Abstract
Context:
An athlete’s health may be endangered if he or she continues to compete after diagnosis of certain cardiovascular conditions. The most worrisome risk is sudden cardiac death; the annual rate in US athletes is 1 in 50 000 to 200 000.
Evidence Acquisition:
Part 2 of this review highlights the current guidelines and controversies surrounding compatibility of participation with a variety of cardiac conditions in competitive and recreational athletics. Data sources were limited to peer-reviewed publications from 1984 to the April 2009.
Results:
The guidelines published by the American College of Cardiology and the European Society of Cardiology provide a framework for safe competitive and recreational sports participation in athletes with a broad spectrum of inherited and acquired cardiovascular disorders. These guidelines are necessarily conservative because it is not currently possible to individualize risk prediction. Few data are available in many areas, particularly in the noncompetitive arena or in older athletes.
Conclusions:
Published national guidelines are currently the foundation governing return-to-play decisions in athletes with cardiovascular conditions. Further studies are needed to refine risk stratification algorithms to allow athletes with cardiovascular conditions to reap the health benefits of regular exercise and sports participation without undue risk.
Keywords: athletes, sports participation guidelines, cardiomyopathies, arrhythmias, defibrillators
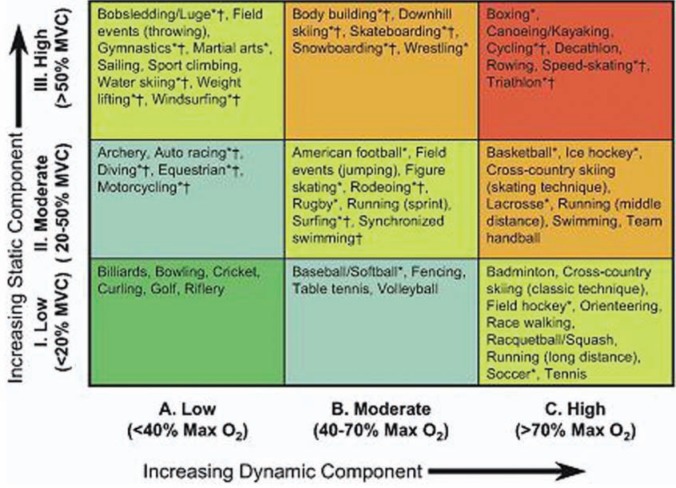
For the purposes of cardiovascular risk stratification, sports are classified according to their degree of dynamic and static exercise. Dynamic exercise, such as distance running, involves changes in muscle length and joint movement with relatively small intramuscular force. Conversely, static exercise, such as weight lifting, involves large intramuscular forces with relatively minor change in muscle length and joint movement. This classification scheme, which is illustrated in Figure 1, was adopted by the 36th Bethesda Conference and is used throughout this article to indicate which sports are suitable for a given medical condition.28
Figure 1.
Classification of sports according to dynamic and static exercise components. Adapted with permission.28 HCM, hypertrophic cardiomyopathy; LV, left ventricular; SAM, systolic anterior motion; FH, family history.
It is important to distinguish recreational sports from competitive sports for the purposes of discussing cardiovascular risk stratification. Competitive sports are defined as individual or team sports that require systematic training and regular competition against others and that place a high premium on athletic excellence and achievement. Competitive athletes tend to push themselves beyond their natural limits for extended periods, regardless of other considerations. In contrast, recreational sports do not involve systematic training; the exercise level can vary from modest to vigorous; and the frequency may vary from inconsistent to regular. Compared to their competitive counterparts, recreational athletes do not experience the same pressure to achieve excellence, and they are less likely to exceed their natural limits.24 Independent of the sports classification, emotional stress and environmental factors, such as temperature and altitude, should be taken into account when assessing risk in an individual athlete.25
General Considerations for Sports Participation in Athletes With Established Cardiovascular Disease
The 36th Bethesda Conference of the American College of Cardiology published a document in 2005 that deals with eligibility recommendations for competitive athletes with cardiovascular abnormalities in the United States.23 A parallel document was devised by the European Society of Cardiology (ESC) to address the same issues for European athletes.31 Two consensus documents from the United States24 and Europe30 provide recommendations for recreational sports participation limited to patients with genetic cardiovascular diseases. These published guidelines for participation in competitive and recreational sports are based on the knowledge of an overall increased risk of sudden cardiac death (SCD) associated with vigorous exercise. In interpreting these guidelines, one need recognize that many recommendations are based on expert opinion in areas where little data are available. The guidelines are designed to be generally conservative as it is not currently possible to predict SCD risk in an individual athlete with a known cardiovascular condition. It is important to note that there are differences between the guidelines published by the American College of Cardiology and the ESC. In general, the ESC guidelines are more restrictive in terms of eligibility and disqualification of athletes. A summary of the differences between these documents was recently published (see Table 1).33
Table 1.
