Abstract
Crimean-Congo hemorrhagic fever (CCHF) is a disease prevalent among humans and animals and is endemic in Iran. Although CCHF has been reported in all of its neighboring provinces, in Mazandaran in northern Iran there have been no reports of any cases of human infection. This research has been carried out to clarify the epidemiological aspects of CCHF infection among sheep in various geographical regions of Mazandaran province. In this survey, 270 blood samples were collected from sheep in eastern, central, and western Mazandaran between 2010 and 2011, and the specific ELISA test for CCHF virus was carried out on the blood samples in the National Reference Laboratory in the Pasteur Institute, Tehran, Iran. The CCHF infection rate according to this study was 3.7%. A weak statistical relationship (p=0.063) was seen between the different geographical regions, with a gradual decrease in the infection rate noted, stretching from the eastern to the western portions of the province (eastern 6.8%, central 2.8%, and western 0%). Older sheep were 2.7 times more likely to be infected with the virus (OR 2.70; 95% CI 1.50,4.87; p<0.001). As the infection rate in Mazandaran is low among sheep, and as yet there have been no reports of human infection in this province, CCHF disease is not considered a serious health problem in Mazandaran. It is recommended that further research be carried out on other animals, high-risk human groups, and ticks, in order to more completely reveal the status of the disease in this province.
Key Words: Arbovirus, Crimean-Congo hemorrhagic fever virus, ELISA, Epidemiology, Zoonotic
Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a zoonotic disease caused by a Nairovirus belonging to the Bunyaviridae family, and transmitted by ticks (Anagnostou and Papa 2009). This disease is transmitted to humans by tick bites or direct contact with the blood or tissues of a viremic animal (Whitehouse 2004; Ergönül 2006). CCHF symptoms in humans include high fever, restlessness, severe headache, weariness, myalgia, stomach ache, nausea, vomiting, and diarrhea. At the next level of disease hemorrhage may also occur. The fatality rate of this disease in humans is reported to be between 10 and 50% (Ergönül 2006; Vorou et al. 2007).
The circulation of the virus in nature is enzootic, via tick-vertebrate-tick, and Hyalomma ticks are considered to be the most important transmitters and source of the virus, determining distribution worldwide (Charrel et al. 2004; Whitehouse 2004; Fisher-Hoch 2005; Ergönül 2006). A wide variety of domestic animals (e.g., sheep, cows, goats, and ostriches), as well as large wild herbivores, hares, and hedgehogs, can become infected with the virus, and these infections are usually asymptomatic and subclinical (Garcia et al. 2006; Chinikar et al. 2010a). Sheep are considered the most important domestic host for the virus in nature. Livestock and other hosts can transmit CCHFV to humans during the viremic period (Papa et al. 2002; Chinikar et al. 2008).
CCHF is endemic in some parts of Africa, Asia, and Europe. In recent years, cases of human infection have increased, and have been reported from endemic countries such as Kenya, Mauritania, Senegal, South Africa, Kosovo, Albania, Bulgaria, Greece, Russia, Georgia, Tajikistan, Turkey, Iran, Afghanistan, and Pakistan (Leblebicioglu 2010). The existence of antibodies against CCHF among domestic animals (sheep, cows, and camels), and in wild animals in Iran, was first reported in 1970 by Chumakov and Smirnova (1972). Until 1999 there had been no clinical reports of the disease in Iran, but in that year, new cases of human infection were reported in Shahrekord City (in Char Mahalo Bakhtiari, central Iran), and subsequently in other provinces in Iran (Chinikar et al. 2002, 2005). Cases of human infection have been reported in 25 of a total of 30 provinces in Iran, including Sistan and Baluchestan, and Isfahan, Fars, Khorasan, and Tehran have a high human infection rate (Chinikar et al. 2010b).
CCHF is currently endemic in Iran. Despite the fact that CCHF was reported in neighboring provinces, as yet there have been no reports of any cases of human infection in Mazandaran, while neighboring provinces (Tehran and Golestan) were among the most highly reported provinces (Chinikar et al. 2010b). With respect to such evidence, and taking into consideration that the existence of Hyalomma ticks has been reported in various regions of Mazandaran (Nabian et al. 2007; Razmi et al. 2007; Youssefi et al. 2008), this research has been carried out as a seroepidemiological survey of CCHF among sheep in different geographical regions, in order to clarify the epidemiological aspects of the disease.
