Abstract
Bone marrow stem cells (BMSCs) have been shown to improve neurological function recovery in cerebral ischemia. Hypoxia-inducible factor-1 (HIF-1) α-AA is a more stable mutant form of HIF-1α, which is a crucial oxygen-sensitive regulator. To investigate the protective effects of HIF-1α-AA-modified BMSCs on neuron survival in cerebral ischemia models, we co-cultured HIF-1α-AA-modified BMSCs with neuron-like cells (PC12 cells) and observed a significant increase in the release of vascular endothelial growth factor (VEGF) from BMSCs, the decreased PC12 cell apoptosis, and the upregulation of Survivin expression reduced by hypoxia in PC12 cells compared to enhanced green fluorescent protein (EGFP) BMSCs. In addition, to explore whether VEGF secreted by HIF-1α-AA-modified BMSCs plays an important role in preventing hypoxia-induced apoptosis and the possible mechanism involved, exogenous VEGF were applied and the similar protective effects on PC12 cells were observed in vitro. Furthermore, hypoxia reduced the expression of phosphorylated Akt and phosphorylated FoxO1, whereas the administration of VEGF reversed these changes. Transfection of FoxO1 H215R, a DNA-binding mutant, abrogated the inhibitory ability on Survivin promoter activity, whereas FoxO1 AAA, the active form of FoxO1, presented further repression on Survivin promoter, indicating that FoxO1 directly binds on Survivin promoter as a transcriptional repressor and that phosphorylation status of FoxO1 affects its inhibition on the Survivin promoter. Transplantation of HIF-1α-AA-modified BMSCs after cerebral ischemia in vivo sufficiently reduced neurons apoptosis, decreased cerebral infarction volume, and induced a significant improvement on the modified neurological severity score compared to the EGFP BMSCs group. In conclusion, HIF-1α-AA-modified MSCs showed an obvious protective effect on neuron-like cells or neuron after ischemia in vitro and in vivo, at least in part, through the VEGF/PI3K/Akt/FoxO1 pathway.
Introduction
Cerebral ischemia is a common cerebrovascular disease with high disability and mortality [1,2]. Bone marrow stem cells (BMSCs) have captured scientists' attention in curing central nervous diseases such as cerebral ischemia, stroke, and spinal cord injury. BMSCs are a kind of adult stem cells with the ability to divide with unlimited self-renewal properties and differentiation potential [3]. It is generally assumed that BMSCs transplanted into the vein or artery can cross the blood–brain barrier and reach the ischemia region and differentiate into neuron-like cells, and then improve neurological function recovery in cerebral ischemia [4]. Meanwhile, BMSCs have been shown to provide an attractive microenvironment for injured and damaged tissues, through the secretion of cytokines and trophic factors, to activate endogenous brain remodeling [5,6]. These processes may include neurogenesis, angiogenesis, and synaptogenesis, reducing neuronal apoptosis and promoting neuronal proliferation in the boundary zone of the ischemic hemisphere. However, these possible therapeutic potentials remain to be characterized and subsequently further enhanced. Many studies on stroke using BMSCs as treatment suggest genetic modification of BMSCs to improve therapeutic potential. Indeed, overexpression of trophic factors such as brain-derived neurotrophic factor (BDNF), fibroblast growth factor-2, and hematopoietic growth factor in gene-modified BMSCs has been demonstrated to improve neurologic outcome in animal models of ischemic stroke [7–9]
Hypoxia-inducible factor-1 (HIF-1) is an oxygen-sensitive transcription factor [10]. HIF-1 is a heterodimeric protein that consists of a functional HIF-1α subunit and a constitutively expressed HIF-1β subunit. The HIF-1α subunit's stability and transcriptional activity are subject to the regulation of intracellular oxygen concentration [11]. Two critical prolines in HIF-1α (Pro402 and Pro564) can be hydroxylated by proline hydroxylase under normoxia conditions. Hydroxylation of HIF-1α leads to its binding to von Hippel-Lindau (VHL) and ubiquitin-mediated degradation [12–14]. It has been shown that HIF-1α mutation in which 2 prolines were substituted to alanine (P402A and P564A) is more stable than wild-type HIF-1α and exhibit higher transcription activities in upregulating its downstream targets [15].
In the cerebral ischemia, as a crucial physiological regulator of the cellular response to low oxygen concentrations, HIF-1α is upregulated and induces the expression level of its downstream target genes [10], such as vascular endothelial growth factor (VEGF), erythropoietin (EPO), and CXC chemokine receptor 4 (CXCR4) [16], and it is also involved in angiogenesis, cell survival, anaerobic metabolism, cell migration, and differentiation, suggesting that HIF-1α plays an important role in the functional recovery of ischemia [10]. Furthermore, the central role of HIF-1 in the cellular response to hypoxia makes this factor an attractive therapeutic target for ischemic stroke [17]. Recently, Dai et al. reported that HIF-1α-induced VEGF overexpression in BMSCs protected cardiomyocytes against ischemia [18]. In a way, HIF-1 can serve as a neurotrophic and neuroprotective factor [19], which raises the possibility that HIF-1 plays essential roles in BMSC-mediated promotion of neurological and functional recovery in ischemic brain.
In the current study, we established the alanine-mutated HIF-1α-modified BMSCs, first applied them into brain ischemia, and observed the neuron protective effects of neuron and brain function. In addition, we specifically investigated the mechanisms of how HIF-1α-AA-modified BMSCs prevent hypoxia-induced apoptosis.
Materials and Methods
Preparation, isolation, and culture of BMSCs
BMSCs were isolated by the density gradient technique as described previously [20]. In brief, the bone marrow was obtained from the femurs and tibias taken from male Sprague-Dawley rats (weighing from 60 to 80 g). The bone marrow mononuclear cells were separated over Ficoll-Hypaque gradients (Sigma Chemical Co.) at 400 g for 30 min and plated into 25-cm2 flasks in Iscove's modified Dulbecco medium: Nutrient Mixture F-12 (GIBCO, Invitrogen) supplemented with 10% fetal bovine serum (Kibbutz Beit Haemek). After the cells were incubated at 37°C in 5% CO2 for 3 days, the culture medium containing nonadherent cells was removed, and the adherent layer was washed once with the fresh medium and then cultured continuously. Cells were passaged at 1:3 dilution when reaching 80% confluence. BMSCs used for intravenous transplantation in these experiments were harvested from passage 5.
Culture and treatment of PC12 cells
PC12 cells, a rat cell line derived from phaeochromocytoma cells, can be induced into neuronal-like cells with nerve growth factor (NGF) [21] and were provided as a kind gift by Professor Jianqiang Feng [22]. Induced PC12 cells were maintained on tissue culture plastic in the RPMI-1640 medium supplemented with 10% heat-inactivated horse serum and 5% fetal bovine serum (FBS) with 50 ng/ml purified recombinant Mouse beta-NGF (R&D Systems) at 37°C under an atmosphere of 5% CO2 and 95% air. 293T cells were obtained from ATCC. 293T cells were maintained with Dulbecco's modified Eagle media (DMEM) and 10% FBS with 100 μg/mL penicillin and streptomycin. All of the cells were grown at 37°C in a humidified atmosphere of 5% CO2. For anoxia treatment, PC12 cells were exposed to anoxic atmosphere (95% N2, 5% CO2 incubator) for 3–24 h. As an alternative, PC12 cells were treated with different doses of CoCl2, a hypoxia mimic compound, for 20 h. To test whether anoxic condition and CoCl2 can cause the comparable effects on PC12 cell fate, we treated PC12 cells under anoxic condition and different doses of CoCl2 and observed similar rates of cell apoptosis between anoxic conditions and 0.6 mM CoCl2 treatment. Then, for the experimental convenience, we performed 0.6 mM CoCl2 treatment in the experiments of exploring the mechanisms of how VEGF protects PC12 cells from apoptosis. For co-culturing of BMSCs and PC12 cells, we cultured PC12 cells on the bottom of transwell plate and enhanced green fluorescent protein (EGFP)-BMSCs or HIF-1α-AA-modified BMSCs on the transwell plate filters in which the pore is 0.4 μm so that only factors secreted by BMSCs, not BMSCs themselves, can go through the filters in the transwell plates.
Cell characterization assessment for Annexin V/propidium iodide flow cytometry assay
PC12 cells were quickly trypsinized to detach from the plastic plates and washed with phosphate-buffered saline (PBS) (PH 7.2), resuspended with 1× Binding buffer, and stained with Annexin V and propidium iodide (PI) for 15 min according to the manufacturer's protocol. The stained cells were processed with flow cytometry by FACSCalibur flow cytometer (BD Biosciences) to assess the apoptosis.