Summary of major differences between US and European guidelines for sports participation for athletes with cardiovascular diseases. Adapted with permission from Elsevier.33
| 36th Bethesda Conference | European Society of Cardiology | |
|---|---|---|
| Gene carriers without phenotypea | All sports | Only recreational sports |
| Long QT syndrome |
|
|
| Marfan syndrome | If aortic root < 40 mm, no mitral regurgitation, no familial sudden death, then low- to moderate-intensity competitive sports permitted | Only recreational sports |
| Asymptomatic Wolff-Parkinson-White syndrome |
|
|
| Premature ventricular complexes | All competitive sports, when no increase in premature ventricular complexes or symptoms occur with exercise | All competitive sports, when no increase in premature ventricular complexes or symptoms occur with exercise, or when couplets with short R-R interval are present |
| Nonsustained ventricular tachycardia |
|
|
Hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, ion channel diseases (long QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia).
Sports in dangerous environments are restricted, given the risk should impaired consciousness occur, such as motor sports, rock climbing, and downhill skiing.
Hypertension
Systemic hypertension is defined as a blood pressure exceeding 140/90 mm Hg recorded on at least 2 occasions. It is the most common cardiovascular condition affecting competitive athletes and is usually discovered on a routine preparticipation physical examination.
Athletes with sustained stage 1 hypertension (systolic, 140-159 mm Hg; diastolic, 90-99 mm Hg) or higher should undergo electrocardiogram (ECG) and echocardiography to evaluate for left ventricular hypertrophy. If left ventricular hypertrophy beyond that expected for an athlete’s heart is discovered, participation should be limited until blood pressure is normalized. Similarly, athletes with stage 2 hypertension should be restricted from activity until their blood pressure has been controlled.13
Treatment for the hypertensive athlete should focus on initial lifestyle modification. Athletes should be instructed to limit dietary sodium intake, avoid tobacco, limit alcohol intake, and avoid drugs of abuse—particularly, sympathomimetics such as cocaine and ephedra. If lifestyle modification fails to achieve adequate control of blood pressure, then medication should be considered. Recommended initial agents include hydrochlorothiazide and vasodilator agents, such as angiotensin converted enzyme inhitors, angiotension II receptor blockers, or alpha-adrenergic blocking agents. Beta-blockers are not as well tolerated in competitive athletes owing to limitations on exercise capacity. Once treatment is initiated, the athlete should initially have follow-up blood pressure checks every few weeks to assess adequacy of therapy and ensure no adverse side effects. Hypertensive athletes on medication should register their medication with the governing body of their sports to obtain a therapeutic exception. Finally, an athlete with severe elevations of blood pressure (> 200 mm Hg systolic) or hypertension that is refractory to multiple classes of medications should be referred to a physician for workup of secondary causes of hypertension (eg, coarctation of the aorta, pheochromocytoma, renal artery stenosis).13
Coronary Artery Disease
Evaluation of the athlete with symptoms suggestive of coronary artery disease (CAD) should include an ECG and a stress test. A treadmill stress echocardiogram or a treadmill nuclear stress test is equally appropriate in this setting. If results of the stress test or ECG are abnormal, consultation with a cardiologist should be obtained before allowing the athlete to return to competitive activity.
Although CAD is an extremely rare occurrence in the young competitive athlete, it is of significant concern in the master athlete. The paradox with CAD and exercise is that whereas exercise is associated with a reduction in cardiac mortality for CAD patients, CAD is the most common cause of exercise-related cardiac events in adults older than 30 years.37 Furthermore, whereas every young competitive athlete must undergo preparticipation screening and examination, the same is not true for the older athlete.
Athletes with CAD can be broadly classified into 2 groups based on their level of risk: mildly increased risk and substantially increased risk (Table 2). Athletes in the former can participate in class IA and IIA competitive sports; however, they should avoid intensively competitive sports. Athletes in the latter should compete in class IA sports only. All athletes with CAD should be counseled to recognize symptoms of myocardial ischemia (chest, arm, or jaw discomfort; diaphoresis; unusual dyspnea; etc). Individuals with a recent myocardial infarction or revascularization procedure (ie, stent placement or bypass surgery) should avoid vigorous physical activity for at least 4 to 6 weeks or as recommended by their cardiologist.37
Table 2.
