Abstract
Context:
Exercise is recommended for individuals with diabetes mellitus, and several facets of the disease must be considered when managing the diabetic athlete. The purpose of this article is to review diabetes care in the context of sports participation.
Evidence Acquisition:
Relevant studies were identified through a literature search of MEDLINE and the Cochrane database, as well as manual review of reference lists of identified sources.
Results:
Diabetics should be evaluated for complications of long-standing disease before beginning an exercise program, and exercise should be modified appropriately if complications are present. Athletes who use insulin or oral insulin secretogogues are at risk for exercise-induced immediate or delayed hypoglycemia. Diabetics are advised to engage in a combination of regular aerobic and resistance exercise. Insulin-dependent diabetics should supplement carbohydrate before and after exercise, as well as during exercise for events lasting longer than 1 hour. Adjustment of insulin dosing based on planned exercise intensity is another strategy to prevent hypoglycemia. Insulin-dependent athletes should monitor blood sugar closely before, during, and after exercise. Significant hyperglycemia before exercise should preclude exercise because the stress of exercise can paradoxically exacerbate hyperglycemia and lead to ketoacidosis. Athletes should be aware of hypoglycemia symptoms and have rapidly absorbable glucose available in case of hypoglycemia.
Conclusion:
Exercise is an important component of diabetes treatment, and most people with diabetes can safely participate in sports at recreational and elite levels with attention to appropriate precautions.
Keywords: diabetic athlete, medication adjustment, insulin pump, benefits and risks of sport
Diabetes mellitus (DM) affects 7% of Americans, according to 2005 data from the Centers for Disease Control and Prevention.9 The prevalence of DM is increasing owing to the national epidemic of obesity.24 Exercise can delay the onset of diabetes in individuals with impaired glucose tolerance.19,21,22,25,34 Exercise aids in diabetes treatment by improving glucose metabolism8,33 and insulin sensitivity,36 and it can reduce the use of oral medications and insulin. Participation in team sports early in life has been associated with decreased macrovascular complications and mortality for type 1 diabetics.20 To avoid harmful effects, exercise may require modifications for individuals with complications of diabetes.3 Physicians must carefully adjust treatment and monitor care of diabetic athletes to maximize their safe participation in sport.
There are separate but related considerations for athletes with type 1 and type 2 DM (respectively, DM1 and DM2). Individuals with DM1 are typically thin and experience onset of the illness at a young age. Their disease is caused by an autoimmune process that destroys the insulin-producing beta cells in the pancreas, rendering them completely dependent on exogenous insulin.14 Management of insulin dosing and glycemic control is an important component of their athletic participation. Type 1 diabetics with long-standing disease often have end-organ complications. Athletes with DM1 may participate in sports at many levels, ranging from recreational to elite, with appropriate care.
DM2 most commonly affects overweight adults, although it is on the rise in younger age groups, secondary to childhood obesity. DM2 is caused by insulin resistance and relative insulin deficiency and can sometimes be controlled through lifestyle changes such as diet and exercise. Type 2 diabetics often require oral medications and in the later stages of the disease may require insulin injection. Just as for DM1, management of exercise includes consideration of diabetes complications. Most athletes with DM2 participate in recreational-level sports.
Physiology Overview
An increase in plasma glucose concentration after a meal stimulates insulin secretion by the beta cells of the pancreas.11 Insulin decreases plasma glucose by decreasing hepatic glycogenolysis, decreasing delivery of glucose precursors to the liver, and increasing glucose uptake by muscle and fat. When blood glucose is low, the counterregulatory hormone of insulin, glucagon, stimulates the liver to generate glucose, and insulin secretion is suppressed.
During exercise, insulin is suppressed to allow for glucose release from the liver. However, the muscle cells are more sensitive to the remaining insulin, allowing for more efficient glucose uptake. Blood glucose levels typically drop modestly but not to the point of hypoglycemia, because of the decrease in insulin secretion and the increase in counterregulatory hormones. Following exercise, insulin levels increase to facilitate the storage of excess glucose (which was released into the bloodstream because of the demand in exercise).
Non-insulin-dependent diabetics do not generally have significant hyper- or hypoglycemic episodes in exercise. Insulin-dependent diabetics are at much higher risk for these issues owing to more dramatic abnormalities of glucose homeostasis and because of the presence of exogenous insulin.
The effects of hypoglycemia include autonomic central nervous system decline. Symptoms may include hunger, sweating, palpitations, anxiety, lightheadedness, fatigue, weakness, and confusion.