Growth inhibition assay
A growth inhibition assay of cells was performed as previously described [23]. Briefly, cells were digested with trypsin, counted, and then plated in a 96-well plate at a density of 5×103 cells per well. The cells were exposed to various concentrations of cobalt chloride or anoxia. After the treated cells were incubated for 16 h or at described time points, 50 μL MTT (1 mg/mL; Sigma-Aldrich) was added and the plates were incubated at 37°C for 4 h. To dissolve formazan, 150 μL dimethyl sulfoxide was added and the plates were measured at 540 nm by spectrometer (Wellscan MK3; Labsystems Dragon).
RNA extraction, cDNA synthesis, and semi-quantitative reverse transcriptase–polymerase chain reaction
Total RNA was extracted from BMSCs and PC12 cells using RNAiso plus reagent (Takara), according to the manufacturer's protocol. The first strand of cDNA was obtained using RevertAid First Strand cDNA Synthesis Kit (MBI). Polymerase chain reaction (PCR) was performed with rTaq polymerase (Takara). The cycling conditions used were as follows: denaturing at 94°C for 5 min, annealing at 60°C for 1 min, and polymerization at 72°C for 1 min. Primers used for PCR are shown in Table 1. The resulting PCR products were analyzed by 1.5% agarose gels with a DL2,000 DNA Marker (Takara) as a size reference. Gel images were obtained with G:BOX gel image machine (Syngene) and the band density was quantified with the application GeneSys image acquisition software (Syngene). All results represented the average density of positive bands obtained from at least 3 independent experiments.
Table 1.
The Primers are Used in Amplifying Vascular Endothelial Growth Factor, SURVIVIN, and β-Actin
| VEGF (forward) | 5′-GCCCATGAAGTGGTGAAGTT-3′ |
| VEGF (reverse) | 5′-ACTCCAGGGCTTCATCATTG-3′ |
| Survivin (forward) | 5′-AACAGAAAGAGTTCGAGGAG-3′ |
| Survivin (reverse) | 5′-ACAATAGAGCAAAGCCACA-3′ |
| β-actin (forward) | 5′-TGTCACCAACTGGGACGATA-3′ |
| β-actin (reverse) | 5′-AACACAGCCTGGATGGCTAC-3′ |
VEGF, vascular endothelial growth factor.
Western blotting
BMSCs and PC12 cells were rinsed with ice-cold PBS (pH 7.2) and lysed in RIPA buffer (Kangcheng). Protein concentrations were measured using the Protein Analysis System (Bio-Rad). After lysis and evaluation of protein content via Bradford protein assay, equal amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to the polyvinylidene fluoride membrane (0.45-μm pore size; Roche). Membranes were blocked in 5% nonfat dry milk for 1 h and incubated with the corresponding primary Ab [anti-HIF-1α (1:1000; Chemicon, Millipore); anti-p-FoxO1 or anti-FoxO1 (1:500 or 1:1000; Cell Signaling Technology); anti-p-Akt or anti-Akt (1:500; Kangcheng); anti-Survivin (1:500; Cell Signaling Technology)] at 4°C overnight. Blots were washed with TBS-T [20 mM Tris (pH 7.5), 0.5 M NaCl, and 0.01% Tween-20] and incubated with horseradish peroxidase (HRP)-conjugated respective secondary Abs (1:2000) for 1 h at room temperature (RT). For western blotting of β-actin, membrane was incubated with HRP-conjugated anti-β-actin (1:10,000, Kaicheng) at 4°C overnight. Antigen-Ab complexes were visualized by enhanced chemiluminescence (PIERCE). β-actin was used as a control for protein loading.
Lentivirus vector construction
The mutant HIF-1α (P402A/P564A) fragment was digested from HA-HIF-1α (P402A/P564A)-pBabe-puro purchased from Addgene and then was sub-cloned into Lentiviral backbone Lv-IRES-EGFP vector. The integrity of plasmids was identified by restriction enzyme analysis and confirmed by DNA sequencing.
Lentivirus production and infection of BMSCs
Recombinant lentiviruses were produced by cotransfection of 293T cells with the packaging plasmids (VSVG, gag-pol) and lentivirus expression plasmids (Lv-HIF-1α-IRES-EGFP or Lv-IRES-EGFP), using the Lipofectamine 2000 reagent (Invitrogen). The virus-containing supernatant was harvested 48 h post-transfection and filtered through a Millex HV filter (0.45 μm Millipore Corp.). BMSCs of passage 3 were infected with the lentivirus in the presence of 10 μg/mL Polybrene (Sigma-Aldrich). BMSCs infected with Lv-HIF-1α-AA-IRES-EGFP were named HIF-1α-AA-BMSCs, and BMSCs infected with Lv-IRES-EGFP were named EGFP-BMSCs.
Immunofluorescence microscopy of BMSCs and PC12 cells
BMSCs and PC12 cells were fixed with 4% formaldehyde for 10 min at RT and then washed with PBS 3 times. After that, cells were incubated with 5% bovine serum albumin for 1 h at RT. Cells were incubated with primary anti-Survivin (1:250; Cell Signaling Technology) overnight at 4°C, followed by Alexa 488 anti-rabbit (1: 500; Cell Signaling Technology) for 45 min. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue; Sigma-Aldrich) for nuclear staining. For detecting apoptosis by Hoechst 33258 (Beyotime Institute of Biotechnology) staining, cells were fixed in 4% formaldehyde for 30 min at RT, washed with PBS 3 times, and then stained with Hoechst 33258 for 1 h at RT, followed by washing with PBS 3 times. Cells were viewed under a Leica DMI4000B microscope (Leica) and pictures were captured with Leica Application Suite (LAS) Microscope Software (Leica).
Measurement of VEGF by Enzyme-Linked Immunosorbent Assay
EGFP-BMSCs and HIF-1α-AA-modified BMSCs were plated in 6-well plates, separately. After 96 h in culture, VEGF released from BMSCs into culture supernatant was directly measured using the enzyme-linked immunosorbent assay (ELISA) kit (RayBiotech) according to manufacturer's instructions.
Co-transfection
Transient transfections in PC12 cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. PC12 cells were seeded at 5×104 cells/well in a 24-well plate 1 day before transfection. The following day, cells were transfected with 0.6 μg of Survivin reporter and/or 0.25 μg pCDNA3, pCDNA3.1/FoxO1, pCDNA3/FoxO1 AAA, or pCDNA3/FoxO1 H215R plasmid as indicated. Total amount of DNA transfected per well was adjusted to be uniform by transfecting empty vectors as necessary. Forty-eight hours later, cells were harvested and the luciferase activity was determined with the dual-Luciferase reporter assay system (Promega). Firefly luciferase was normalized to Renilla luciferase activity.
Animals and pMCAo model
Healthy male Sprague–Dawley rats (weighing 220–250 g) were obtained from the Experimental Animal Center of Sun Yat-Sen University and randomly divided into 3 groups: the HIF-1α-AA-BMSCs (n=9), EGFP-BMSCs (n=9), and the control group (n=9). The animals were kept in a standard light/dark schedule with free access to food and water. All animal procedures were conducted in compliance with the guidelines of the Institutional Animal Care and Use Committee. Permanent middle cerebral artery occlusion was induced by a modification of the intraluminal vascular occlusion method as previously described [24,25]. Briefly, the animals were anesthetized with 10% chloral hydrate (0.4 g/kg, i.p.). The left common carotid artery, external carotid artery (ECA), and internal carotid artery (ICA) were exposed through a ventral midline incision in the neck. A silicone-coated nylon filament at the diameter of 0.234 mm, with its tip rounded by heating near flame, was introduced from the ECA into the lumen of the ICA until it blocked the origin of the middle cerebral artery (MCA).
Intravenous administration of BMSCs
In vivo study, the experimental groups consisted of 3 groups: control group (rats given MCA occlusion [MCAo] without cell administration but with 1 mL PBS), EGFP-BMSCs group (rats transplanted with 2×106 EGFP-BMSCs), and HIF-1α-AA-BMSCs group (rats transplanted with 2×106 HIF-1α-AA-BMSCs) were injected intravenously at 3 h after MCAo. All the transplantation procedures were performed under aseptic conditions.
