Abstract
Germline-competent rat embryonic stem (ES) cell lines are important resources for the creation of mutant rat models using ES-cell-based gene targeting technology. The ability to isolate germline-competent ES cell lines from any rat strain, including genetically modified strains, would allow for more sophisticated genetic manipulations without extensive breeding. Sprague Dawley (SD) males carrying an enhanced green fluorescent protein (EGFP) transgene were used as the founder animals for the derivation of ES cell lines. A number of ES cell lines were established and subjected to rigorous quality control testing that included assessment of pluripotency factor expression, karyotype analysis, and pathogen/sterility testing. Two male ES cell lines, SD-Tg.EC1/Rrrc and SD-Tg.EC8/Rrrc, were injected into blastocysts recovered from a cross of Dark Agouti (DA) males with SD females. Resulting chimeric animals were bred with wild-type SD mates to verify the germline transmissibility of the ES cell lines by identifying pups carrying the ES cell line–derived EGFP transgene. While both ES cell lines gave rise to chimeric animals, only SD-Tg.EC1 was germline competent. This confirms the feasibility of deriving germline-competent ES cell lines from transgenic rat strains and provides a novel ES cell line with a stable green fluorescent protein (GFP) reporter for future genetic manipulations to create new rat models.
Introduction
The rat is an essential animal model of human health and diseases and has traditionally been the preferred model over mice in many areas of biomedical research, such as physiology, toxicology, behavioral, and cardiovascular research [1–3]. However, mouse models have gained popularity over rats as a preferred animal model in the last 2 decades due to the inability to genetically modify the rat genome in the sophisticated ways that are possible in the mouse.
Previously, the creation of knockout rats depended upon random mutagenesis approaches: chemical mutagenesis using N-ethyl-N-nitrosourea and transposon-mediated insertional mutagenesis [4,5]. Recently, a number of new technologies have been developed for the generation of rat models with targeted mutations. The establishment of germline-competent rat embryonic stem (ES) cell lines enables ES-cell-based gene targeting in rats [6–10]. Zinc-finger nuclease and transcription activator-like effector nuclease-mediated gene targeting enables generation of knockout rats through pronuclear injection [11,12]. As a result, the ability to generate mutant rats is rapidly increasing.
ES-cell-based genetic modification has been proven to be an effective method for the production of mutant animal models, especially for the production of models with complicated design, such as conditional or inducible knockouts [6,13]. Currently, several groups have reported the derivation of rat ES cells from different strains but only a subset of these have been proven to be germline competent [13]. The ability to isolate new germline-competent rat ES cell lines from a wide variety of rat strains, including those that already carry genetic modifications will be critical for increasing the utility of rat ES cells for genetic manipulation of the rat genome using many of the targeting strategies for creating mouse models.
The standard procedure to assess germline competence involves injection of ES cells into host blastocysts to make chimeric animals. Chimeric animals are then bred to produce offspring that are assessed for the presence of genetic contribution from the ES cell line. This process requires the production and characterization of multiple generations of animals, which is costly and time consuming [13]. Therefore, it is advantageous to prescreen and exclude cell lines that exhibit characteristics that might negatively impact their germline transmissibility. The ability of an ES cell line to be germline competent is affected by various factors, including the genetic background of the ES cell line, normality of its karyotype, passage number, cell morphology/density, and pathogen status of the cell line including Mycoplasma status [14]. The genetic background of recipient embryos also affects the germline transmissibility of ES cells [13,15].
In these studies, we describe the isolation of a novel germline-competent rat ES cell line derived from transgenic rats carrying an EGFP transgene. We describe the characterization of ES cell lines using various prescreening tests to select rat ES cell lines that have a higher probability for germline transmissibility and the use of hybrid recipient embryos to improve the efficiency of germline competency testing. These studies demonstrate that it is feasible to isolate ES cell lines from a genetically modified rat strain.