The Challenges of Managing the Diabetic Athlete With Complications
Smoking cessation, blood pressure control, and lipid management are important in preventing complications of diabetes.14 Glycemic control is addressed through diet, exercise, and medications. However, reasonable glycemic control is an essential consideration before initiation of an exercise program for the diabetic athlete. The physician should establish a baseline hemoglobin A1c (HbA1c). In type 1 diabetics with levels higher than 9%, no vigorous exercise is advised until baseline control is improved (normal HbA1c in individuals without DM is less than 6%).23 For type 2 diabetics with a poorly controlled HbA1c, the exercise program should begin gradually. The physician may allow sequential increases in exercise intensity as long as diabetic control improves.
Many diabetics develop macrovascular and microvascular complications of their disease, such as coronary artery disease, peripheral vascular disease, autonomic neuropathy, peripheral neuropathy, retinopathy, nephropathy, and musculoskeletal problems.14 American Diabetes Association (ADA) guidelines5-7 recommend evaluating diabetics for complications before initiating an exercise program. Identification of complications will allow physicians to appropriately modify the exercise prescription to minimize risks.3,5,6
Diabetic athletes should wear a MedicAlert-type bracelet or necklace indicating their diagnosis and specifying if it is DM1 or DM2.
Coronary Artery Disease
When contemplating an exercise program, it is important to consider cardiovascular risk factors of the diabetic athlete and to screen for coronary artery disease with exercise treadmill testing, if appropriate.3,5 Screening asymptomatic diabetics for coronary disease remains controversial. Guidelines of the American College of Sports Medicine and the American College of Cardiology recommend screening diabetic patients with exercise treadmill testing before initiation of a moderate to high-intensity exercise program.3,13 However, the most recent position statement of the ADA no longer recommends universal cardiac stress testing based on age or duration of disease, although its recommendation does not directly address the population of diabetics initiating vigorous exercise.6 Physicians should consider screening previously sedentary diabetics for coronary disease, particularly if they are high risk, before initiation of moderate to intense exercise.3,5,13 At a minimum, high-risk patients should be cautioned to embark on an exercise program of low intensity and to gradually increase intensity and duration.6 Table 1 summarizes the characteristics of diabetics at high risk for cardiovascular disease. Diabetics with established coronary disease who do not have ischemia or significant arrhythmias should not generally engage in intense exercise (60% to 80% maximum heart rate). For those with angina, target heart rate should be at least 10 beats below their ischemic threshold.3
Table 1.
Characteristics of diabetics at increased risk for cardiovascular disease.5
| Age > 35 years |
| Age > 25 years; type 2 diabetes mellitus > 10 years |
| Age > 25 years; type 1 diabetes mellitus > 15 years |
| Presence of other risk factors for cardiovascular disease |
| Microvascular complications of diabetes: retinopathy, nephropathy |
| Peripheral vascular disease |
| Autonomic neuropathy |
Peripheral Vascular Disease
Individuals with peripheral vascular disease who have claudication may need to modify activities by decreasing intensity or utilizing intermittent periods of rest during exercise sessions to minimize symptom provocation.3 Walking or other low-intensity exercise programs below the symptomatic level are encouraged as a part of the overall management of these patients.3,5
Autonomic Neuropathy
Autonomic neuropathy may be manifested by elevated resting heart rate, orthostatic hypotension, or abnormal chronotropic and blood pressure responses to exercise.3,5 Diabetics with autonomic neuropathy generally have lower overall fitness levels and are at increased risk of adverse cardiovascular events during exercise (silent myocardial ischemia or sudden death).5 Individuals with autonomic neuropathy should undergo exercise treadmill testing before embarking on an exercise program.5 Autonomic neuropathy disrupts thermoregulation, so exercise in hot or cold environments should proceed with caution and careful monitoring. If gastroparesis is present, delayed carbohydrate absorption may predispose the diabetic to hypoglycemia.6 To monitor exercise intensity, athletes with autonomic neuropathy should use relative perceived exertion rather than heart rate response.18
Peripheral Neuropathy
Individuals with significant peripheral neuropathy lose protective sensation in the feet, which is detectable with the 10-g monofilament test.14 With the loss of protective sensation, repetitive weightbearing exercise can lead to skin ulceration and breakdown, fracture, and/or Charcot joint degeneration, making supportive footwear essential. Significant peripheral neuropathy is a reason to limit weightbearing exercise and suggest safer activities, such as swimming, bicycling, or arm exercises.3,5,6