Behavioral tests
All animals were assessed with modified neurological severity score (mNSS) tests before MCAo and at day 1, 7, 14, or 28 after MCAo (n=6 for each group). Neurological function including motor and sensory systems as well as reflexes and a balance test is graded on a numeric scale from 0 to 18 (normal score, 0; maximal deficit score, 18); the higher the score, the more severe the neurological deficit. Spatial learning and memory was assessed using a modified version of the Morris water-maze test [26]. Briefly, the rats were placed in a circular tank of warm, opaque water with a hidden platform. During training trials, latency to find the platform location is recorded. During probe trials, the platform is removed, and the percentage of time spent in the quadrant that normally contains the platform is compared to the time spent in other quadrants.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
DNA strand breaks were detected using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method. Briefly, brain sections were deparaffinized and digested with proteinase K (Roche Molecular Biochemicals) and then covered with the TUNEL reaction mixture, followed by converter-POD/wash buffer. Finally, sections were covered with the AEC substrate solution and detected with diaminobenzidine (DAB, Chemicon ES005-10ML). The integrated optical density (IOD) of apoptotic neuronal cells was measured in the cortex and hippocampus of ipsilateral hemisphere using a light microscope (Olympus BH2-RFL-T3 Japan 400×) and color image recorder (JVC KY F30B 3-CCD Germany) and analyzed using Pro Plus software 5.1 (Media Cybernetics). The neuronal apoptosis from one section per animal (n=3 each group) was evaluated by immunohistochemistry to determine IOD of the apoptotic cells exhibiting positive staining. For each slide, 6 fields were selected and examined randomly, in a defined rectangular field area (×200 objective), a total of at least 2000 cells per field were counted. The apoptotic index was determined (i.e., IOD of apoptotic cells divided by the total neuronal cells counted ×200) from a total of 18 fields per group. The assays were performed in a double-blinded manner.
Cerebral infarction measurement
Cerebral infarction volume was measured by staining brain slices with triphenyltetrazolium chloride (TTC; Sigma) at day 3 after MCAo. The animals (n=3 for each group) were decapitated under deep anesthesia with 10% chloral hydrate (0.8 g/kg, i.p.). The brains were quickly removed and sliced into 5 coronal sections at 3-mm intervals. The slices were incubated in a 2% solution of TTC at 37°C for 30 min and then fixed in a 10% formaldehyde solution overnight. The infarct area of each brain slice was analyzed using the Image-Pro plus 5.0 analysis software. The total infarction volume for each brain was calculated by summation of the infarcted area of all brain slices.
Statistical analysis
The experimental measurements were performed blindly. SigmaStat (SPSS, Inc.) statistical software was used for all statistical analysis. All data represented at least 3 independent experiments (n=3–8). We used z-tests to determine if the ratiometric data (i.e., normalized against control) are different from controls. Statistical differences between the means for the different groups were evaluated with a one-way analysis of variance. Results are expressed as mean±SEM. P<0.05 was set as statistical significance.
Results
Establishment of lentiviral-mediated HIF-1α-AA-modified BMSCs
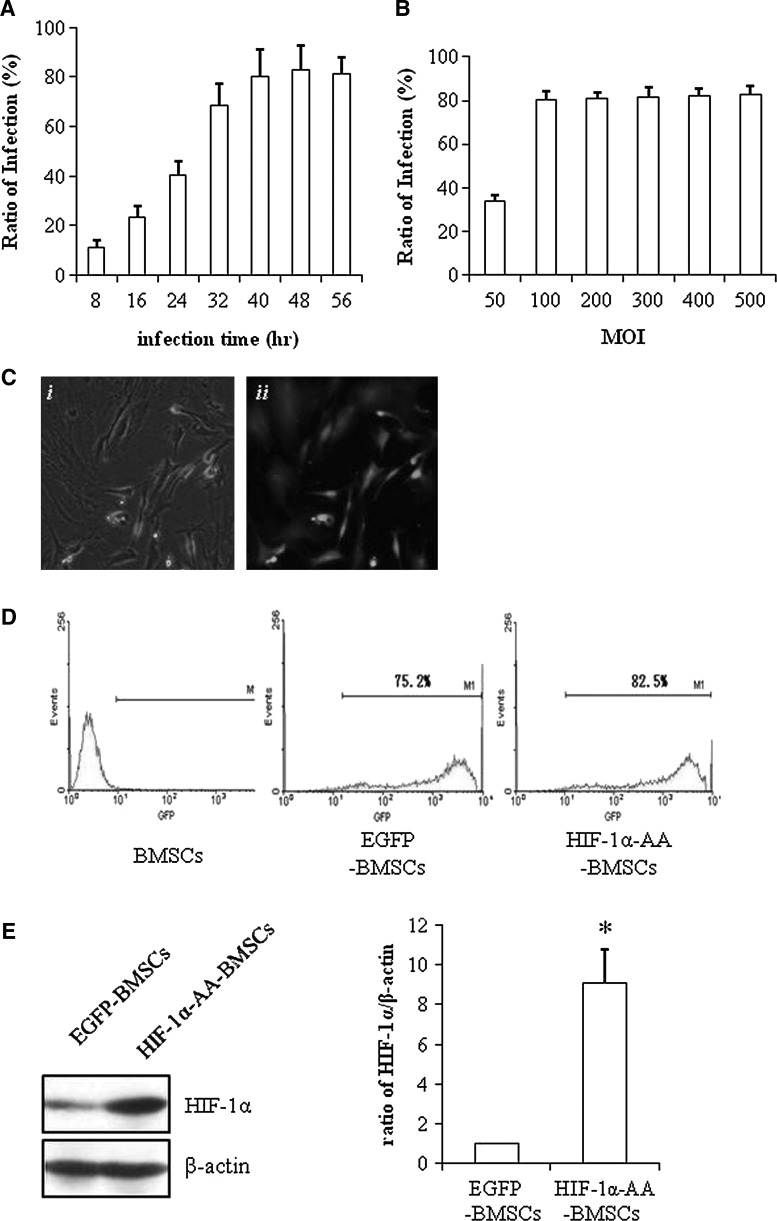
We constructed the lentiviral vector containing HIF-1α-AA, which mutates 2 critical prolines (Pro402 and Pro564) into alanines and then infected BMSCs with lentivirus containing HIF-1α-AA or EGFP as control. As shown in Fig. 1A and B, fluorescence-activated cell sorting (FACS) data examined that overexpression of EGFP in BMSCs mediated by lentivirus transduction achieved the best infection ratio after 40 h and at multiplicity of infection greater than 100. The morphology of BMSCs was not obviously affected by transduction with lentivirus containing EGFP only (Fig. 1Ci) and the EGFP-expressed BMSCs were identified as green cells under fluoresce microscopy (Fig. 1Cii). The ratio of EGFP-expressed BMSCs was examined by FACS analysis and was 75.2% and 82.5% for EGFP-induced BMSCs and HIF-1α-AA-induced BMSCs, respectively (Fig. 1D). Next, we checked the HIF-1α expression in BMSCs and HIF-1α-AA-induced BMSCs and found a significant increase of HIF-1α expression in HIF-1α-AA-induced BMSCs compared to BMSCs only (Fig. 1E), confirming the successful overexpression of HIF-1α-AA in BMSCs.
FIG. 1.
Establishment of lentiviral-mediated hypoxia-inducible factor (HIF)-1α-AA-modified bone marrow stem cells (BMSCs). (A) Flow cytometry analysis of green fluorescent protein (GFP) expression in BMSCs infected with Lv-enhanced green fluorescent protein (EGFP) at different time points when Multiplicity of Infection (MOI)=100. (B) Flow cytometry analysis of GFP expression in BMSCs infected with Lv-EGFP at different MOI after 48 h. (C) Representative pictures of both phase image and fluorescein isothiocyanate image of HIF1α-AA BMSCs (100×). (D) Flow cytometry analysis of GFP expression in BMSCs, EGFP-BMSCs, and HIF1α-AA BMSCs at MOI=100. (E) Representative picture of western blotting of HIF1α expression in EGFP-BMSCs, and HIF1α-AA BMSCs. β-actin was used as a control for protein loading. P≤0.05; data are means±SEM (n=3); *significant difference from EGFP-BMSCs.