Risk stratification for athletes with coronary artery disease.39
| Factor | Mildly Increased Risk | Substantially Increased Risk |
|---|---|---|
| Left ventricular systolic function at rest—ejection fraction (EF) | Preserved (EF > 50%) | Impaired (EF < 50%) |
| Exercise tolerance for age | Normal | Abnormal |
| Presence of hemodynamically significant stenoses in a major coronary artery as measured by angiography | Absent or < 50% of luminal diameter narrowing | Present or > 50% of luminal diameter narrowing |
| Exercise-induced myocardial ischemia or complex ventricular arrhythmias | Absent | Evident |
| Myocardial revascularization by surgical or percutaneous techniques (if necessary) | Successfully performed | Not attempted or not successful |
In the younger athlete, there are 3 conditions that could mimic the presentation of atherosclerotic CAD: coronary artery vasospasm, myocardial bridging, and anomalous origin of coronary arteries. Each of these conditions can be difficult to diagnose, and the last is the third-most-common cause of sudden death in athletes.22 Athletes with coronary anomalies of wrong sinus origin should be excluded from all competitive sports until at least 3 months after surgical repair.9
Valvular Heart Disease
The most common valvular heart defects seen in the young athlete are mitral regurgitation, aortic stenosis, and aortic regurgitation. Mitral stenosis is typically caused by rheumatic fever and is uncommon in the United States. One of the essential components of the preparticipation cardiovascular examination is auscultation of the chest to identify cardiac murmurs associated with valvular heart disease or hypertrophic cardiomyopathy (HCM). When a new murmur is auscultated, the athlete should be restricted from competitive athletic activity until an echocardiogram is obtained and formally interpreted by a cardiologist. In some cases, a murmur may be difficult to appreciate at rest but may become audible after exertion, particularly for stenotic valvular lesions where the pressure gradient across the valve increases with exertion. Even in the absence of a heart murmur, valvular heart disease should be suspected when an athlete presents with symptoms of decreased exercise tolerance, chest pain, chest pressure, excessive dyspnea, syncope, or near syncope. Syncope or near syncope is a particularly ominous feature and should prompt immediate evaluation. Owing to the physical demands of sports, athletes with valvular heart disease often present at an earlier stage of disease.3
Although the cardiologist will dictate appropriate care for the athlete, it is helpful to understand a few general points about caring for individuals with valvular heart disease. Mild valvular defects are usually compatible with unrestricted participation in all competitive sports. Moderate defects that do not result in structural abnormalities of the heart or arrhythmias are compatible with participation in most sports, with the exception of those with high static and high dynamic components. Any athlete with moderate valvular heart disease should have an annual echocardiogram and visit with a cardiologist. Athletes with mild valvular heart abnormalities may be evaluated less frequently. Finally, athletes with severe valvular heart disease should be disqualified from participating in competitive sports until they have undergone the appropriate therapeutic procedure (ie, surgery or balloon dilatation) and have been cleared to return by a cardiologist. Following valve repair or replacement with bioprosthetic or mechanical valves, athletes can return to sports, although they should avoid high static and high dynamic sports. The use of anticoagulants with mechanical valves precludes participation in sports with bodily contact or significant risk of trauma.3
Congenital Heart Disease
As successful surgical and medical therapies for patients with congenital heart defects have emerged, individuals are living longer and some are able to participate in competitive athletics. The care of these individuals is complex and beyond the scope of this review9; however, a few general comments can be made. Patients with congenital heart disease should be seen annually by either a pediatric cardiologist or a cardiologist that specializes in adult congenital heart disease. These individuals are prone to unique complications depending on the nature of congenital heart defect. Athletes with sizable atrial septal defects or patent foramen ovale are susceptible to embolic stroke, as recently evidenced by the case of Tedy Bruschi, the linebacker for the New England Patriots. Athletes with other congenital heart defects may be prone to bradyarrhythmias and may present with syncope or near syncope. It is essential to identify these athletes during the preseason screen and to work closely with their cardiologists to help avoid any foreseeable complications.