Materials and Methods
Derivation of ES cell lines from transgenic rats
SD-Tg(UBC-EGFP)2BalRrrc (RRRC No. 065) male rats were obtained from the Rat Resource and Research Center (University of Missouri) and were used for the derivation of rat ES cell lines. This strain carries a single EGFP transgene under control of an Ubiquitin C promoter on a Sprague Dawley (SD) genetic background [16]. The transgene insertion site is on Chromosome 14 (www.rrrc.us) [17]. Unless specifically indicated, all chemicals were from SigmaAldrich (SigmaAldrich, St. Louis, MO). Wild-type SD females (Harlan, Indianapolis, IN) were mated to hemizygous SD-Tg(UBC-EGFP)2BalRrrc males. Blastocysts were collected on day 4.5 postmating in mRiECM+22 mM HEPES [18]. After collection, blastocysts showing green fluorescence were selected and subjected to ES cell line derivation as previously described [8]. Briefly, EGFP blastocysts were treated briefly with acidic Tyrode's solution to remove zona pellucidae and then cultured in N2B27+3 μM CHIR99021 (Axon Medchem BV, Groeningen, The Netherlands)+0.5 μM PD0325901 (Selleckchem, Houston, TX) [19] on CF-1 mouse feeder cells (Millipore, Billerica, MA) in Nunc 4-well plates (Thermo Scientific, Roskilde, Denmark) at 37°C in an incubator with 5% CO2 and maximal humidity. On day 5, outgrowths of the embryos were individually disassociated into single-cell suspension using accutase and then cultured in 24-well plates. ES cells were passaged every 48–72 h.
Expression of pluripotency factors
The established ES cell lines were screened for the expression of Oct4, Sox2, and Nanog by reverse transcription polymerase chain reaction (RT-PCR) analysis using rat-specific primers. The positive control was germline-competent rat ES cell line DAc8 [8] (RRRC No. 464) obtained from the Rat Resource and Research Center. The negative controls were rat embryonic fibroblasts (REFs) (made in house), mouse embryonic fibroblasts (MEFs) (feeder cells; Millipore), and a no-template control (NTC). RNA was extracted from up to 5×105 cells using RNeasy Plus Micro Kit (QIAGEN, Valencia, CA). High Capacity First Strand Synthesis Kit from Applied Biosystem (Carlsbad, CA) was used to synthesize cDNA from 1 μg of RNA. The rat-specific primers are as follows: Oct4, 5′ CCCAGCGCCGTGAAGTTG-GA 3′ and 5′ ACCTTTCCAAAGAGAACGCCCAGG 3′; Sox2, 5′ ATTACCCGCAGCAAAATGAC 3′ and 5′ AT-CGCCCGGAGTCTAGTTCT 3′; Nanog, 5′ GACTAGCAACGGCCTGACTCA 3′ [8] and 5′ CTGCAATGGATGCTGGGATA 3′; and GAPDH, ATCACTGCCACTCAGAAG 3′ and AAGTCACAGGAGACAACC 3′ [8]. RT-PCR was performed in 25 μL reactions containing 250 pg–250 ng cDNA, 1×polymerase chain reaction (PCR) buffer (Roche, Indianapolis, IN), 1.5 mM MgCl2, 0.2 mM dNTPs, 0.2 μM of each primer, and 2.5 U of Roche FastStart Taq polymerase. Thermal cycling conditions were 1 cycle at 95°C for 2 min; 35 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s; and 1 cycle at 72°C for 5 min. The DNA samples were analyzed using the QIAxcel (QIAGEN) with the QIAxcel DNA Screening Kit, QX Alignment Marker 15 bp/3 kb, and QX DNA Size Marker 100 bp–3 kb. The method was AM320 with an injection of 10 s at 5 kV and a separation of 320 s at 6 kV.
ES cell karyotyping
Rat ES cell lines were cultured in 60-mm culture dishes for ∼48 h or until 60%–70% confluent. The cells were then treated with 0.1 μg/mL colcemid (Irvine Scientific, Santa Ana, CA) for 1 h at 37°C. Cells were disassociated into single-cell suspension with accutase and then pelleted by centrifugation at 200 g for 8 min in a 15 mL conical tube. After removing the supernatant, the cells were resuspended with 4–5 mL hypotonic solution (0.075 M KCl solution) and incubated at room temperature for 15 min. A few drops of freshly made fixative consisting of methanol (Fisher Scientific, Pittsburg, PA) and acetic acid (Fisher Scientific) in a ratio of 3:1 were added to the hypertonically treated cell suspension and mixed by inversion. The cells were centrifuged at 200 g for 8 min to pellet the cells. The pellet was resuspended in 4–5 mL of freshly made fixative and centrifuged at 200 g to re-pellet the cells. After 1 more repetition of the fixation step by resuspending the cells in 4–5 mL fixative, the fixed ES cells were pelleted by centrifugation at 200 g and resuspended in 1 mL fixative. Preparation of chromosome spreads and karyotype analysis of the fixed cells were performed by the Molecular Biology Laboratory, University of Southern California (Los Angelos, CA). ES cell lines were analyzed by Giemsa-Trypsin-Wrights (GTW) banding and at least 20 metaphase spreads were counted. A cell line with 80% or higher normal karyotypes was considered to have a normal karyotype. The passage numbers at the time of karyotyping for each cell line are as follows: SD-Tg.EC1 at P9, SD-Tg.EC2 at P11, SD-Tg.EC7 at P11, SD-Tg.EC8 at P10, SD-Tg.EC11 at P10, and SD-Tg.EC12 at P11.