Materials and Methods
This study was carried out in the province of Mazandaran, northern Iran. Mazandaran has a geographical area of approximately 460,456 km2, and is situated with the Caspian Sea to its north and the Alborz Mountains to the south. The province enjoys a moderate, semi-tropical climate, with average temperatures of 25°C in the summer and 8°C in the winter. The province also enjoys a quasi-Mediterranean climate. Annual rainfall averages 650 mm in eastern Mazandaran, and the province has more than 2,106,300 sheep, 232,700 goats, 7500 buffalo, 21,800 horses, and 886,800 head of cattle (Iranian Veterinary Organization, unpublished data). In this study, sheep blood samples were collected from Ramsar and Chaloos in the western region, Noor and Babol in the central region, and Sari, Savad Kooh, Jooybar, and Ghaemshahr in the eastern region of Mazandaran. The sheep were selected from flocks that did not have any animals imported from neighboring provinces in the previous year. This survey was carried out between 2010 and 2011. In all, 270 blood samples were taken from the jugular veins of the sheep, and their history, including age, gender, and area of habitat was recorded. The samples were immediately taken to the laboratory and their serum extracted. Each blood sample was placed in a micro-tube and kept in the Pasteur Institute in Tehran, Iran, at a temperature below 20°C until analysis.
The serum samples were analyzed by specific ELISA for IgG detection. IgG detection involved coating the ELISA plates with mouse hyper-immune ascitic fluid (diluted at 1:1000) in phosphate-buffered saline (PBS 1×), and incubating them overnight at 4°C. Following the washing step, the native or recombinant antigen (diluted at 1:500) in PBS containing 0.5% Tween (PBST), and 3% skim milk (PBSTM), was added, and the plates were incubated for 3 h at 37°C. Serum diluted at 1:100 in PBSTM was added and the plates were incubated for 1 h at 37°C. Peroxidase-labeled anti-animal immunoglobulin diluted at 1:1000 in PBSTM was added and the plates were incubated for 1 h at 37°C. The plates were washed 3 times with PBST after each incubation. Finally, hydrogen peroxide and 3,3′,5,5′-tetramethyl benzidine (TMB) was added and the plates were incubated for 15 min at room temperature. The enzymatic reaction was stopped by the addition of 4 N sulfuric acid. The plates were read by an ELISA reader (Anathos 2020; Biochrom Ltd., Cambridge, U.K.) at 450 nm (Garcia et al. 2006; Chinikar et al. 2008, 2009).
Data were analyzed with SPSS software, v. 16. We used chi-square testing for the comparison of variables in the analysis, and regression logistic analysis for analyzing the effects of different variables on the disease. p Values<0.05 were considered significant.
Results
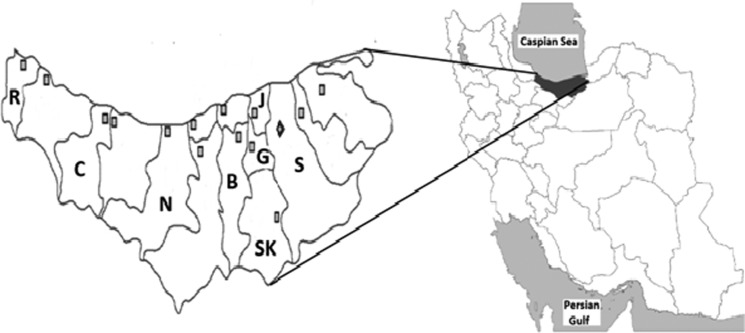
In this study, serum from 270 sheep, from three regions of Mazandaran, including eastern (Sari, Savad Kooh, Jooybar, and Ghaemshahr; n=125), western (Ramsar and Chaloos; n=73), and central (Babol and Noor; n=72), and from both males (n=25), and females (n=254) were collected (Fig. 1). The CCHF infection rate according to this study was reported at 3.7%.
FIG. 1.
Mazandaran province is shown in dark gray on the large map. Sampling was done in Ramsar (R), Chaloos (C), Noor (N), Babol (B), Savad Kooh (SK), Sari (S), Jooybar (J), and Ghaemshahr (G) townships.
A chi-square test showed a weak statistical relationship (p=0.063) among the different geographical areas of the study, as there was a gradual decrease in the infection rate going from the eastern to the western parts of the province (eastern 6.8%, central 2.8%, and western 0%). The most highly infected areas were Ghaemshahr (10%) and Savad Kooh (9.1%), with no infection found in Chaloos and Ramsar (Table 1).
Table 1.
The Infection Rate of Crimean-Congo Hemorrhagic Fever Among Sheep of Mazandaran by District and Region
| Region | Number tested (percent infected) | District name | Number tested (percent infected) |
|---|---|---|---|
| Western | 73 (0%) | Ramsar | 38 (0%) |
| Central | 72 (2.8%) | Chaloos | 35 (0%) |
| Eastern | 125 (6.4%) | Noor | 31 (3.2%) |
| Babol | 41 (2.4%) | ||
| Sari | 51 (3.9%) | ||
| Savad Kooh | 44 (9.1%) | ||
| Jooybar | 20 (5%) | ||
| Ghaemshahr | 10 (10%) | ||
| Total | 270 (3.7%) |
Regression logistic analysis showed that a 1-year increase in the age of a sheep increased the risk of infection 2.7 times (OR 2.70; 95% CI 1.50,4.87; p<0.001).