Recommendations for Athletic Participation in Individuals With Cardiomyopathies
Competitive
Task Force 4 of the 36th Bethesda Conference addresses athletes with cardiomyopathies.23 The panel recommends that all athletes with a “probable or unequivocal clinical diagnosis of HCM should be excluded from most competitive sports with the possible exception of those of low intensity” (eg, golf, bowling, billiards, cricket, curling, riflery). This recommendation is independent of age, sex, and phenotypic appearance, and it does not differ for those athletes with or without symptoms, left ventricular outflow obstruction, or prior treatment with drugs or major interventions with surgery, alcohol septal ablation, pacemaker, or implantable defibrillator.” The panel also comments on athletic participation for individuals who are known to carry a familial genetic mutation for HCM but have no evidence of left ventricular hypertrophy—so-called preclinical HCM or genotype positive–phenotype negative HCM. There are, as yet, no compelling data for an increased risk of SCD in these individuals, and the panel does not recommend exclusion from competitive sports. However, routine surveillance on a 12- to 18-month basis with echocardiography, ECG, holter, exercise stress testing, and possible cardiac magnetic resonance imaging are strongly encouraged for genotype positive athletes.
Recommendations are similar for competitive athletic participation with other cardiomyopathies. The panel advises exclusion of athletes from competition, with the possible exception of low-intensity sports, if they carry a diagnosis of arrhythmogenic right ventricular dysplasia, dilated cardiomyopathy, or other myocardial disease, such as left ventricular noncompaction—a familial or sporadic condition characterized by an embryonic pattern of trabeculated left ventricular myocardium, with a predisposition to systolic dysfunction, heart failure, and ventricular arrhythmias. The panel also advises that athletes with acute myocarditis of any cause (usually viral) be withdrawn from athletic competition for at least 6 months. If all parameters of left ventricular geometry and function have returned to normal after that time, athletes may return to training and competition.
The ESC guidelines provide similar recommendations for competitive athletes with cardiomyopathies. The major difference is the recommended exclusion of individuals who are genotype positive–phenotype negative for inherited cardiomyopathies or arrhythmia syndromes from competitive sports. Although there are few existing data for increased risk of SCD in these individuals, it is conceivable that an arrhythmogenic substrate could exist in the absence of hypertrophy, fibrosis, or electrocardiographic abnormalities; that is, a genetic mutation may invoke early changes at the microscopic level that affect cellular signaling and electrical excitability.17 It is also possible that intense exercise training could promote or accelerate the development of inherited cardiomyopathies in genetically predisposed individuals. The risk of athletic participation for genotype positive–phenotype negative individuals is therefore an area in need of further study. In the meantime, it may be wise to consider each athlete in this category individually, taking into account other factors, such as family history of SCD, as well as the comfort level of the athlete and his or her family with regard to the athlete’s continuing to compete with an uncertain level of risk.
Recreational
The goal of the American Heart Association and ESC, in publishing recommendations for recreational sports participation in patients with inherited cardiovascular diseases, is to supply a framework for devising a safe exercise program that allows patients to take advantage of the well-known health benefits of regular exercise while minimizing risk of SCD.25,31 Both documents encourage patients to be alert to their perceived level of exertion, to be aware of cardiac symptoms, and to be willing to readily terminate physical activity in response. It is recommended that certain types of exercise be avoided, including “burst” exertion, exercising under extremely adverse environmental conditions, systematic or progressive training, and intense isometric exercise (ie, heavy free weights). Yet, many forms of low- to moderate-intensity exercise are considered safe and are strongly encouraged. The American Heart Association scientific statement provides a useful grading system for many common forms of exercise. The system is based on a scale of 0 to 5: 0 to 1, generally not advised; 2 to 3, to be assessed on an individual basis; and 4 to 5, probably permitted. For HCM and arrythmogenic right ventricular cardiomyopathy—the 2 cardiomyopathies associated with the highest rate of SCD—all high-intensity sports (ie, basketball, football, hockey) are scored 0 to 2. Most moderate-intensity sports (ie, biking, hiking, jogging, swimming) are scored 2 to 5, and low-intensity sports (ie, bowling, golf, weight lifting [machine assisted, not free weights]) are scored 3 to 5. Although the grading system provides a highly valuable framework, it does not take into account the variable degree of intensity that individuals bring to their particular activity or sport. Indeed, perceived level of exertion and conditioning will be different for highly trained competitive athletes who are downgrading their activity to a recreational level versus nonathletic individuals seeking to maintain a healthy weight and overall good cardiovascular fitness. The guidelines should therefore be used as a starting point that should be individualized to devise a safe program that strikes a reasonable balance between risks and benefits of exercise. One general rule that many experts use is that regardless of what activity patients choose, their intensity level should be maintained at a level which allows them to carry on a conversation while exercising.