Diabetic Retinopathy
There is no evidence that physical activity affects the progression of diabetic retinopathy.2,10 However, diabetics with any degree of proliferative retinopathy or severe nonproliferative retinopathy should avoid activities that increase intraocular pressure, such as weightlifting or high-intensity aerobic activities, because of the risk of vitreous hemorrhage or retinal detachment.3,5,6
Diabetic Nephropathy
There is no evidence that the elevated blood pressures generated during exercise exacerbate progression of diabetic nephropathy.3 However, considering the serious implications of progressive renal damage, individuals with nephropathy should avoid vigorous activity that could cause a significant rise in systolic blood pressure.3,5
Orthopaedic Complications
Fascial disease, adhesive capsulitis, tendon pathology, Dupuytren contractures, flexor tenosynovitis, and nerve entrapments occur with increased frequency in individuals with diabetes.14
Diffuse idiopathic skeletal hyperostosis (calcification of the paraspinal ligaments) is more common in individuals with diabetes. Neuropathic joint collapse (Charcot joint) can also develop, marked by progressive degeneration of weightbearing joints in the setting of peripheral neuropathy.14
Diabetic amyotrophy is a lumbar polyradiculopathy manifesting with severe pain, dysesthesias, and muscle atrophy of the thighs. The presence of these or other orthopaedic conditions may affect the ability of diabetic individuals to participate in certain exercise activities.14
Medications Used in Diabetes Care
The ultimate goal of medical therapy for diabetes is to prevent complications. Therapy consists of a combination of diet, exercise, and medications. Individuals with DM1 are dependent on insulin to control blood glucose levels. Individuals with DM2 may be treated with oral medications and/or insulin therapy. Most advice regarding pharmacologic treatment of diabetes in athletes is based on expert opinion of treating diabetic athletes rather than on controlled trials. Tables 2 and 3 summarize medication used in diabetes.
Table 2.
Insulin preparations.14
| Insulin | Onset | Peak | Duration |
|---|---|---|---|
| Rapid-acting | |||
| Lispro: Humalog | 10-15 min | 1-2 hr | 3-4 hr |
| Aspart: NovoLog | |||
| Glulisine: Apidra | |||
| Short-acting | |||
| Regular: Humulin R, Novolin R | 30 min | 2-4 hr | 5-8 hr |
| Intermediate-acting | |||
| NPH: Humulin N, Novolin N | 2-4 hr | 6-12 hr | 16-24 hr |
| Long-acting | |||
| Detemir: Levemir | 1 hr | No peak | up to 24 hr |
| Glargine: Lantus | 1-4 hr | No peak | > 30 hr |
Table 3.
Overview of oral diabetes medications.14
| Class of Medication | Examples | Mechanism of Action | Side Effects | Sports Considerations |
|---|---|---|---|---|
| Biguanides | metformin: Glucophage, Fortamet, Glumetza, Riomet | Decrease hepatic glucose release; increase peripheral glucose utilization | Gastrointestinal upset; lactic acidosis (rare) | None known |
| Sulfonylureas | glimepiride: Amaryl glyburide: DiaBeta, Micronase glipizide: Glucotrol chlorpropamide: Diabinese tolazamide tolbutamide |
Stimulate insulin secretion by pancreas | Hypoglycemia | Make plan for prevention and management of hypoglycemia, particularly delayed hypoglycemia |
| Thiazolidinediones | pioglitazone: Actos rosiglitazone: Avandia |
Increase sensitivity to insulin | Edema; increased fracture risk in women; increased cardiovascular events with rosiglitazone | None known |
| Alpha-glucosidase inhibitors | acarbose: Precose miglitol: Glyset |
Delay absorption of complex carbs from small intestine | Gastrointestinal upset | May improve glucose balance during exercise for type 1 diabetics |
| Incretin mimetics | exenatide: Byetta | Increase food-induced insulin secretion; slow gastric emptying; decrease appetite | Gastrointestinal upset; rare hypoglycemia | None known |
| Dipeptidyl peptidase-4 inhibitors | sitagliptin: Januvia | Increase food-dependent insulin release | Minimal | None known |
| Meglitinides | nateglinide: Starlix repaglinide: Prandin |
Stimulate insulin secretion by pancreas | Hypoglycemia (less common than with sulfonylureas) | Plan for prevention and management of hypoglycemia |
| Amylin mimetics | pramlintide: Symlin | Lower blood glucose levels; decrease glucagon levels; curb appetite | Can cause severe hypoglycemia | Plan for prevention and management of hypoglycemia |
Insulin
Injected insulin is a mainstay of diabetes therapy for type 1 diabetics and for type 2 diabetics with moderate to severe disease. Several different preparations are used, varying primarily in pharmacokinetics.14 Frequently, regimens consist of long-acting basal insulin with a short-acting multidose regimen taken at meals and bedtime (Table 2).14
Insulin pump technology continues to evolve, providing a device for continuous subcutaneous infusion with preprogrammed basal insulin infusion and patient-controlled bolus dosing of rapid-acting insulin. Pump advantages include rapid blood glucose adjustment and flexibility. Disadvantages include local infection, pump malfunction,14 and localized lipodystrophy.
