μl of the above solution. The cells were then cultured at 37°C in a 5% (volume fraction) CO2 incubator and incubated for 72 h. MTT (5 mg/ml) was then added at 20 μl/well and the cells were cultured for 4 h. Following a period of 24, 48 or 72 h, cell viability was determined by a microplate reader at 5 nm single wavelength optical density (OD). The experiments were repeated three times. The inhibition rate of cell proliferation was calculated using the following formula (where A is the absorbance at 5 nm):
Total RNA isolation, real-time quantitative PCR (qPCR)
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. We synthesized first-strand cDNA in a volume of 20 μl using 1 μg of total RNA and TaqMan reverse transcription reagents (Applied Biosystems, Carlsbad, CA, USA). The target gene sequences were obtained from the National Center for Biotechnology Information GenBank databases. The RNA was quantified at 260 nm in a NanoDrop spectrophotometer at an OD 260/280 ratio of 1.7–2.0 for all samples. PCR was performed with an ABI PRISM 7000 (Applied Biosystems) and 2X qPCR MasterMix (Eurogentec, Seraing, Belgium) according to the manufacturer’s instructions. To quantify the target mRNA levels, bax, bcl-2, caspase-3 and survivin gene expression assays were used (Applied Biosystems). The primer sequences were: Bax (F), 5′-AAGAAGCTGAGCGAGTGT-3′; Bax (R), 5′-GGCGGCAATCATCCTCTG-3′; Bcl-2 (F), 5′-GAT GACTGAGTACCTGAA-3′; Bcl-2 (R), 5′-AGGAGAAAT CAAACAGAG-3′; Caspase-3 (F), 5′-TGACATCTCGGTCTG GTA-3′; Caspase-3 (R), 5′-AACATCACGCATCAATTCC-3′; Survivin (F), 5′-CGCTTTCCTTTCTGTCAA-3′; Survivin (R), 5′-ATTCTTTCTTCTTATTGTTGGTTT-3′. All primers spanned an intron to ensure discrimination between cDNA and genomic DNA. The relative amount of specific mRNA was normalized to GAPDH using the following primer sequences: GAPDH (F), 5′-CTCTGGTAAAGTGGATAT TGT-3′; GAPDH (R), 5′-GGTGGAATCATATTGGAACA-3′. All PCR assays were run in duplicate and performed with 40 cycles. A dilution series was carried out for each gene and the 18S ribosomal subunit was used as an internal control. Real-time qPCR analysis was performed using the 2−ΔΔCt method (7).
Detection of Bax, Bcl-2, Caspase-3 and Survivin protein expression by western blot analysis
Western blots of whole-cell lysates composed of a known number of cells were prepared. The whole-cell lysates were made by lysing cells in the buffer. Protein (50 mg) was separated by SDS-PAGE and transferred to membranes. Bax, Bcl-2, Caspase-3 and Survivin were detected in 100 μg of protein from the whole-cell extracts, according to the procedure described previously (8,9).
We loaded a total of 20 μg of the proteins and a standard prestained protein marker as a molecular weight control. We performed electrophoresis with a 5% stacking gel at 75 V and a 10% separating gel at a constant voltage of 90 V. The proteins were transferred to a PVDF membrane at 0.4 mA/cm for 2 h upon the completion of electrophoresis. We then incubated the transferred PVDF membrane in 5% skim milk/TBST blocking solution at room temperature for 1 h. We placed the membrane proteins upward in the primary antibody [Bax and Bcl-2 monoclonal antibodies were purchased from Epitomics, Inc. (Burlingame, CA, USA), 1063-1 and 1017-1; Caspase-3 and Survivin monoclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), sc-56047 and sc-17779] and incubation was carried out at room temperature for 2 h. The membrane was washed with PBST at room temperature, then agitated and decolorized using the decolorization shaker 4 times, for 8 min each time. The membrane was then developed with a chemiluminescent substrate (Invitrogen, WP20005). We captured the images using the gel imaging system, the A values of the bands were analyzed and the ratio of β-actin to the Bax, Bcl-2 Caspase-3 and Survivin proteins was calculated.