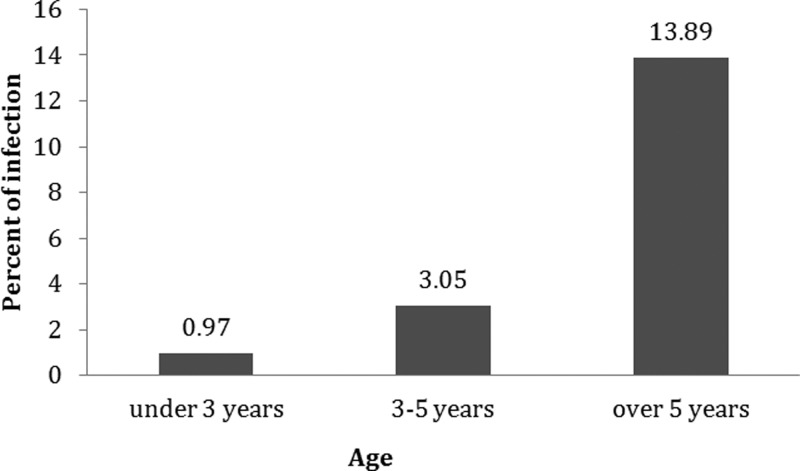
Figure 2 shows that the infection rate was highest in animals older than 5 y (13.89%), and was lowest in animals aged 3 y or under (0.97%).
FIG. 2.
Distribution of Crimean-Congo hemorrhagic fever infection in the different age groups.
In this research no significant relationship between gender and infection rate was found.
Discussion
In this study 3.7% of sheep had a history of CCHF infection. Compared to similar research carried out in other regions of Iran, this is a lower infection rate. For example, the results of two such studies of sheep in Isfahan province were 54.2% of 170 sheep and 76.6% of 286 sheep (Izadi et al. 2004; Ataei et al. 2008). Another study carried out in Ardabil province found an infection rate of 39% in a livestock sample of 56 animals (Telmadarraiy et al. 2010), and a study in northeast Iran found the rate of infection of 286 sheep to be 77.5%, and 150 goats to be 46% (Bokaie et al. 2008). The results of another study from the district of Bahar in the province of Hamadan showed that 27.8% of a livestock sample of 58 animals had IgG antibodies in their serum (Telmadarraiy et al. 2008).
As the infection rate among sheep in Mazandaran is low in all parts of the province, and as yet there are no reports of human infection in this region, it would appear that CCHF is not a serious health problem in this province.
In this survey, the infection rate increased with increasing age. The highest infection rate was seen among sheep older than 5 y (13.89%). As IgG antibody remains in an animal's body for at least 4–5 y, a higher rate of infection was expected in older animals, and this corresponds to other research findings (Wilson et al. 1990). For example, a study carried out in Egypt on 1022 sheep showed that the probability of infection significantly increases with increasing age (Mohamed et al. 2008).
As the infection rate increases from west to east, the theory that the infection is spreading from the eastern boundaries is highly likely, and mirrors findings in similar studies (Bokaie et al. 2008). One aspect influencing the findings is that the eastern part of the province is on the border with the province of Golestan, which has a high infection rate of CCHF.
Although there was no significant relationship between gender and infection rate, which also reflects the findings of similar studies (Bokaie et al. 2008), the lower number of males (25) compared to females (245) was problematic for comparing the infection rate between the genders.
It is recommended that further studies be carried out on additional livestock, high-risk groups of humans, and ticks, to determine the CCHF disease status in Mazandaran. It is also suggested that a similar study be carried out on other northern neighboring provinces (Golestan and Gilan), to clarify the epidemiological features of CCHF disease in the provinces bordering the Caspian Sea.
Acknowledgments
We appreciate the financial support of the research committee of the Pasteur Institute in Tehran (grant no. 1548), and wish to thank Mr. Hadi Mahmoudi, D.V.M., who helped us with the sampling process.