General Considerations for Sports Participations in Athletes With Arrhythmias
The initial evaluation of an athlete with a suspected arrhythmia should include a 12-lead ECG, an echocardiogram, an exercise test, and an ambulatory ECG monitor. Management of athletes with documented rhythm disturbances involves individualized risk stratification and, usually, consultation with a cardiologist or electrophysiologist. Oftentimes, patients with heart rhythm abnormalities are told that participation in sports is strictly contraindicated, but this is not always the case. In this portion of the review, we discuss the relevance of various electrophysiological abnormalities to athletic participation. Task Force 7 of the 36th Bethesda Conference provides the framework for the discussion to follow.39
Sinus Bradycardia
The normal heart rate ranges from 60 to 100 beats per minute in the resting state. Electrical conduction begins in the atria at the sinoatrial node and travels to atrioventricular node, ending with the Purkinje fibers in the ventricles. It is not unusual for athletes to have resting heart rates lower than 60 beats per minute (Figure 2A). Various media outlets have cited Lance Armstrong’s resting heart rate to be around 30 beats per minute. In addition, pauses up to 2 or 3 seconds occur in up to 20% of athletes.5 This is a physiologic response to increases in vagal parasympathetic tone resulting in depressed sinoatrial node function or an intrinsic alteration of the sinoatrial node itself as a result of recurrent strenuous exercise.36 Participation in competitive sports is unrestricted in athletes with sinus bradycardia, except in the presence of symptoms such as dizziness or syncope or in the failure of the heart rate to elevate appropriately with exercise, which should prompt further evaluation.
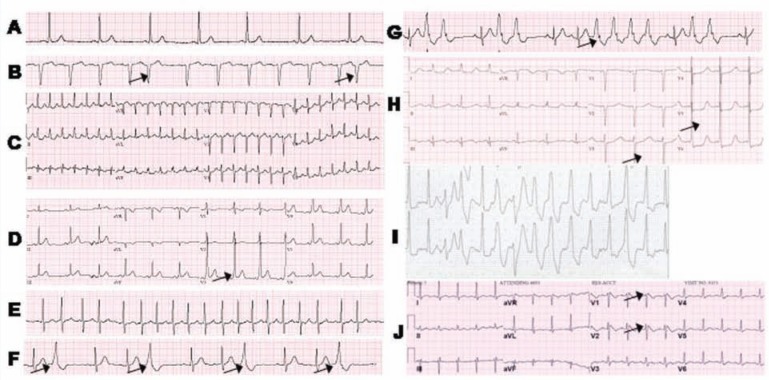
Figure 2.
Representative ECGs of various arrhythmias: A, sinus bradycardia at a heart rate of 51; B, premature atrial contractions (indicated by arrows); C, supraventricular tachycardia with a heart rate of 173 beats per minute at rest; D, Wolff-Parkinson-White (arrows indicate characteristic short PR intervals with delta waves); E, atrial fibrillation with a rapid ventricular response; F, frequent premature ventricular complexes (indicated by arrows); G, couplets and a 4-beat run of nonsustained ventricular tachycardia (arrow); H, inherited long QT syndrome (prolonged QT interval indicated by arrows); I, polymorphic ventricular tachycardia; J, Brugada syndrome (with arrows indicating ST elevation).
Premature Atrial Complexes
Premature atrial complexes are benign “extra” beats that originate from nonsinoatrial nodal atrial tissue (Figure 2B). In the absence of symptoms or structural heart disease, full participation in competitive sports is permissible without further evaluation.39
Supraventricular Tachycardia
Supraventricular tachycardia encompasses atrioventricular nodal reentrant tachycardia, atrioventricular reentry tachycardia, and atrial tachycardia. Atrioventricular nodal reentrant tachycardia and atrioventricular reentry tachycardia are arrhythmias with accessory pathways within the atrioventricular node and between the atria and ventricles, respectively. When these accessory pathways are activated, a closed circuit is formed that creates perpetual rapid impulses to the ventricles (Figure 2C). Accordingly, the ventricles contract at a rapid rate and can cause symptoms of palpitations, lightheadedness, or even syncope. Atrial tachycardia occurs when an atrial focus other than the sinus node sends impulses to the ventricles, resulting in a rapid heart rate.