Statistical analysis
The data are shown as mean ± SD. A two-factor design analysis of variance (ANOVA) was used for the MTT assay and a one-factor ANOVA was used for the qPCR and western blot analysis. P<0.05 was considered to indicate a statistically significant result. All statistical analyses were performed using the SPSS 11.0 software for Windows.
Results
Growth inhibition effect of TAT-Apoptin on the EJ cell line
Following incubation with different concentrations of TAT-Apoptin for 24, 48 or 72 h, the proliferation of the EJ cell line was significantly inhibited in a concentration-dependent manner. The details are listed in Table 1.
Table I.
Growth inhibition rate of human bladder cancer EJ cells treated with modified TAT-Apoptin at different concentrations for various time periods.
| 24 h
|
48 h
|
72 h
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TAT-Apoptin (μg/ml) | OD mean ± SD | Inhibition rate (%) | P-value | OD mean ± SD | Inhibition rate (%) | P-value | OD mean ± SD | Inhibition rate (%) | P-value |
| 0 | 0.003±0.001 | 0 | - | 0.003±0.001 | 0 | - | 0.003±0.001 | 0 | - |
| 0.1 | 0.861±0.007 | 1.61±0.87 | 0.003 | 1.350±0.003 | 2.60±1.74 | 0.000 | 0.865±0.003 | 15.57±1.83 | 0.000 |
| 0.5 | 0.861±0.006 | 1.61±0.60 | 0.003 | 1.302±0.013 | 6.07±2.14 | 0.000 | 0.798±0.020 | 22.06±2.40 | 0.000 |
| 1 | 0.827±0.004 | 5.50±1.92 | 0.000 | 1.242±0.006 | 10.41±1.64 | 0.000 | 0.756±0.007 | 26.25±1.09 | 0.000 |
| 10 | 0.799±0.004 | 8.72±1.83 | 0.000 | 1.110±0.009 | 20.06±1.87 | 0.000 | 0.446±0.003 | 56.61±1.91 | 0.000 |
| 50 | 0.483±0.005 | 44.95±2.07 | 0.000 | 0.451±0.006 | 67.61±2.34 | 0.000 | 0.212±0.002 | 79.53±2.06 | 0.000 |
| 100 | 0.215±0.005 | 75.69±2.58 | 0.000 | 0.204±0.008 | 80.33±2.47 | 0.000 | 0.223±0.011 | 84.09±2.21 | 0.000 |
OD, optical density.
Effect of TAT-Apoptin on Bax, Bcl-2, Caspase-3 and Survivin mRNA expression
The RNA was quantified at an OD 260/280 ratio of 1.7 to 2.0 for all specimens. The expression of Bax, Bcl-2, Caspase-3 and Survivin was presented as 2−ΔΔCt. Bax, Bcl-2, Caspase-3 and Survivin mRNA expression at 24, 48 and 72 h after the addition of TAT-Apoptin was detected by qPCR.