Pathogen screening of rat ES cells
For bacterial testing, 1 mL of the culture medium from each cell line was submitted to the Research Animal Diagnostic Laboratory (RADIL) (Columbia, MO) for microbiological evaluation. The medium was placed on blood agar (BA) and brain heart infusion (BHI) broth for 10 days to evaluate bacterial growth. One million cells from each of the ES cell lines SD-Tg.EC1 and SD-Tg.EC8 were submitted to RADIL for a comprehensive pathogen testing. This included screening for the presence of H1 parvovirus, Kilham's rat virus, Mycoplasma spp., rat minute virus, and rat parvovirus in the cell lines. A portion of the cell sample was also grown on BA/BHI broth for 10 days to examine any potential bacterial contamination in the cell lines.
Chimeric animal production and breeding
To investigate whether the ES cells were able to contribute to the formation of germ cells in vivo, the ES cell lines were used for the production of chimeric animals via blastocyst injections. Two male ES cell lines, SD-Tg.EC1 and SD-Tg.EC8, were investigated in this experiment. Prior to blastocyst injection, the cells were cultured in N2B27+2i in 60 mL culture dishes and cultured for 3 passages after thawing to ensure that the ES cells were fully recovered from any stress resulting from cryopreservation. On the day of injection, rat ES colonies were detached from the feeders by gently pipetting the media up and down followed by collection into a 15 mL centrifugation tube. The ES cell colonies were pelleted through centrifugation at 200 g for 3 min. After removing the supernatant, the pelleted ES cell colonies were disassociated with accutase into a single-cell suspension followed by centrifugation at 200 g for 3 min. The cell pellet was resuspended in N2B27+20 mM HEPES and incubated on ice. Donor blastocysts were collected from day-4.5 pregnant SD females that had been mated with Dark Agouti (DA) males (Harlan). These females were synchronized using gon adotrophin releasing hormone at 40 μg/rat 4 days before the mating. Donor blastocysts were cultured in mRiECM+10% fetal bovine serum after collection. For blastocyst injection, 10–12 rat ES cells were injected into single blastocysts using a beveled Transfertip (Eppendorf, Hauppauge, NY). Injected blastocysts were cultured for about 1 h in mRiECM+10% FBS and then approximately 20–30 blastocysts were transferred into the uterine horns of day-3.5 pseudo-pregnant SD females (10–15 blastocysts per uterine horn). All surgical procedures were approved by the Animal Care and Use Committee of the University of Missouri-Columbia.
Chimerism of the resulting pups was assessed by coat color chimerism (presence of albino hairs against an agouti coat color background), fluorescence microscopy of tail snips to detect GFP fluorescence, and the presence of the EGFP transgene using an insertion-site-specific PCR genotyping assay developed for SD-Tg(UBC-EGFP)2BalRrrc by the RRRC (protocol at www.rrrc.us). DNA was extracted from tail biopsies using the Extract-N-Amp Tissue PCR kit and PCR was performed using the manufacturer's protocol and reagents. Primers were R52 int 1F: 5′ AGCAATGAATAGCCTCTCTCCT 3′, R52 int 1R: 5′ CCCATATGTGCCAAGCACTTTACC 3′, and U3r-0: 5′ GTCTGAAGGGATGGTTGTAGCTGT 3′. Thermal cycling conditions were 1 cycle at 94°C for 3 min; 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min 15 s; and 1 cycle at 72°C for 10 min. The wild-type product is 799 bp and the mutant product is 1,050 bp. Upon sexual maturation, chimeric animals were bred with SD mates to verify germline transmissibility. DNA was extracted from tail snips of all offspring and the transgene-specific PCR assay described previously was performed. Recovery of animals that inherited the transgene from their chimeric parent was evidence of germline competency of the ES cell line. Failure to produce any transgenic offspring in 3 consecutive litters (n>30) was taken as lack of germline competency.
Results
Derivation of ES cell lines from SD-Tg(UBC-EGFP)2BalRrrc transgenic rats
The efficiency of isolating rat ES cells from SD-Tg(UBC-EGFP)2BalRrrc blastocysts is shown in Table 1. Three independent experiments were performed and blastocysts were successfully cultured and showed outgrowths in all 3 experiments. The number of ES cell lines recovered was highly variable among experiments because a portion of the outgrowths failed to form ES cell colonies on subsequent plating.