Atrioventricular nodal reentrant tachycardia is the cause of supraventricular tachycardia in greater than 50% of athletes.10 Termination of an acute episode can by achieved by increasing vagal parasympathetic tone with carotid massage or a Valsalva maneuver. Radiofrequency ablation to eliminate the accessory pathway and disable the reentrant circuit is highly successful (97%) with a low complication rate and should therefore be strongly considered in the symptomatic athlete.34
Athletes without structural heart disease and supraventricular tachycardia with or without exercise may participate in all sports if the supraventricular tachycardia is short-lived and controlled with maneuvers or pharmacologic therapy, such as atrioventricular nodal blocking agents. Structural heart disease or supraventricular tachycardia resulting in syncope or near syncope will require further evaluation and treatment by a cardiologist. Athletes without structural heart disease may return to play after successful ablation, without restrictions.39
Wolff-Parkinson-White
Wolff-Parkinson-White is a preexcitation syndrome that involves an accessory conduction pathway from the atria to the ventricle that bypasses the atrioventricular node. This accessory pathway can be seen as a delta wave pattern on ECG (Figure 2D). Wolff-Parkinson-White can cause atrioventricular reciprocating tachycardia, but it is also associated with atrial fibrillation with rapid ventricular rates. Although sudden death with Wolff-Parkinson-White is rare, there is concern that athletic competition can increase conduction through accessory pathways, particularly in those individuals with short refractory periods.27
The consensus statement from the 36th Bethesda Conference states that athletes with Wolff-Parkinson-White but no structural heart disease, palpitations, or tachycardia can participate in all competitive sports. All other patients should be referred to an electrophysiologist, with strong consideration given to electrophysiologic study and radiofrequency ablation. Following successful ablation, athletes without structural heart disease can resume participation in all competitive sports.39
Atrial Fibrillation and Flutter
Atrial fibrillation is an arrhythmia that uncommonly affects young individuals but may be triggered by physical exertion.6 The prevalence in athletes is reported to be 0.3%.32 Atrial fibrillation results from generation of disorganized impulses from multiple electrical foci that overwhelm the normal conduction system. As a result, the heart rate becomes rapid and irregular and is associated with symptoms ranging from palpitations to syncope (Figure 2E). Athletes are thought to develop this arrhythmia at a younger age because of the increased cardiac output and, possibly, inflammation.18 Sustained atrial flutter is an uncommon arrhythmia in athletes with structurally normal hearts. Symptoms can often mimic those of atrial fibrillation.
Task Force 7 from the 36th Bethesda Conference recommends that athletes with atrial fibrillation and no evidence of structural heart disease can compete without restrictions if they are able to maintain a normal dynamic range of their heart rate with exercise. Full participation is not recommended in athletes with untreated atrial flutter, owing to the risk of 1:1 atrioventricular conduction. If an athlete is on anticoagulation for stroke prevention, then he or she should abstain from competition associated with body collisions, because of the increased risk of bleeding.39 For those athletes with symptomatic atrial fibrillation or flutter that prevents them from competing, radiofrequency ablation can be effective in restoring sinus rhythm. Sports participation is permitted 4 to 6 weeks after ablation without recurrence.7
Premature Ventricular Complexes and Ventricular Tachycardia
Premature ventricular complexes (PVCs) are extrasystolic single wide complex beats that originate from the ventricle (Figure 2F). Although PVCs are observed in up to 75% of healthy patients on Holter monitoring, progression to ventricular tachycardia is extremely rare.16 Although PVCs are more common in athletes, they are usually not associated with structural heart disease or an increased risk for SCD.2 Athletes without structural heart disease who have asymptomatic PVCs at rest or with exercise can participate in all competitive events. If the PVCs increase with exercise and cause significant symptoms, participation should be restricted to class IA sports.39
Those athletes with nonsustained ventricular tachycardia (Figure 2G) (3 or more consecutive ventricular beats at a rate greater than 120 beats per minute with duration less than 30 seconds) or sustained ventricular tachycardia should undergo a thorough evaluation for occult structural heart disease, including advanced imaging such as magnetic resonance imaging. Athletes without structural heart disease who have asymptomatic nonsustained ventricular tachycardia may participate in all sports, without further evaluation, if the tachycardia is of short duration (< 8-10 beats), if it comprises less than 150 beats per minute, and if it extinguishes or does not increase with exercise. Athletes with a structurally normal heart but more significant ventricular tachycardia may be candidates for radiofrequency ablation that could restore their sports eligibility. Any ventricular tachycardia in the presence of structural heart disease, regardless of ablation or other suppressive therapies, is reason for exclusion from all sports except class 1A.