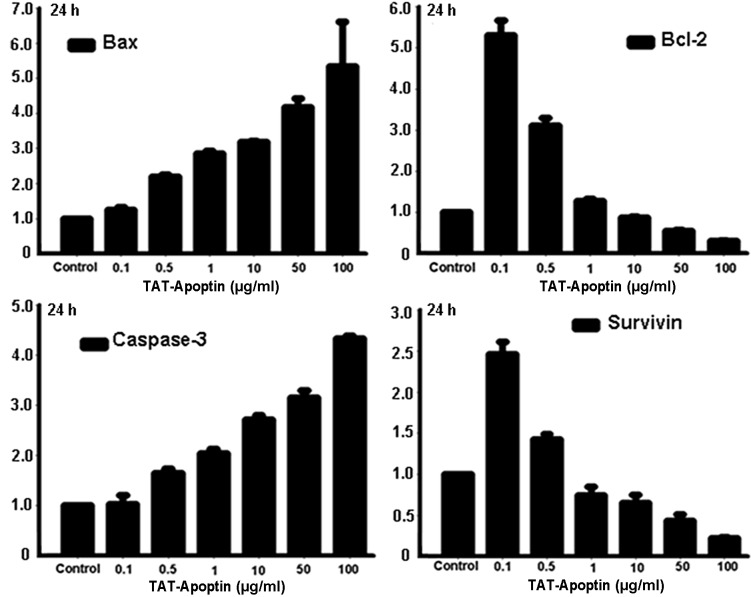
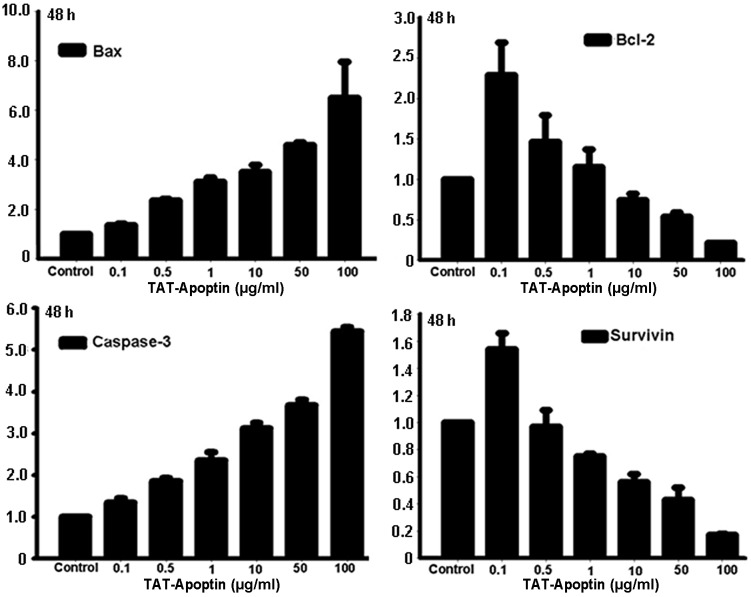
Following the treatment of the EJ cells with TAT-Apoptin for 24, 48 or 72 h, the expression level of Bcl-2 and Survivin mRNA gradually and significantly decreased and Bax and Caspase-3 mRNA levels gradually and significantly increased compared with the control group. The differences in Bax, Bcl-2, Caspase-3 and Survivin mRNA expression level at each concentration of TAT-Apoptin and for all treatment periods were statistically significant (P<0.001) and are shown in Figs. 1–3.
Figure 1.
Bax, Bcl-2, Caspase-3 and Survivin mRNA expression analysis following treatment of EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 24 h. The differences in expression after treatment with the various concentrations of TAT-Apoptin were calculated using the ANOVA method. The level of mRNA expression of Bcl-2 (F=392.011, P=0.000) and Survivin (F=217.929, P=0.000) in EJ cells was significantly decreased, while that of Bax (F=30.185, P=0.000) and Caspase-3 (F=385.565, P=0.000) increased significantly with increasing concentration of TAT-Apoptin. ANOVA, analysis of variance.
Figure 3.
Bax, Bcl-2, Caspase-3 and Survivin mRNA expression analysis following treatment of EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 72 h. The differences in expression after treatment with the various concentrations of TAT-Apoptin were calculated using the ANOVA method. The level of mRNA expression of Bcl-2 (F=72.065, P=0.000) and Survivin (F=70.933, P=0.000) in EJ cells was significantly decreased, while that of Bax (F=52.522, P=0.000) and Caspase-3 (F=663.839, P=0.000) increased significantly with increasing concentration of TAT-Apoptin. ANOVA, analysis of variance.