Table 1.
Derivation of Rat Embryonic Stem Cell Lines from Blastocysts
| No. of blastocysts cultured | No. of outgrowths | No. of ES lines | |
|---|---|---|---|
| Experiment 1 | 12 | 12 | 0 |
| Experiment 2 | 14 | 14 | 10 |
| Experiment 3 | 11 | 11 | 2 |
ES, embryonic stem.
Characterization of the novel ES cell lines
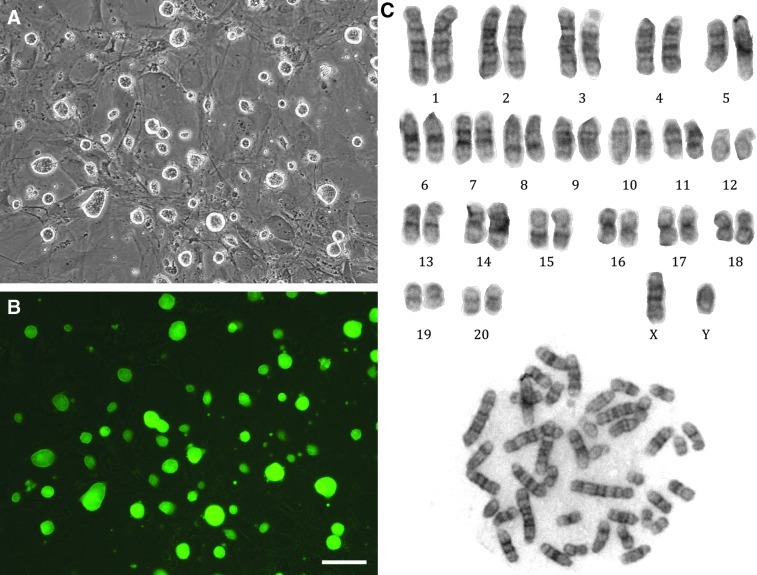
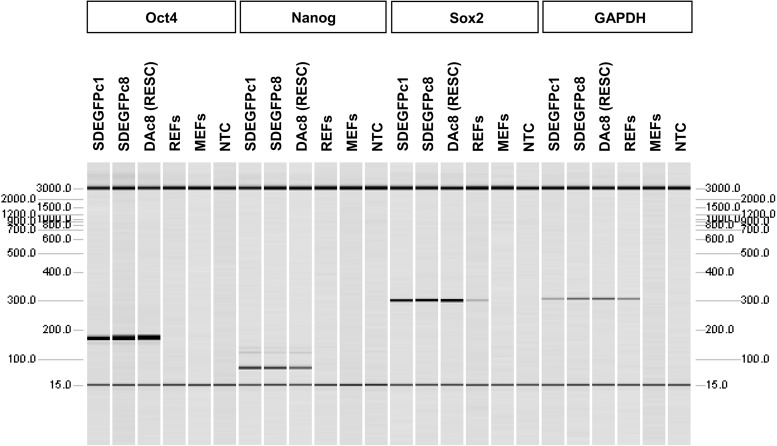
In total, 12 ES cell lines were derived from 37 EGFP blastocysts. All 12 cell lines could be maintained in an undifferentiated state in rat ES medium (N2B27+2i) and showed compact colonies with smooth boundaries and retained GFP fluorescence (Fig. 1A, B). Based on RT-PCR analysis, 6 of the SD ES cell lines (SD-Tg.EC1, EC2, EC7, EC8, EC11, and EC12) were confirmed to express Oct4, Sox2, and Nanog; the remaining 6 were not analyzed as we felt that 6 stem cell marker–positive cell lines were a sufficient number to study further (Fig. 2). Karyotyping analysis indicated that all 6 ES cell lines had normal karyotypes (Fig. 1C). Two lines, SD-Tg.EC1 and SD-Tg.EC8, were male while the remaining 4 lines were female. Pathogen screening indicated the 2 male lines (SD-Tg.EC1 and SD-Tg.EC8) were free of H1 parvovirus, Kilham's rat virus, Mycoplasma spp., rat minute virus, and rat parvovirus. There was also no bacterial or fungal growth after 10 days of sterility testing for both the culture media as well as the cell lines.
FIG. 1.