Induction of apoptosis in PC12 cells
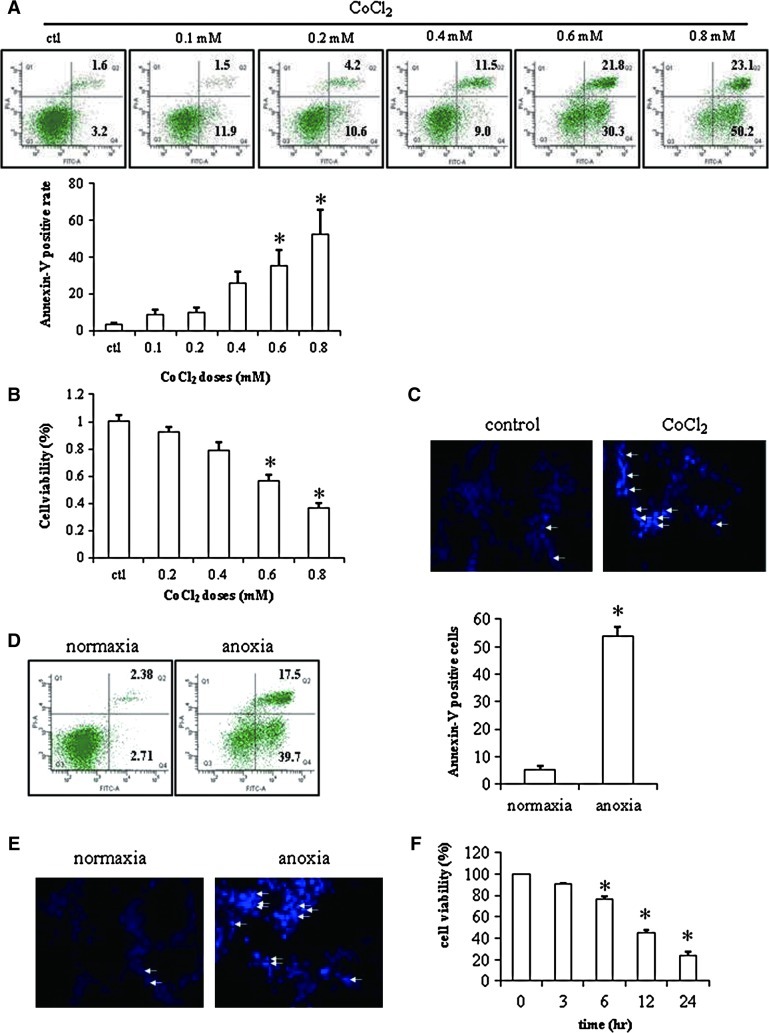
Cellular apoptosis in neuron-like cells (PC12 cells) was induced under anoxic condition or chemically induced hypoxia by cobalt chloride (CoCl2) [27]. At the beginning, the apoptotic rates with different doses of CoCl2 by FACS analysis of Annexin-V/PI staining were 3.8%±0.7%, 8.9%±2.5%, 10.1%±2.4%, 25.9%±6.4%, 35.4%±8.5%, and 52.2%±13.2%, respectively (Fig. 2A). The similar results were observed in the MTT assay (Fig. 2B), suggesting that the apoptosis was obviously induced beyond the concentration of 0.4 mM CoCl2. Therefore, we chose the dose of 0.6 mM CoCl2 for further hypoxia experiments due to less toxicity and good apoptosis effect in PC12 cells, which was confirmed with an increase of Hoechst-positive stained cells (Fig. 2C).
FIG. 2.
Apoptosis of PC12 cells. (A) Histograms and quantitative analysis of flow cytometry of Annexin-V/propidium iodide (PI) staining in PC12 cells treated with different doses of CoCl2 (n=3). (B) MTT assay of PC12 cells treated with different doses of CoCl2 (n=8). (C) Hoechst staining of PC12 cells treated with or without 0.6 mM CoCl2 (100×) (n=2). (D) Histograms and quantitative analysis of flow cytometry of Annexin-V/PI staining in PC12 cells under normoxic or anoxic condition for 12 h (n=3). (E) Hoechst staining of PC12 cells under normoxic or anoxic condition for 12 h (100×) (n=2). Hoechst-positive staining is denoted by white arrows. (F) MTT assay of PC12 cells under anoxic condition at different time points (n=3). Data are means±SEM; P≤0.05; *significant difference from control. Color images available online at www.liebertonline.com/scd
To make a comparison of anoxia and chemically induced hypoxia, we also applied anoxic conditions to PC12 cells. The apoptotic rate of PC12 cells was 53.8%±3.3%, under anoxia by FACS analysis of Annexin-V/PI staining (Fig. 2D) and more positively stained nucleoli were found in PC12 cells under anoxic conditions by Hoechst staining (Fig. 2E), indicating the comparable effects of apoptosis in PC12 cells by either anoxia or CoCl2-induced hypoxia. In addition, we examined the cell viability at different time points by MTT assay and found that cell viability was reduced to 23.3%±2.5% at 24 h (Fig. 2F).
HIF-1α-AA-modified BMSCs protect PC12 cells from apoptosis
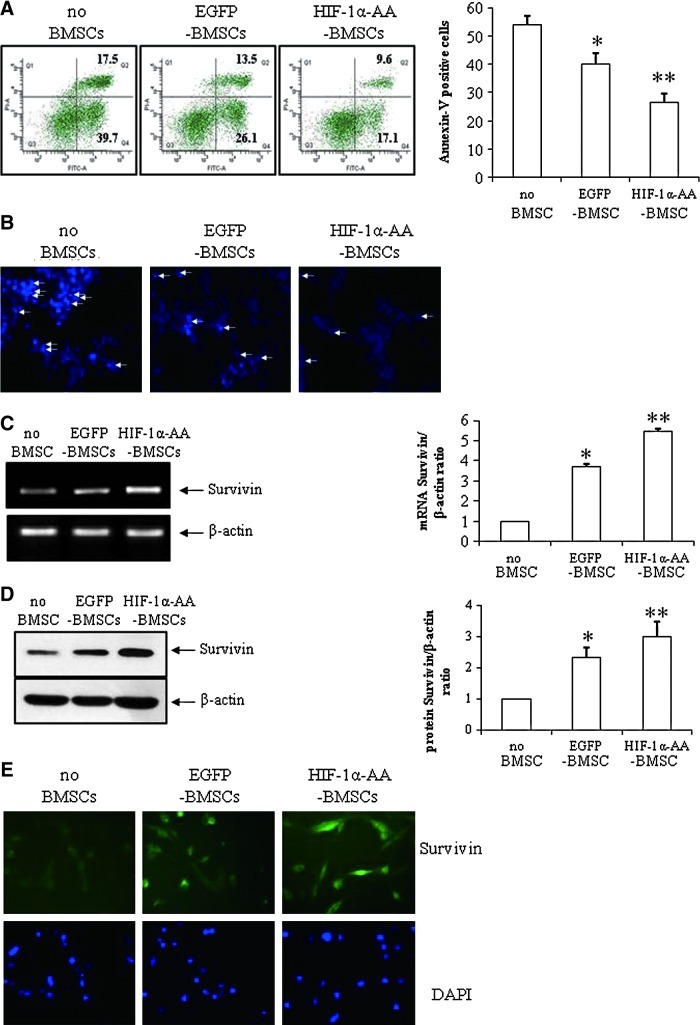
To investigate the protective effects of HIF-1α-AA-modified BMSCs, we co-cultured HIF-1α-AA-modified BMSCs with PC12 cells in transwell plates with 0.4 μm pore under anoxia and used EGFP-modified BMSCs as control BMSCs. As shown in Fig. 3, the apoptosis rates under hypoxia were 53.8%±3.3%, 39.7%±4.1%, and 26.6%±3.9% in the groups of PC12 cells, PC12+EGFP-BMSCs, and PC12+HIF-1α-AA- BMSCs, respectively. This result suggested that both control BMSCs and HIF-1α-AA-modified BMSCs presented protective effects of preventing apoptosis on PC12 cells. In addition, HIF-1α-AA-modified BMSCs provided a more effective protection than control BMSCs did. Then, we observed the lowest number of the positive Hoechst-stained nucleus with co-culture of HIF-1α-AA-modified BMSCs (Fig. 3B), further confirming the better protective effect of HIF-1α-AA-modified BMSCs.
FIG. 3.