Effect of TAT-Apoptin on Bax, Bcl-2, Caspase-3 and Survivin protein expression
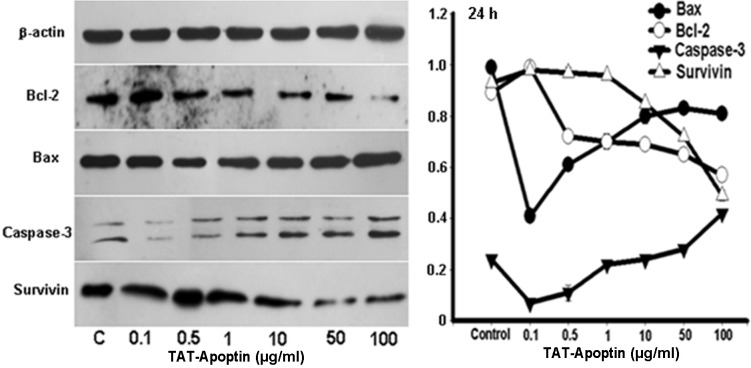
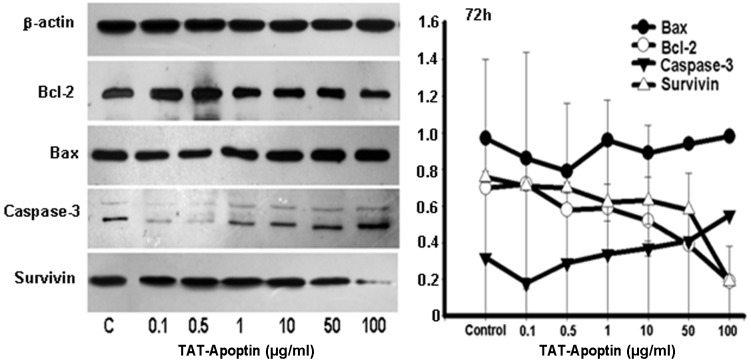
Following the treatment of the EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml), the protein expression level of Bcl-2 and Survivin gradually and significantly decreased and that of Bax and Caspase-3 gradually and significantly increased compared with the negative control group. The differences in the expression level of Bax, Bcl-2, Caspase-3 and Survivin proteins at each concentration of TAT-Apoptin and for all treatment periods were statistically significant (P<0.001) and are shown in Figs. 4–6.
Figure 4.
Expression levels of Bax, Bcl-2, Caspase-3 and Survivin proteins and the gray values of those proteins detected by western blot analysis. Bax, Bcl-2, Caspase-3 and Survivin protein expression analysis following treatment of EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 24 h. The protein expression level of Bcl-2 (F=280.348, P= 0.000) and Survivin (F=401.617, P= 0.000) in the EJ cells decreased significantly, while that of Bax (F=300.795, P=0.000) and Caspase-3 (F=207.562, P=0.000) increased significantly with increasing concentration of TAT-Apoptin.
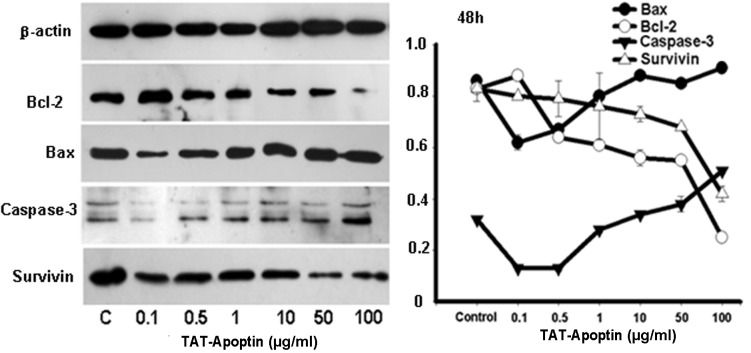
Figure 6.
Expression levels of Bax, Bcl-2, Caspase-3 and Survivin proteins and the gray values of those proteins detected by western blot analysis. Bax, Bcl-2, Caspase-3 and Survivin protein expression analysis following treatment of EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 72 h. The protein expression level of Bcl-2 (F=194.736, P=0.000) and Survivin (F=27.545, P=0.000) in the EJ cells decreased significantly, while that of Bax (F=39.569, P=0.000) and Caspase-3 (F=81.303, P=0.000) increased significantly with increasing concentration of TAT-Apoptin.