Embryonic stem (ES) cell morphology and karyotype. The morphology and karyotype of SD-Tg.EC1 is shown and is representative of the other ES cell lines. (A) Phase-contrast image shows cultured ES cells forming compact colonies with smooth edges. (B) Fluorescence microscopy image of same field of view as (A). Cultured ES cells express the EGFP transgene. Scale bar represents 100 μm. (C) Cytogenetic analysis. ES cells have a normal male karyotype (42, XY).
FIG. 2.
Reverse transcription polymerase chain reaction (RT-PCR) analysis of Oct4, Nanog, and Sox2 gene expression using rat-specific primers. Results of SD-Tg.EC1 and EC8 are shown and are representative of the other ES cell lines analyzed. DAc8, a proven germline-competent rat ES cell line [8], is included as a positive control; rat embryonic fibroblasts (REFs), mouse embryonic fibroblasts (MEFs), as well as a no-template control (NTC) are also shown. The molecular size marker in base pairs is shown to the left and right of the image. The bands at 3,000 bp and 15 bp represent the alignment marker. Sox2 is known to be expressed in both rat ES cells and REFs.
Generation of chimeras
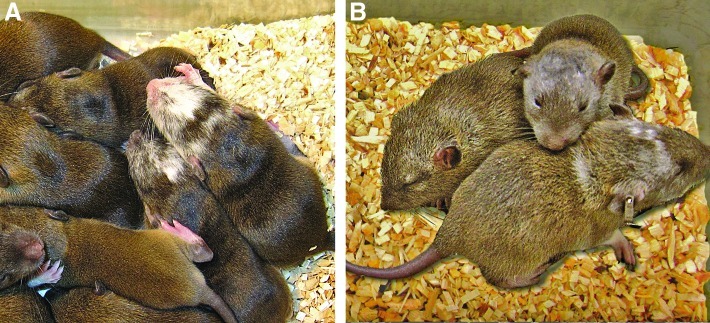
Ten to twelve SD-Tg.EC1 and SD-Tg.EC8 ES cells at passage 12 were injected into hybrid DA×SD blastocysts. For SD-Tg.EC1, 119 blastocysts were injected and transferred. However, 1 recipient female did not recover from the transfer surgery, resulting in the loss of 30 blastocysts for further analysis (Table 2). Twenty-five live pups were recovered. Inheritance of the EGFP transgene was confirmed by GFP fluorescence in tail snips and confirmation of a transgene-positive genotype using DNA isolated from tail snips. A total of 11 animals were positive for the transgene. However, only 10 showed coat color chimerism. One transgene-positive female obtained from injection of SD-Tg.EC8 showed no coat color chimerism. Of the 10 transgene-positive animals that did have coat color chimerism, 5 (4 males and 1 female) were obtained from injection of SD-Tg.EC1 cells and 5 males were obtained from injection of SD-Tg.EC8 cells (Table 2). The degree of coat color chimerism was noticeably different in chimeras derived from SD-Tg.EC1 versus SD-Tg.EC8 ES cells (Fig. 3). SD-Tg.EC1 chimeras had a denser pattern of albino hairs on the head region (Fig. 3A), whereas SD-Tg.EC8 chimeras had sparser, more diffuse albino hairs (Fig. 3B).
Table 2.
Generation of Chimeric Animals Via Blastocyst Injection with Rat Embryonic Stem Cell Lines
| |
|
No. of blastocysts |
|
No. of chimeras (%)2 |
|||
|---|---|---|---|---|---|---|---|
| ES cell line | Passage number | Collected | Injected | Transferred | No. of live pups (%)1 | Coat color | PCR |
| SD-Tg.EC1 | P12 | 119 | 119 | 893 | 14 (15.7) | 5 (35.7) | 5 (35.7) |
| SD-Tg.EC8 | P12 | 111 | 111 | 111 | 11 (9.9) | 5 (45.5) | 6 (54.5) |
Number of live pups divided by actual number of blastocysts transferred.
Percent of live pups exhibiting chimerism.
One recipient animal received 30 injected blastocysts but did not recover after anesthesia.
PCR, polymerase chain reaction.
FIG. 3.
Coat color chimeras. (A) SD-Tg.EC1. (B) SD-Tg.EC8.