HIF1α-AA-modified BMSCs protect PC12 cells from apoptosis. (A) Histograms and quantitative analysis of flow cytometry of Annexin-V/PI staining in PC12 cells under anoxic condition for 12 h without co-culturing of BMSCs (no BMSCs), or with co-culturing of EGFP-BMSCs or HIF1α-AA-BMSCs (n=3). (B) Hoechst staining of PC12 cells under anoxic condition for 12 h without co-culturing of BMSCs (no BMSCs), or with co-culturing of EGFP-BMSCs or HIF1α-AA-BMSCs (100×) (n=2). Polymerase chain reaction (PCR) (n=3) (C), western blotting (n=3) (D), and immunostaining (n=2) of Survivin (green) (E) in PC12 cells under anoxic condition for 12 h without co-culturing of BMSCs (no BMSCs), or with co-culturing of EGFP-BMSCs or HIF1α-AA-BMSCs (400×). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Data are means±SEM; P≤0.05; *significant difference from no BMSCs; **significant difference from other conditions. Color images available online at www.liebertonline.com/scd
It is well known that Survivin inhibits cell apoptosis as a member of the inhibitor of apoptosis [28]. Therefore, we explored the role of Survivin in apoptosis with co-culture of BMSCs. As expected, BMSCs increased the level of Survivin expression measured with reverse transcriptase-PCR (Fig. 3C), western blotting (Fig. 3D), and immunostaining (Fig. 3E). Moreover, there is a further increase of Survivin expression with co-culture of HIF-1α-AA-modified BMSCs (Fig. 3C, D, E), consistent with the reduction of apoptosis induced by hypoxia, indicating that Survivin might repress hypoxia-induced apoptosis in PC12 cells.
VEGF secreted from HIF-1α-AA-modified BMSCs prevent apoptosis
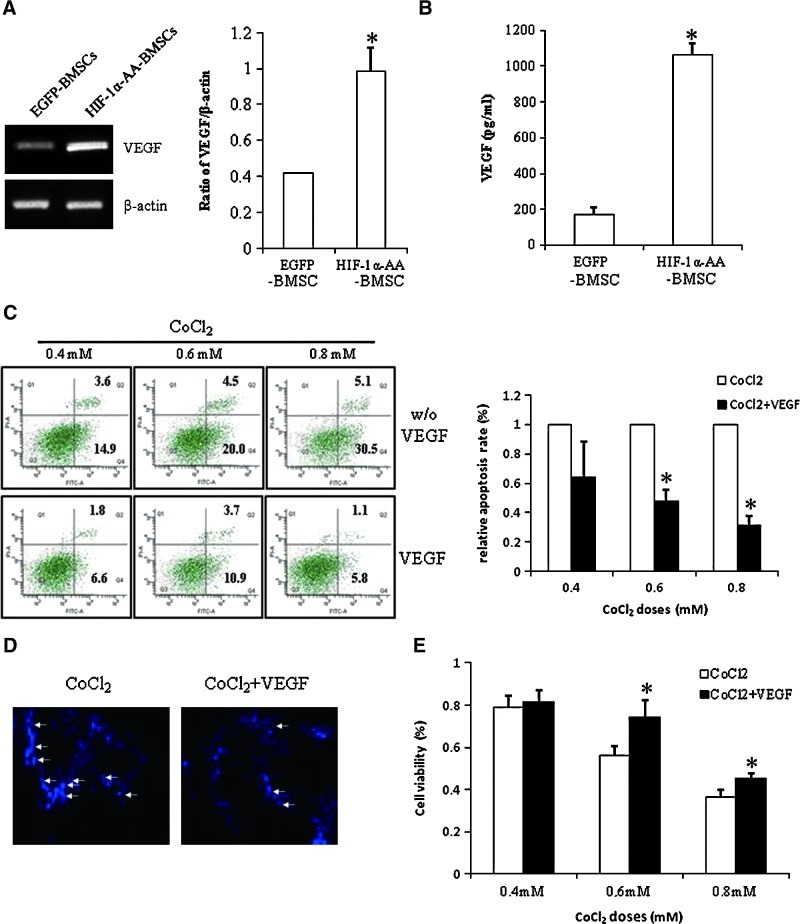
BMSCs have been shown to benefit neurological recovery after cerebral ischemia [6,29]. The possible mechanisms are differentiating into neuron-like cells [4,30] and excreting trophic factors [5,6]. It is well recognized that VEGF was upregulated by HIF-1α [31]. Our previous study showed that VEGF released from BMSCs plays an important role in mediating BMSCs' beneficial effects of neuronal survival and proliferation [32]. To explore whether VEGF is also involved in the cytoprotection of HIF-1α-AA-modified BMSCs, we cultured PC12 cells on the bottom of transwell plate and HIF-1α-AA-modified BMSCs on the transwell plates filters with the 0.4 μm pore. Therefore, only factors secreted by BMSCs will go through the filters and act on PC12 cells grown on the bottom layer of transwell plate. We analyzed the expression of VEGF in BMSCs and found that VEGF expression was enhanced in HIF-1α-AA-modified BMSCs, suggesting that overexpression of HIF-1α-AA increased the expression level of VEGF (Fig. 4A). In addition, we quantified the VEGF level in the culture medium of EGFP-BMSCs and HIF-1α-AA-modified BMSCs and observed that the release of VEGF from HIF-1α-AA-modified BMSCs was obviously higher than that from EGFP-BMSCs (Fig. 4B). Then, we applied exogenous VEGF to examine the protective effects in PC12 cells. VEGF decreased the cell apoptosis induced by 0.4 mM, 0.6 mM, or 0.8 mM CoCl2 (Fig. 4C) and reduced the number of positively stained PC12 cells by Hoechst staining (Fig. 4D), indicating that VEGF secreted by BMSCs might play an important role in protection of PC12 cells from hypoxia. Likewise, cell viability also increased with the addition of VEGF at different doses of CoCl2 (Fig. 4E).
FIG. 4.
Vascular endothelial growth factor (VEGF) secreted from HIF1α-AA-modified BMSCs prevents apoptosis. (A) Representative picture of PCR of VEGF expression in EGFP BMSCs or HIF1α-AA-BMSCs. β-actin was used as a control for protein loading. Data are means±SEM (n=3); P≤0.05; *significant difference from EGFP BMSCs. (B) The level of the released VEGF from EGFP BMSCs or HIF1α-AA-BMSCs by ELISA. Data are means±SEM (n=4); P≤0.05; *significant difference from EGFP BMSCs. (C) Histograms of flow cytometry of Annexin-V/PI staining and relative apoptotic ratio of PC12 cells treated with different doses of CoCl2 and with or without VEGF (n=3). Data are means±SEM; P≤0.05; *significant difference from CoCl2 treatment only at the same dosage. (D) Hoechst staining of PC12 cells treated with 0.6 mM CoCl2 and with or without VEGF (100×) (n=2). Hoechst-positive staining is denoted by white arrows. (E) Histograms and quantitative analysis of MTT assay (n=8) of PC12 cells treated with different doses of CoCl2 and with or without VEGF. Data are means±SEM; P≤0.05; *significant difference from CoCl2 treatment only at the same dosage. Color images available online at www.liebertonline.com/scd
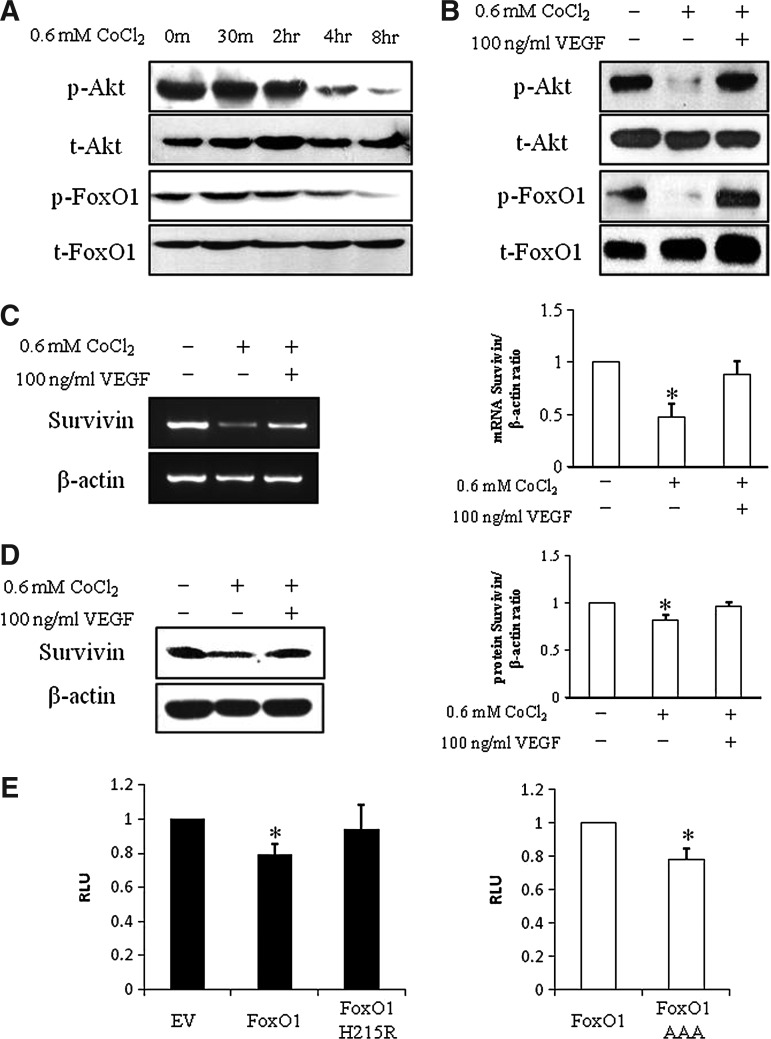
VEGF regulates Survivin through the p-AKT/p-FoxO1 pathway
Although VEGF has been shown to promote endothelial cell survival [33], the exact mechanisms of how VEGF regulates neuron survival were still elusive. We examined VEGF downstream pathways p-Akt and p-ERK1/2 after treatment with CoCl2, with or without VEGF. It showed that p-Akt was slightly decreased at the beginning with the addition of CoCl2, then was dramatically repressed after 4 h and p-FoxO1 was reduced at 8 h with the treatment of CoCl2 (Fig. 5A), whereas there was no change of p-ERK 1/2 under CoCl2-induced hypoxia (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd), suggesting that hypoxia depressed the p-Akt/p-FoxO1 pathway but not the p-ERK 1/2 pathway. Administration of VEGF increased the expression of p-Akt at 4 h and induced phosphorylated FoxO1 at 8 h (Fig. 5B), indicating that VEGF activated the p-Akt pathway, further leading to FoxO1 inactivation by phosphorylation.