Discussion
Bladder cancer is a common type of genitourinary cancer and approximately 70% of cases are superficial (10,11). Currently, the main therapy used to treat bladder cancer is transurethral electroresection of bladder tumors (TURBT) or partial cystectomy. Following surgery, long-term intravesical instillation with chemotherapeutic drugs is often performed to inhibit the recurrence of the cancer. However, due to the natural or acquired drug resistance of tumor cells, the instillation of a single drug often fails to prevent recurrence. Although combined chemotherapy may achieve a better result than single drug instillation, the rate of recurrence remains high (11,12). Therefore, it is necessary to develop drugs with a novel antitumor mechanism.
Apoptin is a viral protein from the chicken anemia virus (CAV) which is able to induce apoptosis in tumor cells. This protein, with a molecular weight of 13,600 Da, is composed of 121 amino acids and contains 2 functional regions (13). In previous studies, the following merits of Apoptin in antitumor therapy were revealed: Apoptin is able to induce apoptosis in numerous types of malignant tumor cells (13); Apoptin is capable of killing tumor cells but not normal cells (4); Apoptin is able to induce high levels of apoptosis in tumor cells (14); the antitumor effect of Apoptin does not depend on the expression of p53 (15); Apoptin is able to induce high levels of apoptosis in tumor cells expressing the anti-apoptosis proteins Bcl-2 and Bcl-xL (16). This viral protein has been studied as a candidate for a novel antitumor drug.
In nature, tumors are heterogenous and during the process of malignant transformation possibly hundreds of genes are altered in expression in some way.
In this study, we conducted experiments on in vitro cultured EJ cells which were treated with TAT-Apoptin. The results of the MTT assay revealed that TAT-Apoptin was able to significantly inhibit the growth of the EJ cells. Following treatment with TAT-Apoptin for 24 h, 1 μg/ml TAT-Apoptin had a significant inhibitory effect on the EJ cells (P<0.001). In a certain range of concentration and time, TAT-Apoptin inhibited the proliferation of the EJ cells time- and dose-dependently. Its inhibitory effect was maximal at 100 μg/ml and 72 h. Using qPCR, the expression level of Bax, Bcl-2, Caspase-3 and Survivin mRNA was detected in the EJ cells. Following treatment with TAT-Apoptin, the level of expression of Bcl-2 and Survivin mRNA in EJ cells decreased significantly, while that of Bax and Caspase-3 mRNA increased significantly. Using western blot analysis, it was observed that, following treatment of the EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml), the expression level of the Bcl-2 and Survivin proteins decreased gradually, while that of the Bax and Caspase-3 proteins increased gradually.
In summary, TAT-Apoptin is able to inhibit the proliferation of the human bladder cancer EJ cell line, possibly by inducing apoptosis. TAT-Apoptin is capable of killing tumor cells through apoptosis. Consequently, this protein may provide a novel choice of therapy for multi-drug resistant bladder cancer and our study has laid a theoretical foundation for the choice of clinical drugs in the prevention and treatment of bladder cancer.
Figure 2.
Bax, Bcl-2, Caspase-3 and Survivin mRNA expression analysis following treatment of EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 48 h. The differences in expression after treatment with the various concentrations of TAT-Apoptin were calculated using the ANOVA method. The level of mRNA expression of Bcl-2 (F=30.181, P=0.000) and Survivin (F=102.542, P=0.000) in EJ cells was significantly decreased, while that of Bax (F=33.304, P=0.000) and Caspase-3 (F=464.560, P=0.000) increased significantly with increasing concentration of TAT-Apoptin. ANOVA, analysis of variance.
Figure 5.
Expression levels of Bax, Bcl-2, Caspase-3 and Survivin proteins and the gray values of those proteins detected by western blot analysis. Bax, Bcl-2, Caspase-3 and Survivin protein expression analysis following treatment of EJ cells with TAT-Apoptin (0.1, 0.5, 1, 10, 50 and 100 μg/ml) for 48 h. The protein expression level of Bcl-2 (F=550.113, P=0.000) and Survivin (F=16.364, P=0.000) in the EJ cells decreased significantly, while that of Bax (F=97.963, P=0.000) and Caspase-3 (F=311.731, P=0.000) increased significantly with increasing concentration of TAT-Apoptin.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (no. 30760251).