Inherited Long QT Syndrome
Inherited long QT syndrome is a potentially life-threatening disorder that affects ~1 in 2500 individuals.35 On ECG, the interval between ventricular depolarization (QRS) and repolarization (T wave) is prolonged. Although debatable, a QT calculated greater than 470 milliseconds in men or 480 milliseconds in women is considered abnormal and requires further evaluation (Figure 2H). Long QT syndrome may cause syncope or SCD owing to polymorphic ventricular tachycardia (torsades de pointes), which may be triggered by physical exertion in some long QT syndrome subtypes.12 Many patients can be treated effectively with beta blockers, but high-risk features such as previous cardiac arrest, syncope, and exaggerated QT calculated length should prompt consideration for implantable cardioverter defibrillator (ICD) implantation.8 Diagnosis, therapeutic interventions, and risk stratification can be aided by genetic testing for long QT syndrome 1-4, 5, and 6, which is of relatively high yield (approximately 75%).20,38
Task Force 7 of the Bethesda Conference recommends that athletes who have experienced a cardiac arrest or syncopal episode owing to suspected long QT syndrome should abstain from all competitive sports except those classified as class IA.39 Asymptomatic patients with evidence of prolonged QT calculated interval on ECG should also be restricted to class IA activities, but they may be good candidates for genetic testing for diagnosis confirmation and risk stratification. Athletes who carry a genetic mutation associated with long QT syndrome but with a normal or nondiagnostic QT calculated are probably at low but not zero risk. In the absence of available data, the American Heart Association document does not preclude competitive sports in this population (with the exception of swimming in genetically confirmed long QT1), but the ESC recommends only class IA sports.31 Guidelines for recreational sports are similar to those described for HCM above, with the exception of exclusion from swimming for all long QT patients in the absence of genetic information.25,31
Catecholaminergic Polymorphic Ventricular Tachycardia
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an uncommon exercise-induced arrhythmia syndrome that is linked to genetic mutations in calcium channels.14 Syncope related to emotional or physical stress is usually the presenting symptom in patients with CPVT.21 Diagnosis usually requires exercise stress testing (Figure 2I) given that the majority of patients have a normal physical examination and ECG at rest. Athletes with CPVT should be restricted from all competitive sports except class 1A. Symptomatic patients should be referred to an electrophysiologist for consideration of medical therapy with beta blockers or ICD implantation. Like long QT syndrome, genetically affected, clinically unaffected athletes with CPVT may be allowed to compete. Although there are no formal guidelines for allowable recreational activity, patients with CPVT should be advised to exercise cautiously at the discretion of their cardiologist.
Brugada Syndrome
Brugada syndrome is an uncommon arrhythmogenic condition characterized by incomplete right bundle branch block, accentuated J wave, and ST elevation in leads V1 to V3 (Figure 2J).1 SCD occurs in up to 33% of patients and may be the initial presentation of the disease. Ventricular tachycardia is not clearly provoked by exercise, but it can be by hyperthermia.4 Therefore, athletes with Brugada should be restricted from all competitive sports except class 1A. Guidelines for recreational activity are more lenient than they are for other inherited cardiovascular disease.24
Marfan Syndrome
Marfan syndrome is an autosomal dominant disorder affecting approximately 1 in 5000 individuals, most often caused by mutations in the fibrillin gene.11 Clinical sequelae include elongated limbs and digits, joint hypermobility, ocular abnormalities, and aortic root dilation (Figure 3), resulting in aortic valve regurgitation and predisposing to aortic dissection. The body habitus of Marfan syndrome is highly advantageous in many competitive sports, but athletic participation for these individuals can have potentially fatal consequences.15
Figure 3.
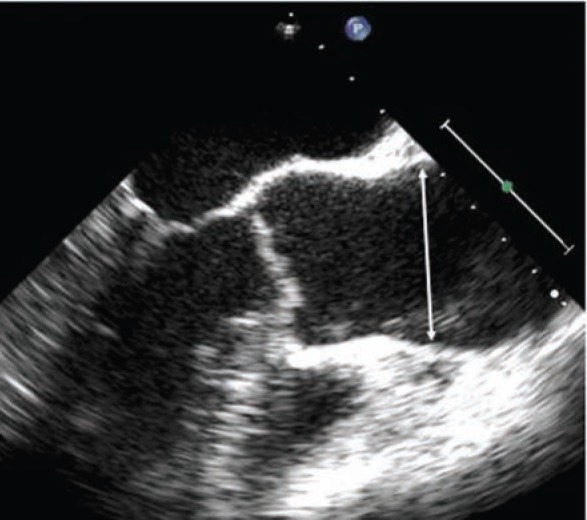
Marfan syndrome and dilated ascending aorta measuring 49 mm at the sinuses (arrow).