FIG. 5.
VEGF regulates Survivin through the p-AKT/p-FoxO1 pathway. (A) Representative picture of western blotting of p-Akt, Akt, p-FoxO1, and FoxO1 in PC12 cells treated with 0.6 mM CoCl2 at the indicated time points (n=3). (B) Representative picture of western blotting of p-Akt, Akt, p-FoxO1, and FoxO1 in PC12 cells treated with 0.6 mM CoCl2 and with or without VEGF (n=3). Representative pictures of PCR (C) and western blotting (D) of Survivin in PC12 cells treated with 0.6 mM CoCl2 and with or without VEGF. β-actin was used as an internal control. Data are means±SEM (n=3); *significant difference from control. (E) PC12 cells were co-transfected with the Survivin-luc reporter construct and with empty vector, FoxO1, or a FoxO1 construct lacking the DNA binding capacity (FoxO1 H215R) as indicated. Data are means±SEM (n=3); P≤0.05; *significant difference from empty vector. (F) PC12 cells were co-transfected with the Survivin-luc reporter construct and with either with FoxO1 or a dephosphorylated form of FoxO1, which renders it constitutive activity (FoxO1 AAA) as indicated. About 48 h later, dual luciferase assay was performed. Data are means±SEM (n=3); P≤0.05; *significant difference from transfection of FoxO1.
The addition of VEGF also rescued Survivin expression that it is repressed by CoCl2 treatment at both mRNA level (Fig. 5C) and protein level (Fig. 5D), which was consistent with the cell protection from hypoxia-induced apoptosis. To investigate whether FoxO1 directly regulates Survivin expression, we co-transfected wild-type FoxO1 or FoxO1 H215R, a FoxO1-binding mutant, into PC12 cells, respectively. As shown in Fig. 5E, wild-type FoxO1 repressed about 30% Survivin promoter activity, but FoxO1 H215R lost the inhibitory ability on Survivin promoter activity, suggesting that FoxO1 regulates Survivin expresssion as a transcriptional repressor. Then, we further explored whether the phosphorylation of FoxO1 is involved in the regulation of the Survivin promoter, FoxO1 or FoxO1 AAA, which is an activated form of FoxO1, was transfected into PC12 cells, respectively. Interestingly, FoxO1 AAA repressed further 30% Survivin promoter activity compared to wild-type FoxO1 (Fig. 5F), indicating that nonphosphorylated FoxO1 has a better efficiency in repressing Survivin and the phophorylation of FoxO1 plays an important role in the regulation of Survivin.
HIF-1α-AA-modified BMSCs protect neurons from ischemia
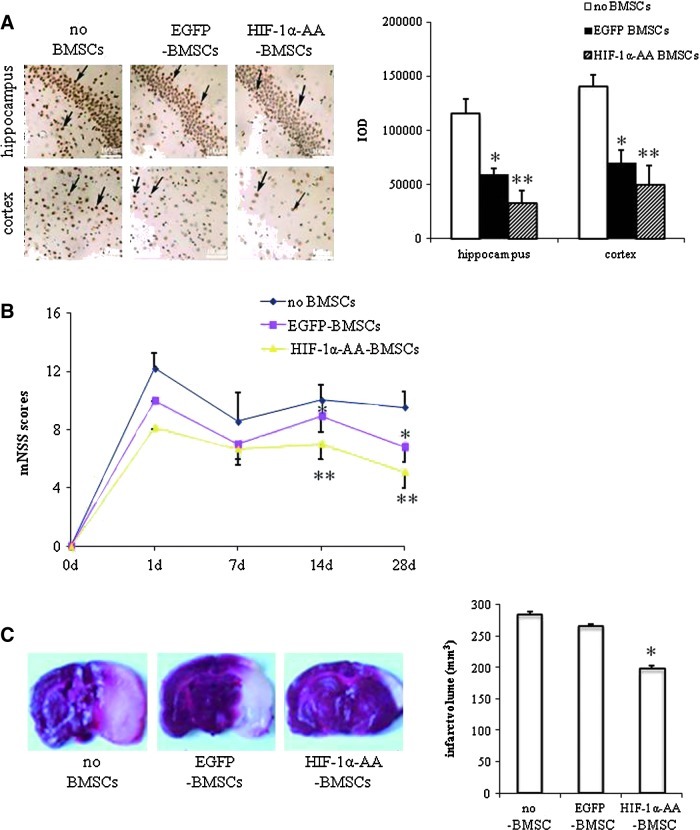
To investigate the effects of HIF-1α-AA-modified BMSCs in protecting neurons from ischemia, we established ischemic stroke models that were induced by permanent MCAo in Sprague-Dawley rats, and then transplanted HIF-1α-AA-modified BMSCs into these rats intravenously. At the beginning, we examined the neuronal apoptosis with TUNEL immunohistochemistry staining in the ischemia cortex and hippocampus region in 14 days after transplantation. At day 14 after MCAo, neuronal apoptosis was prominently less in both hippocampus (∼7.2×104, P<0.05) and cortex (3.7×104, P<0.01) of the HIF-1α-AA-BMSCs group compared with EGFP-BMSCs rats (1.4×105 and 1.8×105, respectively), or the no BMSCs group of rats (sham group) (1.6×105 and 1.3×105, respectively) (Fig. 6A), suggesting the neuronal protective effects of HIF-1α-AA-modified BMSCs to PC12 cells.
FIG. 6.
HIF1α-AA-modified BMSCs protect neuron from ischemia. (A) Representative photographs and quantitative analysis of hippocampus and cortex tissue using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay in 3 groups of rats (no BMSCs group [sham group], EGFP-BMSCs group, and HIF1α-AA-BMSCs group) at day 14 after middle cerebral artery occlusion (MCAo) (n=3). Scale bar, 100 μm; data are means±SEM; P≤0.05; *significant difference from sham group (no BMSCs); **significant difference from EGFP-BMSCs group. (B) Comparison of neurological outcome on the modified neurological severity score (mNSS) among the 3 groups of rats at days 1, 7, 14, and 28 after MCAo (n=6). Data are means±SEM; P≤0.05; *significant difference from sham group (no BMSCs); **significant difference from EGFP-BMSCs group. (C) Cerebral infarct volume of 3 groups of rats measured by straining brain slices with TTC at day 7 after MCAo (n=3). Data are means±SEM; P≤0.05; *significant difference from sham group (no BMSCs) and EGFP-BMSCs group. Color images available online at www.liebertonline.com/scd
Next, we evaluated neurological function using a modified neurological severity score (mNSS) and only included the moderate and severely injured rats (mNSS>6) in subsequent experiments (>90% of rats in each group). No significant difference was detected among 3 groups of animals before surgery and before treatment. However, the rats treated with HIF-1α-AA-modified BMSCs had significant improvement on their mNSS compared to those without BMSCs treatment (sham group) from day 7 after MCAo. Additionally, the significant neurological functional recovery was observed in the EGFP-BMSCs group compared to the sham-group rats 14 days after MCAo. At days 14 and 28 after MCAo, significant neurological functional recovery in rats with HIF-1α-AA-BMSCs was observed when compared to either the EGFP-BMSCs or sham groups (Fig. 6B).