Demonstration of germline competency
Ten chimeric animals derived from the SD-Tg.EC1 and SD-Tg.EC8 cell lines were bred to SD mates. One male chimeric animal from the SD-Tg.EC8 line was euthanized before sexual maturation due to congenital malocclusion and megaesophagus with secondary aspiration pneumonia. At least 30 offspring produced by each chimera were analyzed for inheritance of the EGFP transgene (Table 3). The results showed that cell line SD-Tg.EC1 is able to transmit through the germline, although of 4 chimeric males tested, 1 failed to produce offspring that inherited the transgene. The female chimera (258RBB) from SD-Tg.EC1 was sterile and did not produce any offspring. Necropsy was performed on this female at the end of the study and she was found to be a hermaphrodite with abdominal undescended testes as well as a uterus and vaginal opening. Cell line SD-Tg.EC8 did not demonstrate germline competency as indicated by the lack of transgene-positive pups from any of the 4 chimeric males and 1 chimeric female tested. As described previously, this female (302RV) showed no coat color chimerism but was EGFP transgene positive by PCR analysis and fluorescent microscopy.
Table 3.
Breeding Results for Chimeric Animals
| ESC line | Chimera's ID | Sex | No. of pups | No. of GFP+ pups (%) | Germline transmissibility |
|---|---|---|---|---|---|
| SD-Tg.EC1 | 258RBB | F | 0 | NA | NA |
| 259RBB | M | 45 | 2 (4.4) | + | |
| 260RBB | M | 39 | 3 (7.7) | + | |
| 261RBB | M | 43 | 21 (48.8) | + | |
| 263RBB | M | 33 | 0 (0) | − | |
| SD-Tg.EC8 | 302RV | F | 41 | 0 (0) | − |
| 304RV | M | 46 | 0 (0) | − | |
| 305RV | M | 33 | 0 (0) | − | |
| 306RV | M | 45 | 0 (0) | − | |
| 307RV | M | 39 | 0 (0) | − |
Discussion
Germline-competent ES-cell-based genetic modification technology is proven to be an effective method for making mouse and rat models with targeted mutations [6,13]. Therefore, the availability of germline-competent ES cell lines from many strains or lines will be very valuable resources for making rat models of various genetic backgrounds. In the present study, we reported derivation of a novel rat ES cell line with germline transmissibility from transgenic SD rats carrying a ubiquitously expressed EGFP gene on Chromosome 14. We also demonstrated that hybrid recipient embryos with an SD×DA genetic background were able to support the germline competence testing of SD-Tg.EC lines.
While a number of groups have derived ES cell lines and generated chimeras, it is clear that several lines then failed to demonstrate germline transmissibility [20,21]. Several factors, such as the genetic background, stemness, normality of karyotype, pathogen status of the ES cell line, as well as the genetic background of recipient embryos, affect the ES cells' ability to transmit their genetic material through the germline [13,14]. The utmost test for germline transmission is to inject the ES cell lines into recipient blastocysts to produce chimeric animals and then breed these animals to look for resulting pups with the ES cell genetic background. However, this process is time consuming and costly. Confirming expression of key pluripotency factors, such as Oct4, Sox2, and Nanog, as well as confirming that a cell line has a high proportion of cells retaining a normal karyotype are essential preliminary screening tools to eliminate cell lines with characteristics that may negatively affect their germline transmissibility [8,22,23]. Pathogen status of the ES cell line also affects various properties of ES cells, including their germline transmissibility. It has been demonstrated in mouse ES cells that the presence of virus, bacteria, or Mycoplasma affects germline transmissibility [24,25]. In our study, we conducted a comprehensive test for rat pathogens, such as H1 parvovirus, Kilham's rat virus, Mycoplasma spp., rat minute virus, and rat parvovirus. Prior to freezing, samples of the culture media were also tested for the presence of any pathogens, especially slow-growing bacteria. After these tests, we selected only the 2 male lines SD-Tg.EC1 and SD-Tg.EC8 to conduct in vivo testing to investigate their germline competency. Only male cell lines were chosen because we wanted to ultimately work with primarily male chimeras for breeding efficiency.
The genetic background of recipient embryos also affects the germline transmissibility of ES cells [13–15]. Ideally, the host background should allow the ES cells to have an optimal developmental advantage when injected into the blastocyst. This allows the ES cells to contribute to the germline of the chimeric animals that consequently transmit the ES-cell-derived genetic material to their offspring [8,13,20]. Because a relatively few number of ES cell lines have been isolated to date and even fewer have been shown to be germline competent, relatively little is known about the optimal combinations of ES cell genetic background and recipient blastocyst genetic background in the rat. Recipient embryos from SD rats have been used as recipient embryos for DA ES cell lines to generate chimeric animals; however, these chimeric animals failed to produce offspring with an ES cell genetic contribution [8]. Similarly, chimeric animals resulting from SD blastocysts injected with ES cells from Brown Norway rats also failed to produce offspring with the Brown Norway genetic background [20].