References
- 1.Han SX, Ma JL, Lu Y, Huang C, Duan KM. SP-TAT-Apoptin induces G1 arrest in HepG2 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:864–866. (In Chinese). [PubMed] [Google Scholar]
- 2.Han SX, Zhao J, Ma JL, et al. The effect of the fused gene of SP-TAT-Apoptin transfected by lentivirus on HepG2 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26:310–312. (In Chinese). [PubMed] [Google Scholar]
- 3.Han SX, Zhu Q, Ma JL, et al. Apoptin sensitizes radiation-induced cell death via classic mitochondrial, caspase and p53-dependent signaling in HepG2 cells. Mol Med Report. 2011;4:59–63. doi: 10.3892/mmr.2010.391. [DOI] [PubMed] [Google Scholar]
- 4.Schoop RA, Baatenburg de Jong RJ, Noteborn MH. Apoptin induces apoptosis in an oral cancer mouse model. Cancer Biol Ther. 2008;7:1368–1373. doi: 10.4161/cbt.7.9.6419. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Zhang Y. Apoptin gene transfer via modified wheat histone H4 facilitates apoptosis of human ovarian cancer cells. Cancer Biother Radiopharm. 2011;26:121–126. doi: 10.1089/cbr.2010.0858. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Yue X, Han J, Yang W. Improved MTT assay for activity of antitumor agents. Chin J Pharmaceut. 1993;24:455–457. [Google Scholar]
- 7.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 8.Calogero A, Arcella A, De Gregorio G, et al. The early growth response gene EGR-1 behaves as a suppressor gene that is down-regulated independent of ARF/Mdm2 but not p53 alterations in fresh human gliomas. Clin Cancer Res. 2001;7:2788–2796. [PubMed] [Google Scholar]
- 9.Baron V, Adamson ED, Calogero A, et al. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGF-β1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu FL. Changing constituents of genitourinary cancer in recent 50 years in Beijing. Chin Med J (Engl) 2003;116:1391–1393. [PubMed] [Google Scholar]
- 11.Pruthi RS, Baldwin N, Bhalani V, Wallen EM. Conservative management of low risk superficial bladder tumors. J Urol. 2008;179:87–90. doi: 10.1016/j.juro.2007.08.171. [DOI] [PubMed] [Google Scholar]
- 12.Schulze M, Stotz N, Rassweiler J. Retrospective analysis of transurethral resection, second-look resection, and long-term chemo-metaphylaxis for superficial bladder cancer: indications and efficacy of a differentiated approach. J Endourol. 2007;21:1533–1541. doi: 10.1089/end.2007.9866. [DOI] [PubMed] [Google Scholar]
- 13.Backendorf C, Visser AE, de Boer AG, et al. Apoptin: therapeutic potential of an early sensor of carcinogenic transformation. Annu Rev Pharmacol Toxicol. 2008;48:143–169. doi: 10.1146/annurev.pharmtox.48.121806.154910. [DOI] [PubMed] [Google Scholar]
- 14.Rohn JL, Noteborn MHM. The viral death effector Apoptin reveals tumor-specific processes. Apoptosis. 2004;9:315–322. doi: 10.1023/b:appt.0000025808.48885.9c. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang SM, Shvarts A, van Ormondt H, et al. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res. 1995;55:486–489. [PubMed] [Google Scholar]
- 16.Schoop RA, Kooistra K, Baatenburg de Jong RJ, Noteborn MHM. Bcl-xL inhibits p53-but not apoptin–induced apoptosis in head and neck squamous cell carcinoma cell line. Int J Cancer. 2004;109:38–42. doi: 10.1002/ijc.11675. [DOI] [PubMed] [Google Scholar]