In addition, cerebral infarction volume was measured by TTC staining. Significant reductions of infarction volume were found in rats of the HIF-1α-AA-BMSCs group on day 3 after MCAo (Fig. 6C), further indicating beneficial effects of neuronal protection and neurological improvement with transplantation of HIF-1α-AA-modified BMSCs.
Discussion
Cerebral hypoxia is defined as the reduction of the oxygen supply in the brain, often caused by brain ischemia [10]. The prolonged shortness of oxygen in the brain usually leads to neuronal disorder, even apoptosis and necrosis. Therefore, the severity of the cerebral hypoxia is dependent on the neuron survival and improved neurological outcome. During the past decade, administration of BMSC has been shown to be a potential cell therapy in brain ischemia [34,35]. Perrasso L. also showed that intravenous administration of BMSCs after global cerebral ischemia decreases hippocampal neural damage, suggesting that BMSCs increase neuron survival by intravenous infusion [3]. In our previous study, we demonstrated that EGFP-BMSCs migrate to hippocampus and cortex regions under ischemia condition after intravenous injection [36], indicating that BMSCs could pass blood–brain barrier and go to hippocampus and cortex. After transplantation into the body, MSCs have shown the ability to differentiate into neuron-like cells that secrete trophic factors such as VEGF [32], BDNF [8], and NGF [37], which play important roles in protecting the injured neurons.
In the current study, we induced the HIF-1α mutant (Pro402Ala and Pro564Ala) in BMSCs by lentiviral transduction and observed better neuronal protection and improvement of neurological deficits. Mutant HIF-1α is more stable than wild type and enhances the regulation of various HIF-1α downstream targets such as VEGF [31], CXCR4 [16], and EPO [38]. VEGF is upregulated by HIF-1α by directly binding to the HRE element on the VEGF promoter [31]. It is well known that VEGF is the main contributor for angiogenesis by promoting endothelial cell survival, proliferation, and migration [33,39,40]. Furthermore, Oosthuyse et al. (2001) found that VEGF also acts on motor neurons as a neuroprotective factor [41]. We examined a further increase of VEGF expression identified in the HIF-1α mutant expressed BMSCs (Fig. 4A), leading to an increased release of VEGF from HIF-1α-AA-modified BMSCs (Fig. 4B). Administration of VEGF in vitro protected neuron-like PC12 cells from hypoxia-induced apoptosis. Similarly, our previous study showed that knocking down VEGF expression attenuated the BMSCs' beneficial effects of neuronal survival and proliferation [32]. Taken together, these results suggest that VEGF might play an essential role in neuronal protection by the HIF-1α mutant expressed BMSCs. The interaction of CXCR4 expression on the BMSC surface and SDF-1 expression in the injured brain area attracts BMSCs to the ischemic lesion of brain [36]. EPO has been shown to get rid of ROS and have anti-inflammation effects in ischemia brain [38]. Whether the HIF-1α mutant expressed BMSCs also have a further increased expression on CXCR4 or EPO need to be investigated in future.
The exact mechanisms of how VEGF regulates neuron survival were not fully understood. Survivin, a member of the inhibitors of apoptosis proteins (IAP) [28], inhibits cell apoptosis by interfering with the function of caspases [42]. Many studies have presented the upregulated expression of Survivin in vascular endothelial cells after brain ischemia [43,44]. However, a couple of reports mentioned that there are less colocalization of TUNEL-labeled neurons and Survivin staining in brain tissue with traumatic brain injury [45], suggesting that Survivin represses neuron apoptosis after TBI. Our study showed that hypoxia decreased the expression of Survivin with an increase of cell apoptotic rates, but the administration of the HIF-1α-AA-BMSCs increased the expression of Survivin and reduced PC12 cell apoptosis. Furthermore, VEGF, which was further enhanced by the HIF-1α-AA-BMSCs, also upregulated the expression of Survivin under hypoxia conditions and was accompanied with a decreased apoptotic rate, indicating that the HIF-1α-AA-modified BMSCs promote cellular survival through the regulation of Survivin by VEGF.
We further investigated the underlying mechanisms of how VEGF regulates Survivin. Phosphatidylinositol 3- kinase (PI-3K) and serine-threonine protein kinase (Akt) pathway [46–48] and Raf/MEK/ERK pathway [49] have been reported to be involved in VEGF promoted cell survival. We checked the expression of p-Akt, p-FoxO1, and p-ERK 1/2 under hypoxia and further applied exogenous VEGF to examine whether VEGF activates these pathways in PC12 cells. Interestingly, we found that the hypoxia condition only affected the expression level of p-Akt and p-FoxO1 (Fig. 5A) but not p-ERK 1/2 (Supplementary Fig. S1), and then with the addition of the VEGF activated p-Akt pathway (Fig. 5B), indicating that VEGF activates p-Akt, which was downregulated by hypoxia, thereby leading to neuron survival.
Transcription factor FoxOs belong to the Fox family, which is involved in many important cellular processes, such as regulation of mammalian cell apoptosis, stress response, angiogenesis, and glucose metabolism [50]. It has shown that p-Akt phosphorylates FoxO in various cell lines to transfer phosphorylated FoxO from the nucleus to the cytoplasm, which causes the inactivation of FoxO transcriptional activity and suppresses the expression of the downstream gene of FoxO [51]. FoxO1 is an important transcriptional factor involved in cell apoptosis [52] and stress response [53]. FoxO1 and FoxO3 have been reported to bind directly on the Survivin promoter as a transcription repressor [54]. In our study, we presented that FoxO1 is involved in apoptosis of PC12 cells by detecting the reduction of p-FoxO1 under hypoxia, whereas this reduction was reversed by VEGF, suggesting that phosphorylation of Foxo1 is regulated by VEGF. We transfected wild-type FoxO1 into PC12 cells, resulting in 30% reduction of Survivin promoter activities. In addition, a FoxO1 mutant—FoxO1 AAA—as an activated form of FoxO1 with 3 mutated phosphorylation sites resulted in further repression on the Survivin promoter activities, supporting the role of phosphorylation of FoxO1 in the regulation of Survivin.
Conclusion
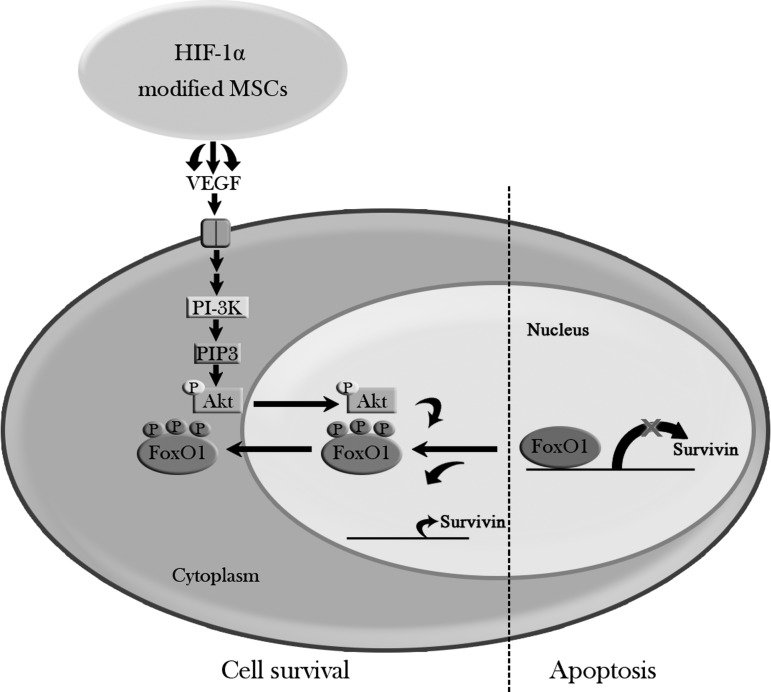
Our research work showed that HIF-1α-AA-modified BMSCs presented better neuron protection and an improvement of neurological function under hypoxic conditions, which might provide a better therapy of BMSC administration to cerebral ischemia. We have also demonstrated the possible mechanism of the beneficial effects of HIF-1α-AA-modified BMSCs acting, at least in part, through secretion of VEGF, activating its downstream signaling pathways—Akt/FoxO1 (Fig. 7).
FIG. 7.