Contact sports, water sports, and endurance events are relative contraindications to pump use.14 Pumps can be temporarily disconnected for contact or water sports participation or padded for sports with minimal contact. One small single-blind randomized crossover trial compared exercising with and without the pump. It showed no significant difference in hypoglycemic events during or after exercise, although there was a trend toward increased delayed hypoglycemia with the pump on.1 Athletes must carry injectable insulin as a backup in case the pump is damaged during sport or lost while disconnected.
The longer-acting insulins, such as glargine or detemir, have been used as pseudo-pumps, making them ideally suited for basal glycemic control. Bolus dosing can be supplemented, depending on the level of exercise. This can be a better strategy than a pump for athletes involved in water or contact sports.
Biguanides
Metformin (Glucophage, Fortamet, Glumetza, Riomet) is the only biguanide available in the United States. It is the first-line therapy for individuals with type 2 diabetes, acting to decrease hepatic gluconeogenesis and increase glucose utilization in peripheral tissues.14 Metformin can improve mortality rates and decrease myocardial infarction in DM2.31 Adverse effects include gastrointestinal distress and lactic acidosis. Hypoglycemia is rare.14 There is a theoretical increased risk of lactic acidosis with metformin usage in endurance athletes because exercise at the aerobic threshold and dehydration may increase lactate levels, but no published studies are available. Most athletes with DM2 exercise at lower-intensity levels than those required to generate significant lactic acidosis.
Sulfonylureas
Sulfonylureas are insulin secretogogues acting to stimulate pancreatic beta-cell insulin production.14 Second-generation agents are glimepiride (Amaryl), glyburide (DiaBeta, Micronase), and glipizide (Glucotrol), and first-generation agents include chlorpropamide (Diabinese), tolazamide, and tolbutamide.14 There are no studies published on the use of sulfonylureas in athletes. The risk of hypoglycemia, particularly delayed hypoglycemia, could be exacerbated by the effects of exercise.
Thiazolidinediones
Thiazolidinediones—pioglitazone (Actos) and rosiglitazone (Avandia)—act to increase insulin sensitivity.14 Hypoglycemia is uncommon,14 but there are recent concerns about the increased risk of cardiovascular events and edema with rosiglitazone. There are limited data regarding the efficacy of these medications in preventing long-term complications of diabetes; the risk:benefit ratio is unclear.29,30 Caution should be used with these medications owing to concerns for cardiovascular disease, heart failure, and the unknown effects of the medications with exercise.
Alpha-glucosidase Inhibitors
Alpha-glucosidase inhibitors, including acarbose (Precose) and miglitol (Glyset), act by delaying absorption of complex carbohydrates in the small intestine. Their use is limited by gastrointestinal side effects.14 A small study of type 1 diabetics (also on insulin) found a decrease in the postprandial glycemic rise, resulting in a more desirable glycemic profile with acarbose versus placebo during exercise. Despite a decrease in the glycemic nadir during exercise, there was no difference in episodes of hypoglycemia between the 2 groups.28 The role of alpha-glucosidase inhibitors in athletes is unclear.
Dipeptidyl Peptidase-4 Inhibitors
Dipeptidyl peptidase-4 inhibitors inhibit breakdown of incretins (hormones that increase food-induced insulin secretion, slow gastric emptying, and decrease appetite), resulting in increased food-dependent insulin release. Sitagliptin (Januvia) is the sole agent currently approved in the United States,14 but there are no published data regarding its use in athletes.
Incretin Mimetics
Exenatide (Byetta), an injectable incretin mimetic, is a relatively new treatment for diabetes,14 with nothing published regarding its use in athletes. One concern is gastric upset during exercise because of delayed emptying.
Meglitinides
Meglitinides are insulin secretogogues similar to sulfonylureas—nateglinide (Starlix) and repaglinide (Prandin)—which are fast-acting and require multiple doses per day just before meals.14 Hypoglycemia is less common than with sulfonylureas but is still significant and could exacerbate exercise-associated hypoglycemia. Cost can also be a limiting factor, with meglitinides being approximately 6 to 8 times more expensive than generic sulfonylureas. Nothing is published regarding use in athletes.
Amylin Mimetics
Amylin is a hormone that is secreted with insulin by beta cells to lower blood glucose, decrease glucagon, and curb appetite. Pramlintide (Symlin) is available as an injectable adjunct to insulin therapy.14 The counterregulatory response to insulin, which is needed in exercise, may be blunted because of decreased glucagon levels. There are no data regarding its use in athletes.