References
- Anagnostou V. Papa A. Evolution of Crimean-Congo hemorrhagic fever virus infection. Genet Evolution. 2009;9:948–954. doi: 10.1016/j.meegid.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Ataei B Touluei HR et al. Seroepidemiology of Crimean-Congo hemorrhagic fever in the local and imported sheep in Isfahan province, Iran, 2002. Iranian J Clin Infect Dis. 2008;1:1. [Google Scholar]
- Bokaie S Mostafavi E et al. Crimean Congo Hemorrhagic fever in northeast of Iran. J Animal Veterinary Adv. 2008;7:354–361. [Google Scholar]
- Charrel R Attoui H et al. Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 2004;10:1040–1055. doi: 10.1111/j.1469-0691.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- Chinikar S Fayaz A et al. The specific serological investigation of suspected humans and domestic animals to have Crimean-Congo Hemorrhagic Fever in various parts of Iran using ELISA techniques. Hakim. 2002;4:294–300. [Google Scholar]
- Chinikar S Ghiasi S et al. Crimean-Congo hemorrhagic fever in Iran and neighboring countries. J Clin Virol. 2010a;47:110–114. doi: 10.1016/j.jcv.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Chinikar S Ghiasi S et al. Situation of Crimean-Congo Hemorrhagic Fever in Iran and region countries. Arbo-Zoonet Newsletter. 2009;02:7. [Google Scholar]
- Chinikar S Goya M et al. Surveillance and laboratory detection system of Crimean-Congo Haemorrhagic fever in Iran. Transboundary Emerg Dis. 2008;55:200–204. doi: 10.1111/j.1865-1682.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- Chinikar S Mazaheri V et al. A serological survey in suspected human patients of Crimean-Congo hemorrhagic fever in Iran by determination of IgM specific ELISA method during 2000–2004. Arch Iran Med. 2005;8:52–55. [Google Scholar]
- Chinikar S Mojtaba GS et al. Phylogenetic analysis in a recent controlled outbreak of Crimean-Congo haemorrhagic fever in the south of Iran, December 2008. Euro Surveill. 2010b;15:47. doi: 10.2807/ese.15.47.19720-en. [DOI] [PubMed] [Google Scholar]
- Chumakov M. Smirnova S. Detection of antibodies to CHF virus in wild and domestic animal blood sera from Iran and Africa. Inst Polio Viral Encephalitis Moscow. 1972:367–368. [Google Scholar]
- Ergönül Ö. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Hoch SP. Lessons from nosocomial viral haemorrhagic fever outbreaks. Br Med Bull. 2005;73:123. doi: 10.1093/bmb/ldh054. [DOI] [PubMed] [Google Scholar]
- Garcia S Chinikar S et al. Evaluation of a Crimean-Congo hemorrhagic fever virus recombinant antigen expressed by Semliki Forest suicide virus for IgM and IgG antibody detection in human and animal sera collected in Iran. J Clin Virol. 2006;35:154–159. doi: 10.1016/j.jcv.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Izadi S Naieni KH et al. Crimean-Congo hemorrhagic fever in Sistan and Baluchestan Province of Iran, a case-control study on epidemiological characteristics. Int J Infect Dis. 2004;8:299–306. doi: 10.1016/j.ijid.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Leblebicioglu H. Crimean-Congo haemorrhagic fever in Eurasia. Int J Antimicrob Agents. 2010;36:S43–S46. doi: 10.1016/j.ijantimicag.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Mohamed M Said AR et al. A serological survey of Crimean-Congo haemorrhagic fever in animals in the Sharkia Governorate of Egypt. Veterinaria Italiana. 2008;44:513–517. [PubMed] [Google Scholar]
- Nabian S Rahbari S et al. Current status of tick fauna in North of Iran. Iranian J Parasitol. 2007;2:1. [Google Scholar]
- Papa A Bozovic B et al. Genetic detection and isolation of Crimean-Congo hemorrhagic fever virus, Kosovo, Yugoslavia. Emerg Infect Dis. 2002;8:852. doi: 10.3201/eid0808.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmi GR Glinsharifodini M et al. Prevalence of ixodid ticks on cattle in Mazandaran province, Iran. Korean J Parasitol. 2007;45:307. doi: 10.3347/kjp.2007.45.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telmadarraiy Z Ghiasi SM et al. A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand J Infect Dis. 2010;42:137–141. doi: 10.3109/00365540903362501. [DOI] [PubMed] [Google Scholar]
- Telmadarraiy Z Moradi A et al. Crimean-Congo hemorrhagic fever: a seroepidemiological and molecular survey in Bahar, Hamadan province of Iran. Asian J Anim Vet Adv. 2008;3:321–327. [Google Scholar]
- Vorou R Pierroutsakos IN et al. Crimean-Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495. doi: 10.1097/QCO.0b013e3282a56a0a. [DOI] [PubMed] [Google Scholar]
- Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Wilson ML LeGuenno B et al. Distribution of Crimean-Congo hemorrhagic fever viral antibody in Senegal: environmental and vectorial correlates. Am J Trop Med Hyg. 1990;43:557–566. doi: 10.4269/ajtmh.1990.43.557. [DOI] [PubMed] [Google Scholar]
- Youssefi M Keighobadi M et al. Ixodid tick species infesting sheep and cattle in Kelardasht Part (Chalóos), Iran. J Entomol. 2008;5:56–58. [Google Scholar]