Model of anti-apoptotic effects of HIF-1α-modified MSCs. HIF1α-AA-modified BMSCs act on PC12 cells, at least in part, through secretion of VEGF, further activating its downstream signaling pathways—PI3K/p-Akt. Then, p-Akt phophorylates FoxO1 and causes p-FoxO1 translocated out from nucleus to cytoplasm, leading to the loss of FoxO1 repression on Survivin promoter, thereby promoting cell survival.
Supplementary Material
Acknowledgments
We are thankful to Helge Chrisopher Leukel for correcting the English of this article. This work was supported by the National Natural Science Foundation of China (NSFC) Grants (30901547, 30973093, and 81171711), Doctoral Fund of Ministry of Education 20090171110048, Guangdong Province Natural Science Fund (C030307 and 2009B050200010), Yi-xian Innovators' Training Project by Outstanding Mentor(s) at Sun Yat-sen University (50000-3126203), and the Graduates' internationally cooperated research project of Sun Yat-sen University (2008–2009).
Author Disclosure Statement
None of the authors have any potential conflicts of interest.
References
- 1.Donnan GA. Fisher M. Macleod M. Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization The world health report 2004. Annex Table 2: Deaths by cause, sex, and mortality stratum in WHO regions, estimates for 2002. 2004.
- 3.Perasso L. Cogo CE. Giunti D. Gandolfo C. Ruggeri P. Uccelli A. Balestrino M. Systemic administration of mesenchymal stem cells increases neuron survival after global cerebral ischemia in vivo (2VO) Neural Plast. 2010;2010:534925. doi: 10.1155/2010/534925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J. Moochhala S. Moore XL. Ng KC. Tan MH. Lee LK. He B. Wong MC. Ling EA. Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398:12–17. doi: 10.1016/j.neulet.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 5.Chen X. Li Y. Wang L. Katakowski M. Zhang L. Chen J. Xu Y. Gautam SC. Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Chopp M. Li Y. Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda N. Nonoguchi N. Zhao MZ. Watanabe T. Kajimoto Y. Furutama D. Kimura F. Dezawa M. Coffin RS, et al. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36:2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- 8.Kurozumi K. Nakamura K. Tamiya T. Kawano Y. Kobune M. Hirai S. Uchida H. Sasaki K. Ito Y, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhao MZ. Nonoguchi N. Ikeda N. Watanabe T. Furutama D. Miyazawa D. Funakoshi H. Kajimoto Y. Nakamura T, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 10.Sharp FR. Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 11.Iyer NV. Leung SW. Semenza GL. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. doi: 10.1006/geno.1998.5416. [DOI] [PubMed] [Google Scholar]
- 12.Ivan M. Kondo K. Yang H. Kim W. Valiando J. Ohh M. Salic A. Asara JM. Lane WS. Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola P. Mole DR. Tian YM. Wilson MI. Gielbert J. Gaskell SJ. Kriegsheim A. Hebestreit HF. Mukherji M, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 14.Masson N. Willam C. Maxwell PH. Pugh CW. Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu CJ. Wang LY. Chodosh LA. Keith B. Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X. Li C. Chen Y. Hao Y. Zhou W. Chen C. Yu Z. Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation. Biochem Biophys Res Commun. 2008;371:283–288. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 17.Giaccia A. Siim BG. Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y. Xu M. Wang Y. Pasha Z. Li T. Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–1044. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vangeison G. Carr D. Federoff HJ. Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci. 2008;28:1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colter DC. Sekiya I. Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polak M. Scharfmann R. Seilheimer B. Eisenbarth G. Dressler D. Verma IM. Potter H. Nerve growth factor induces neuron-like differentiation of an insulin-secreting pancreatic beta cell line. Proc Natl Acad Sci U S A. 1993;90:5781–5785. doi: 10.1073/pnas.90.12.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang XQ. Feng JQ. Chen J. Chen PX. Zhi JL. Cui Y. Guo RX. Yu HM. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005;1057:57–64. doi: 10.1016/j.brainres.2005.07.072. [DOI] [PubMed] [Google Scholar]
- 23.Alsayed Y. Ngo H. Runnels J. Leleu X. Singha UK. Pitsillides CM. Spencer JA. Kimlinger T. Ghobrial JM, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longa EZ. Weinstein PR. Carlson S. Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;21:89–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 25.Belayev LAO. Busto R. Zhao W. Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 26.Puurunen K. Jolkkonen J. Sirvio J. Haapalinna A. Sivenius J. Selegiline combined with enriched-environment housing attenuates spatial learning deficits following focal cerebral ischemia in rats. Exp Neurol. 2001;167:348–355. doi: 10.1006/exnr.2000.7563. [DOI] [PubMed] [Google Scholar]
- 27.An WG. Kanekal M. Simon MC. Maltepe E. Blagosklonny MV. Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosini G. Adida C. Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 29.Chen J. Li Y. Katakowski M. Chen X. Wang L. Lu D. Lu M. Gautam SC. Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 30.Deng YB. Yuan QT. Liu XG. Liu XL. Liu Y. Liu ZG. Zhang C. Functional recovery after rhesus monkey spinal cord injury by transplantation of bone marrow mesenchymal-stem cell-derived neurons. Chin Med J (Engl) 2005;118:1533–1541. [PubMed] [Google Scholar]
- 31.Forsythe JA. Jiang BH. Iyer NV. Agani F. Leung SW. Koos RD. Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng YB. Ye WB. Hu ZZ. Yan Y. Wang Y. Takon BF. Zhou GQ. Zhou YF. Intravenously administered BMSCs reduce neuronal apoptosis and promote neuronal proliferation through the release of VEGF after stroke in rats. Neurol Res. 2008;32:148–156. doi: 10.1179/174313209X414434. [DOI] [PubMed] [Google Scholar]
- 33.Gerber HP. Condorelli F. Park J. Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 34.Chopp M. Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 35.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y. Deng Y. Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz G. Alexander JS. Erkuran Yilmaz C. Granger DN. Induction of neuro-protective/regenerative genes in stem cells infiltrating post-ischemic brain tissue. Exp Transl Stroke Med. 2010;2:11. doi: 10.1186/2040-7378-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solaroglu I. Solaroglu A. Kaptanoglu E. Dede S. Haberal A. Beskonakli E. Kilinc K. Erythropoietin prevents ischemia-reperfusion from inducing oxidative damage in fetal rat brain. Childs Nerv Syst. 2003;19:19–22. doi: 10.1007/s00381-002-0680-2. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau S. Houle F. Landry J. Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 40.Neufeld G. Cohen T. Gengrinovitch S. Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 41.Oosthuyse B. Moons L. Storkebaum E. Beck H. Nuyens D. Brusselmans K. Van Dorpe J. Hellings P. Gorselink M, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 42.Shin S. Sung BJ. Cho YS. Kim HJ. Ha NC. Hwang JI. Chung CW. Jung YK. Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and −7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 43.Conway EM. Zwerts F. Van Eygen V. DeVriese A. Nagai N. Luo W. Collen D. Survivin-dependent angiogenesis in ischemic brain: molecular mechanisms of hypoxia-induced up-regulation. Am J Pathol. 2003;163:935–946. doi: 10.1016/S0002-9440(10)63453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y. Xia ZL. Chen LB. HIF-1-alpha and survivin involved in the anti-apoptotic effect of 2ME2 after global ischemia in rats. Neurol Res. 2011;33:583–592. doi: 10.1179/1743132810Y.0000000013. [DOI] [PubMed] [Google Scholar]
- 45.Johnson EA. Svetlov SI. Pike BR. Tolentino PJ. Shaw G. Wang KK. Hayes RL. Pineda JA. Cell-specific upregulation of survivin after experimental traumatic brain injury in rats. J Neurotrauma. 2004;21:1183–1195. doi: 10.1089/neu.2004.21.1183. [DOI] [PubMed] [Google Scholar]
- 46.Dimmeler S. Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z. Jiang C. Ganther H. Lu J. Antimitogenic and proapoptotic activities of methylseleninic acid in vascular endothelial cells and associated effects on PI3K-AKT, ERK, JNK and p38 MAPK signaling. Cancer Res. 2001;61:7171–7178. [PubMed] [Google Scholar]
- 48.Dormond O. Madsen JC. Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282:23679–23686. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podar K. Tai YT. Davies FE. Lentzsch S. Sattler M. Hideshima T. Lin BK. Gupta D. Shima Y, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–435. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- 50.Obsil T. Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27:2263–2275. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- 51.Greer EL. Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 52.Tang ED. Nunez G. Barr FG. Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 53.Tran H. Brunet A. Griffith EC. Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 54.Guha M. Plescia J. Leav I. Li J. Languino LR. Altieri DC. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res. 2009;69:4954–4958. doi: 10.1158/0008-5472.CAN-09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.