Guidelines
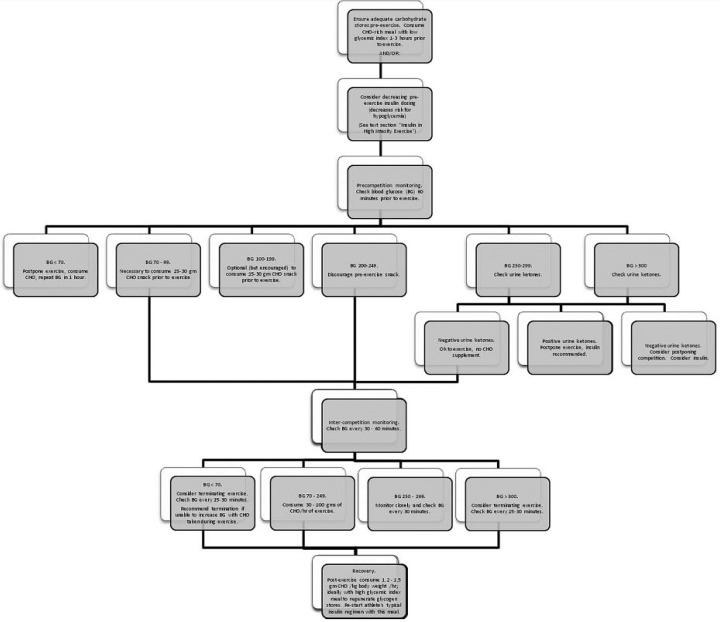
Recommendations vary based on the level of athleticism and on the type of diabetes: DM1 or DM2. In general, athletes with DM1 tend to be younger and are often more competitive. There are elite and endurance athletes with DM1. Figure 1 gives a preexercise algorithm for athletes with DM1.
Figure 1.
Exercise algorithm for athletes with type 1 diabetes mellitus, designed for distance/endurance events longer than 30 minutes. This algorithm represents general guidelines; approach should be tailored to meet the needs of the individual athlete. It is intended to summarize treatment recommendations from multiple sources and does not represent a validated clinical decision rule. Refer to text for evidence supporting each step.
Most patients diagnosed with DM2 are middle aged and often have comorbidities of obesity, hyperlipidemia, and hypertension. They are most commonly recreational-level athletes or sedentary.
Exercise Guidelines
Frequency of exercise
Patients with DM should have 5 or more days (and a minimum of 150 minutes) of aerobic exercise weekly and 3 days of resistance training weekly.6 Evidence for optimal frequency continues to evolve. Current frequency recommendations are based on expert opinion put forth in consensus statements, after review of randomized controlled trials.4-6,8,12
Intensity of exercise
Sedentary individuals beginning an exercise regimen should start exercising at 50% to 70% of their predicted maximum oxygen consumption (VO2 max).6 A simple guideline for patients is to exercise to a level where they are still able to carry on a conversation with a workout partner.
For patients with suboptimal diabetes control, intense exercise can cause paradoxical blood glucose elevation owing to sympathoadrenal activation.23 If glucose increases to more than 200 mg/dL during exercise, exercise intensity should be scaled back until baseline DM is better controlled. For insulin-dependent diabetics, insulin regimen would require adjustment.5,7,26
Type of exercise
For the beginner or recreational athlete, the likelihood of compliance and risks for injury (eg, foot injuries with running) should be considered. Compliance increases if the type of exercise is enjoyable and practical.
Traditionally, medical recommendations for people with diabetes have focused on aerobic exercise. Recent studies show that resistance exercise (eg, weight lifting or push-ups) improves insulin sensitivity similar to that of aerobic exercise.4,6,12,32 In a randomized controlled trial, an aerobic program with resistance training lowered A1C levels more than either form alone (and more than in sedentary controls).32 Consequently, the ADA recommends both types of exercise.6 Some patients with obesity, DM complications, or significant comorbidities may tolerate resistance training better than aerobic exercise.35 Resistance training consensus statements encourage a program for all major muscle groups (6 to 8 separate exercises) progressing to 3 sets of 8 to 10 repetitions. These should be gradually increased to maximal tolerated exertion.4,12
Higher-level athletes (high school, collegiate, or elite/professional) can exercise at the same frequency, intensity, and duration as their nondiabetic peers if their blood glucose is well controlled.16,23
Carbohydrate Intake
For insulin-dependent diabetic athletes, a carbohydrate-rich meal with a low glycemic index should be consumed a few hours before exercise17 with 15 to 30 g of additional carbohydrate ingested in the hour before exercise (see Table 4 for examples). For events longer than 1 hour, athletes should supplement an additional 30 to 100 g of carbohydrate for every hour of exertion, depending on the intensity of the exercise.15,23 One study that looked at maintaining euglycemia in athletes with DM1 found that carbohydrate supplementation was more important than insulin adjustment.15
Table 4.