In our study, we successfully used DA×SD hybrid blastocysts as recipient embryos. SD female rats were selected for generation of the hybrid embryos because of their high fecundity. Male DA rats were selected in order to generate offspring that would have a pigmented coat color to aid in detection of chimeric animals. DA rats lack mutations in both the agouti and tyrosinase genes, and do not carry mutations associated with the hooded locus. Interestingly, all offspring recovered following embryo transfer, including nonchimeric offspring had variable amounts of white on their belly, paws, and tail tip, which is indicative of the homozygous hooded mutation in the SD female blastocyst donors. Chimeric offspring mated to SD rats produced offspring with a variety of coat colors, including albino, agouti, black, and hooded. This confirms that SD stock populations not only carry the albino mutation in the tyrosinase gene, but also harbor mutations in the agouti gene and the hooded locus. While an advantage to using hybrid embryos is the relatively high yield of high-quality embryos that can be obtained, careful consideration of both reproductive performance and coat color genetics is needed when selecting the breeding cross.
We selected 2 male cell lines that passed our selection criteria (proper morphology, expression of pluripotency markers, normal karyotype, and pathogen-free status) to test for germline transmissibility. SD.Tg.EC1-derived chimeras produced offspring that carried the EGFP transgene and therefore confirmed the germline transmissibility of this cell line. In contrast, even after analyzing 3 litters for each chimeric rat, no EGFP-transgene-positive pups were produced using SD-Tg.EC8. This outcome might have been predicted based on the low level of coat color chimerism seen for this cell line when compared with the degree of coat color chimerism seen in SD-Tg.EC1 chimeras.
Interestingly, of the 2 female chimeras produced, the noncoat color female derived from SD-Tg.EC8 was reproductively normal. On the other hand, the female chimera derived from SD-Tg.EC1 was a hermaphrodite. The introduction of XY ES cells into an XX blastocyst results in an embryo with a mixture of XY and XX cells. If the contribution of the XY cells in the developing gonad is high, male gonad development can occur but if the contribution is low, the chimera will develop as a female or a hermaphrodite [26]. In our experiments, the SD-Tg.EC8 female probably had a very low contribution from the ES cells whereas the SD-Tg.EC1 female probably had a much higher contribution but not enough to allow completely male gonad development to occur. Given the potential issues, it is advisable to consider culling female chimeras when working with an XY ES cell line, especially if enough male chimeras are recovered.
In conclusion, novel rat ES cell lines were established from transgenic rats carrying a ubiquitously expressed EGFP gene. One of the ES cell lines, SD-Tg.EC1, was demonstrated to be able to transmit through the germline and this fluorescently tagged ES cell line will be extremely useful for investigators who want to make genetically engineered rat models. Hybrid blastocysts from a cross of DA and SD rats were able to support the differentiation of SD-Tg.EC1 ES cells into germ cells in chimeric animals. And finally, it is clear from our analysis that while preliminary screening of new ES cell lines for expression of pluripotency markers, normal karyotype, and pathogen-free status is a prudent quality control measure before proceeding to generate chimeric animals, there is, as yet undetermined, a variable that plays an important role in determining whether a cell line is capable of being transmitted through the germline. Until that variable can be identified, the degree of coat color chimerism remains an important measure of the likely success of a cell line to be successfully transmitted through the germline.
Acknowledgments
This project was supported by grant funding to the Rat Resource and Research Center (RRRC) from the National Institutes of Health (2P40 RR016939). The authors thank Angela Goerndt for assistance with animal care, Denise Bouvrette and Miriam Hankins for technical assistance, and Howard Wilson for assistance with graphics.
Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Gill TJ., 3rd Smith GJ. Wissler RW. Kunz HW. The rat as an experimental animal. Science. 1989;245:269–276. doi: 10.1126/science.2665079. [DOI] [PubMed] [Google Scholar]
- 2.Abbott A. Laboratory animals: the Renaissance rat. Nature. 2004;428:464–466. doi: 10.1038/428464a. [DOI] [PubMed] [Google Scholar]
- 3.Voigt B. Serikawa T. Pluripotent stem cells and other technologies will eventually open the door for straightforward gene targeting in the rat. Dis Model Mech. 2009;2:341–343. doi: 10.1242/dmm.002824. [DOI] [PubMed] [Google Scholar]
- 4.Kitada K. Ishishita S. Tosaka K. Takahashi R. Ueda M. Keng VW. Horie K. Takeda J. Transposon-tagged mutagenesis in the rat. Nat Methods. 2007;4:131–133. doi: 10.1038/nmeth1002. [DOI] [PubMed] [Google Scholar]
- 5.Zan Y. Haag JD. Chen KS. Shepel LA. Wigington D. Wang YR. Hu R. Lopez-Guajardo CC. Brose HL, et al. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol. 2003;21:645–651. doi: 10.1038/nbt830. [DOI] [PubMed] [Google Scholar]
- 6.Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- 7.Buehr M. Meek S. Blair K. Yang J. Ure J. Silva J. McLay R. Hall J. Ying QL. Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Li P. Tong C. Mehrian-Shai R. Jia L. Wu N. Yan Y. Maxson RE. Schulze EN. Song H, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong C. Li P. Wu NL. Yan Y. Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G. Tong C. Kumbhani DS. Ashton C. Yan H. Ying QL. Beyond knockout rats: new insights into finer genome manipulation in rats. Cell Cycle. 2011;10:1059–1066. doi: 10.4161/cc.10.7.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesson L. Usal C. Menoret S. Leung E. Niles BJ. Remy S. Santiago Y. Vincent AI. Meng X, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotech. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 12.Geurts AM. Cost GJ. Freyvert Y. Zeitler B. Miller JC. Choi VM. Jenkins SS. Wood A. Cui X, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong C. Huang G. Ashton C. Li P. Ying QL. Generating gene knockout rats by homologous recombination in embryonic stem cells. Nat Protoc. 2011;6:827–844. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carstea AC. Pirity MK. Dinnyes A. Germline competence of mouse ES and iPS cell lines: chimera technologies and genetic background. World J Stem Cells. 2009;1:22–29. doi: 10.4252/wjsc.v1.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartzberg PL. Goff SP. Robertson EJ. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989;246:799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- 16.Lois C. Hong EJ. Pease S. Brown EJ. Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 17.Bryda EC. Pearson M. Agca Y. Bauer BA. Method for detection and identification of multiple chromosomal integration sites in transgenic animals created with lentivirus. Biotechniques. 2006;41:715–719. doi: 10.2144/000112289. [DOI] [PubMed] [Google Scholar]
- 18.Oh SH. Miyoshi K. Funahashi H. Rat oocytes fertilized in modified rat 1-cell embryo culture medium containing a high sodium chloride concentration and bovine serum albumin maintain developmental ability to the blastocyst stage. Biol Reprod. 1998;59:884–889. doi: 10.1095/biolreprod59.4.884. [DOI] [PubMed] [Google Scholar]
- 19.Nichols J. Ying QL. Derivation and propagation of embryonic stem cells in serum- and feeder-free culture. Methods Mol Biol. 2006;329:91–98. doi: 10.1385/1-59745-037-5:91. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X. Lv Z. Liu L. Wang L. Tong M. Zhou Q. Derivation of embryonic stem cells from Brown Norway rats blastocysts. J Genet Genom. 2010;37:467–473. doi: 10.1016/S1673-8527(09)60066-7. [DOI] [PubMed] [Google Scholar]
- 21.Hong J. He H. Weiss ML. Derivation and characterization of embryonic stem cells lines derived from transgenic Fischer 344 and dark agouti rats. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0370. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers I. The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells. 2004;6:386–391. doi: 10.1089/clo.2004.6.386. [DOI] [PubMed] [Google Scholar]
- 23.Chen L. Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–R27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 24.Mahabir E. Reindl K. Mysliwietz J. Needham J. Bulian D. Markoullis K. Scherb H. Schmidt J. Impairment of germline transmission after blastocyst injection with murine embryonic stem cells cultured with mouse hepatitis virus and mouse minute virus. Transgenic Res. 2009;18:45–57. doi: 10.1007/s11248-008-9216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markoullis K. Bulian D. Holzlwimmer G. Quintanilla-Martinez L. Heiliger KJ. Zitzelsberger H. Scherb H. Mysliwietz J. Uphoff CC, et al. Mycoplasma contamination of murine embryonic stem cells affects cell parameters, germline transmission and chimeric progeny. Transgenic Res. 2009;18:71–87. doi: 10.1007/s11248-008-9218-z. [DOI] [PubMed] [Google Scholar]
- 26.Nagy A. Gertsenstein M. Vintersten K. Behringer R. Manipulation of the Mouse Embryo, A Laboratory Manual. Cold Spring Harbor Laboratory Press; Plainview: 2003. Production of chimeras; pp. 453–506. [Google Scholar]