Examples of dietary options for type 1 diabetes mellitus.
| Foods and Glycemic Index (GI) | |
|---|---|
| Low GI: ≤ 55 | High-fiber fruit (eg, apples, oranges, stone fruits) |
| Fruit juices | |
| Legumes | |
| Milk | |
| Unsweetened oatmeal | |
| Most whole-grain multigrain breads | |
| Most pastas | |
| Some high-fiber breakfast cereals (eg, All-Bran) | |
| Chocolate bar | |
| Milk | |
| Moderate GI: 56-69 | Whole-wheat breads |
| Lower fiber fruit | |
| Most vegetables | |
| Pizza | |
| Energy bars (eg, Power Bar) | |
| Some candy bars | |
| Brown rice | |
| Popcorn | |
| Soft drinks/soda | |
| High GI: ≥ 70 | Foods high in starch and white flour |
| Most breakfast cereals | |
| Pancakes/syrup | |
| Corn chips | |
| Pretzels | |
| Baked potato | |
| White bread products | |
| White rice | |
| Sports drinks (eg, Gatorade) | |
| Food Examples for Use During Exercise/Competition | |
| Carbohydrate snacks: ~ 30 ga | Banana, large (30 g) |
| Pretzels, 1 ounce (22 g) | |
| Sports drink, 6% carbohydrate (eg, Gatorade: 28 g per 16 oz) | |
| Clif Shot (25 g) | |
| Power Gel (27 g) | |
| Jelly Belly Sport Beans (25 g) | |
| Pretzels | |
| Carbohydrate snacks: ~ 45 gb | Large bagel (white flour) |
| PowerBar (Performance) | |
| Clif Bar | |
Most are 1.0 ounce.
Most are 2.5 ounces.
Postexercise, type 1 diabetics should consume 1.2 to 1.5 g of carbohydrate per kilogram per hour during the first 4 to 5 hours to allow for adequate glycogen synthesis: ideally, a carbohydrate-rich meal with a high glycemic index. Insulin should be restarted with this meal.17
Individuals with diabetes who take insulin or insulin secretagogues (sulfonylureas) are at risk for preexercise hypoglycemia. If glucose levels are less than 100 mg/dL, 1 hour before exercise/competition, a 15- to 30-g carbohydrate snack should be consumed, and glucose should be rechecked in 30 to 60 minutes. If glucose levels are less than 70 mg/dL, exercise should be postponed.6
Diabetics with DM2 (or recreational/low-intensity athletes with well-controlled DM1) should avoid increased caloric intake for moderate-intensity exercise, particularly if they are interested in losing weight. If significant hypoglycemia is detected in the low-intensity DM1 patient, the insulin regimen should be adjusted before increased calories are consumed.6 Patients with DM2 are not usually hypoglycemic; therefore, carbohydrate supplementation is usually unnecessary.
Fluid intake recommendations for diabetics are the same as for nondiabetic athletes: Consume only water in events 1 hour or less; alternate water and sports beverages (6% to 8% carbohydrate) in events longer than 1 hour.3
Insulin Use
Physiology
Hyperinsulinemia suppresses glucose generation by the liver. During exercise, insulin-dependent and independent mechanisms cause significant glucose uptake by muscles. The counterregulatory hormones that restore blood glucose are released during exercise. If these hormones become depleted, they are unable to prevent hypoglycemia in athletes who exercise after insulin use. Thus, diabetic athletes require hypoglycemia monitoring in the immediate postexercise period and for up to 24 hours after exercise (to watch for so-called late-onset hypoglycemia).23
Hypoinsulinemia can cause severe hyperglycemia. Without circulating insulin, glucose is not absorbed by exercising muscle cells, and they must rely on free fatty acid and ketoacids as energy substrates. This results in ketone formation and possibly ketoacidosis. If the liver continues gluconeogenesis and if muscle uptake of glucose is not taking place (ie, paradoxical hyperglycemia of exercise), severe hyperglycemia results and impairs performance.6 In nondiabetic athletes without DM, insulin secretion after exercise is increased to rebuild liver glycogen stores. In DM athetes, hyperglycemia can persist unless exogenous insulin is given.23
Insulin in moderate-intensity exercise
Athletes who use a basal-acting insulin (eg, insulin glargine) should not make adjustments when starting a moderate-intensity exercise regimen but should monitor glucose consistently. Owing to the improved insulin sensitivity associated with consistent exercise, their dose may need to decrease gradually as their exercise regimen is established.
For short-acting insulins, the preexercise dose should be decreased by 20% to 50%.4,15,27
Insulin in high-intensity exercise
Some authors recommend preventing hypoglycemia through increased carbohydrate preexercise, as explained above.15 Other authors recommend decreasing insulin in the preexercise period. If this approach is taken, insulin in the preexercise period should be decreased 20% to 75% for elite athletes or intense exercise regimens.4,15,27
Both short- and intermediate-acting insulins are adjusted by simply decreasing the dose by the same percentage as the estimated percentage of VO2 max of the exercise (eg, 75% decrease in preexercise short-acting insulin for a workout estimated at 75% of predicted VO2 max).27 No change is made to the long-acting (basal) insulin regimen.
A diabetic individual who uses a 24-hour insulin in the evening and a short-acting insulin with meals should use less of the short-acting insulin with breakfast if exercising midmorning (the evening insulin dose remains the same). A diabetic individual who uses NPH (12-hour insulin) at breakfast should decrease this dose (as well as lunchtime short-acting insulin) before afternoon exercise. Administering short-acting insulins 1 to 3 hours before exercise in a nonexercising area of the body (eg, the abdomen) is preferred.3,23
Whether decreasing insulin dose or increasing carbohydrate intake or both, each athlete should test his or her regimen during training.
Blood Glucose Monitoring
In DM1, the insulin-dependent diabetic individual should work closely with a qualified physician to monitor glucose levels before, during, and after exercise. Monitoring as frequently as every 30 minutes while training toward competition intensity may be necessary.23,26
If the blood glucose is above 250 before exercise, urine ketones should be checked and, if present, exercise postponed. If glucose is above 300, exercise is inadvisable, even without urine ketones.16
Some elite athletes erroneously think that starting exercise in a moderately hyperglycemic state (an attempt at preloading) lowers their risk for hypoglycemia. Grimm et al showed that plasma glucose dropped to similar levels within minutes of starting exercise regardless of the preexercise glucose level.15 As mentioned previously, if the athlete is severely hyperglycemic preexercise, she or he may remain dangerously hyperglycemic.23
Insulin regimens and exercise schedules will vary individually, and adjustment is a trial-and-error process aided by frequent blood glucose monitoring. During prolonged exercise, glucose should be checked every 30 to 60 minutes.23
Athletes should be aware of the symptoms and treatment of hypoglycemia. Mild hypoglycemia can be treated with rapidly absorbed glucose, hard candy, sugared beverage, or fruit juice.
Severe hypoglycemia (mental status changes, significant autonomic changes, and/or collapse) should be treated with glucagon (1-mg subcutaneous or intramuscular injection). Kits for home administration are available and should be available at athletic events for athletes with DM1. Glucagon will not be effective if liver glucose stores are depleted. Management includes concomitant treatment with glucose administration (usually 25 to 50 g, one half to one ampule) of 50% dextrose intravenously, followed by normal saline infusion, with clinical and blood glucose reassessment.
Conclusion
Physical activity does reduce the complications of diabetes.4-6,8,32 Many people with diabetes require exercise modifications and restrictions owing to risk for injury or worsening disease. Little is known about oral diabetic medication use in exercise, and oral medications are rarely used in elite-level sports.
Type 2 diabetics are advised to exercise at an appropriate level of intensity and duration, according to current ADA guidelines.
The greatest risks to the elite or endurance athlete with insulin-dependent diabetes are hyperglycemia and hypoglycemia. Hypoglycemia should be avoided through insulin reduction or increased carbohydrate consumption. Both have been effective in maintaining euglycemia.15,27 Guidelines are available to estimate carbohydrate needs and insulin adjustments for athletes.15,16,23,26,27 Blood glucose control for type 1 diabetics in intense exercise and athletic competition is a trial-and-error process with variations between individual athletes.
Footnotes
No potential conflict of interest declared.
References
- 1. Admon G, Weinstein Y, Falk B, et al. Exercise with and without an insulin pump among children and adolescents with type 1 diabetes mellitus. Pediatrics. 2005;116:e348-e355 [DOI] [PubMed] [Google Scholar]
- 2. Albert SG, Bernbaum M. Exercise for patients with diabetic retinopathy. Diabetes Care. 1995;18:130-132 [DOI] [PubMed] [Google Scholar]
- 3. Albright A, Franz M, Hornsby G, et al. American College of Sports Medicine position stand: Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32:1345-1360 [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association Consensus statement: Physical activity/exercise and type 2 diabetes. Diabetes Care. 2006;29:1433-1438 [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association Position statement: Physical activity/exercise and diabetes. Diabetes Care. 2004;27:S58-S62 [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2008;31:S12-S54 [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association Technical review: Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27:2518-253915451933 [Google Scholar]
- 8. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218-1227 [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005 [Google Scholar]
- 10. Cruickshanks KJ, Moss SE, Klein R, Klein BE. Physical activity and the risk of progression of retinopathy or the development of proliferative retinopathy. Ophthalmology. 1995;102:1177-1182 [DOI] [PubMed] [Google Scholar]
- 11. Cryer PE. Glucose homeostasis and hypoglycemia. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, eds. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders-Elsevier; 2008:1503-1536 [Google Scholar]
- 12. Eves ND, Plotnikow RC. Resistance training and type 2 diabetes. Diabetes Care. 2006;29:1933-1941 [DOI] [PubMed] [Google Scholar]
- 13. Gibbons RJ, Balady GJ, Bricker JT, et al. American College of Cardiology / American Heart Association Task Force on Practice Guidelines. (Committee to Update the 1997 Exercise Testing Guidelines.) ACC/AHA 2002 guideline update for exercise testing: summary article. J Am Coll Cardiol. 2002;40:1531-1540 [DOI] [PubMed] [Google Scholar]
- 14. Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, PA: Saunders Elsevier; 2007 [Google Scholar]
- 15. Grimm JJ, Ybarra J, Berné C, Muchnick S, Golay A. A new table for prevention of hypoglycemia during physical activity in type 1 diabetic patients. Diabetes Metab. 2004;30:465-470 [DOI] [PubMed] [Google Scholar]
- 16. Hornsby WG, Chetlin RD. Management of competitive athletes with diabetes. Diabetes Spectrum. 2005;18:102-107 [Google Scholar]
- 17. Jensen J. Nutritional concerns in the diabetic athlete. Curr Sports Med Rep. 2004;3:192-197 [DOI] [PubMed] [Google Scholar]
- 18. Kahn JK, Zola B, Juni JE, Vinik AI. Decreased exercise heart rate and blood pressure response in diabetic subjects with cardiac autonomic neuropathy. Diabetes Care. 1986;9:389-394 [DOI] [PubMed] [Google Scholar]
- 19. Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LaPorte RE, Dorman JS, Tajima N, et al. Pittsburgh Insulin-Dependent Diabetes Mellitus Morbidity and Mortality Study: physical activity and diabetic complications. Pediatrics. 1986;78:1027-1033 [PubMed] [Google Scholar]
- 21. Lindstrom J, Eriksson JG, Valle TT, et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study: results from a randomized clinical trial. J Am Soc Nephrol. 2003;14:S108-S113 [DOI] [PubMed] [Google Scholar]
- 22. Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673-1679 [DOI] [PubMed] [Google Scholar]
- 23. Lisle DK, Trojian TH. Managing the athlete with type 1 diabetes. Curr Sports Med Rep. 2006;5:93-98 [DOI] [PubMed] [Google Scholar]
- 24. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195-200 [DOI] [PubMed] [Google Scholar]
- 25. Orozco LJ, Buchleitner AM, Gimenez-Perez G, et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2008, Issue 3 Art. No. CD003054 DOI: 10.1002/14651858.CD003054.pub3 10.1002/14651858.CD003054.pub3 [DOI] [PubMed] [Google Scholar]
- 26. Peirce NS. Diabetes in exercise. Br J Sports Med. 1999;33:161-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care. 2001;24(4):625-630 [DOI] [PubMed] [Google Scholar]
- 28. Rabasa-Lhoret R, Burelle Y, Ducros F, et al. Use of an alpha-glucosidase inhibitor to maintain glucose homoeostasis during postprandial exercise in intensively treated Type 1 diabetic subjects. Diabet Med. 2001;18(9):739-744 [DOI] [PubMed] [Google Scholar]
- 29. Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Pioglitazone for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2006, Issue 4 Art. No. CD006060 DOI: 10.1002/14651858.CD006060.pub2 10.1002/14651858.CD006060.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Rosiglitazone for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2007, Issue 3 Art. No. CD006063 DOI: 10.1002/14651858.CD006063.pub2 10.1002/14651858.CD006063.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2005, Issue 3 Art. No. CD002966 DOI: 10.1002/14651858.CD002966.pub3 10.1002/14651858.CD002966.pub3 [DOI] [PubMed] [Google Scholar]
- 32. Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes. Ann Intern Med. 2007;147:357-369 [DOI] [PubMed] [Google Scholar]
- 33. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2006, Issue 3 Art. No. CD002968 DOI: 10.1002/14651858.CD002968.pub2 10.1002/14651858.CD002968.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350 [DOI] [PubMed] [Google Scholar]
- 35. Willey KA, Fiatarone Singh MA. Battling insulin resistance in elderly obese people with type 2 diabetes. Diabetes Care. 2003;26:1580-1588 [DOI] [PubMed] [Google Scholar]
- 36. Winnick JJ, Sherman WM, Habash DL, et al. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab. 2008;93:771-778 [DOI] [PMC free article] [PubMed] [Google Scholar]