Task Force 4 of the Bethesda Conference recommends that athletes with Marfan syndrome participate in class IA and IIA sports if they do not have any of the following clinical features: aortic root dilation (> 40 mm or 2 standards deviations from the mean in a transverse dimension), moderate to severe mitral regurgitation, or family history of aortic dissection or SCD. These athletes must have an echocardiographic assessment of the aortic root every 6 months. Athletes with any of the previous features can participate in class IA sports only.23 Sports with bodily collisions24 or static strenuous exercise, such as weight lifting, are contraindicated owing to the high propensity for aortic trauma.31 The ESC guidelines are more conservative and restrict all patients with definite or clinically suspected Marfan syndrome from all competitive sports, independent of aortic root dimensions.31 In general, guidelines for recreational activity are similar to other inherited cardiovascular conditions.24
Commotio Cordis
Commotio cordis, or ventricular fibrillation resulting from blunt chest wall trauma, is the second-leading cause of SCD in young athletes.22,27 Outcome depends on recognition and access to an automatic external defibrillator. Automatic external defibrillators with trained responders should be available at all athletic facilities. These devices should be deployed with a response time of less than 5 minutes to provide greatest survival benefit.29 Efforts are also underway to design safer sports equipment to prevent commotion cordis. Return to sports participation in survivors of commotion cordis is left up to personal discretion.
Implantable Cardioverter Defibrillators and Sports
ICDs are frequently used as primary and secondary preventive therapies for many of the diseases discussed. The 36th Bethesda Conference and ESC are in agreement that individuals with an ICD should participate in Class IA competitive sports only. The basis for excluding athletes with ICDs from competition is increased risk of ICD discharges, the unpredictable performance of these devices under the extreme environmental and physiological conditions associated with intense competition, device misfiring or lead fracture, and the possibility of syncopal events secondary to ICD discharge or the arrhythmia itself.25,26 However, because data are largely anecdotal at this stage, opinions regarding sports participation are often conflicting.19 Currently, an ICD Safety Sports Registry (see http://icdsports.org/) is enrolling patients with ICDs who choose to participate in competitive or recreational sports.
Conclusion
National guidelines provide a framework for safe competitive and recreational sports participation in athletes with cardiovascular disease. In situations of uncertainty about level of risk, coordinated decision making among sports medical personnel, cardiologists, the athletes, and their families is advisable. Further studies are needed among many areas to determine reliability of algorithms to more precisely predict risk in each athlete and for each sport, as well as level of safety for sports participation with ICDs.
Part 1 of this article, “Cardiovascular Health, Part 1: Preparticipation Cardiovascular Screening,” appeared in the November/December 2009 issue of Sports Health.
NATA Members: Receive 3 free CEUs each year when you subscribe to Sports Health and take and pass the related online quizzes! Not a subscriber? Not a member? The Sports Health–related CEU quizzes are also available for purchase. For more information and to take the quiz for this article, visit www.nata.org/sportshealthquizzes.
Footnotes
No potential conflict of interest declared.
References
- 1. Benito B, Brugada R, Brugada J, Brugada P. Brugada syndrome. Prog Cardiovasc Dis. 2008;51:1-22 [DOI] [PubMed] [Google Scholar]
- 2. Biffi A, Pelliccia A, Verdile L, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446-452 [DOI] [PubMed] [Google Scholar]
- 3. Bonow RO, Cheitlin MD, Crawford MH, Douglas PS. Task Force 3: valvular heart disease. J Am Coll Cardiol. 2005;45:1334-1340 [DOI] [PubMed] [Google Scholar]
- 4. Corrado D, Basso C, Buja G, Nava A, Rossi L, Thiene G. Right bundle branch block, right precordial st-segment elevation, and sudden death in young people. Circulation. 2001;103:710-717 [DOI] [PubMed] [Google Scholar]
- 5. Ector H, Bourgois J, Verlinden M, et al. Bradycardia, ventricular pauses, syncope, and sports. Lancet. 1984;2:591-594 [DOI] [PubMed] [Google Scholar]
- 6. Elosua R, Arquer A, Mont L, et al. Sport practice and the risk of lone atrial fibrillation: a case-control study. Int J Cardiol. 2006;108:332-337 [DOI] [PubMed] [Google Scholar]
- 7. Furlanello F, Lupo P, Pittalis M, et al. Radiofrequency catheter ablation of atrial fibrillation in athletes referred for disabling symptoms preventing usual training schedule and sport competition. J Cardiovasc Electrophysiol. 2008;19:457-462 [DOI] [PubMed] [Google Scholar]
- 8. Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291-2300 [DOI] [PubMed] [Google Scholar]
- 9. Graham TP, Driscoll DJ, Gersony WM, Newburger JW, Rocchini A, Towbin JA. Task Force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333 [DOI] [PubMed] [Google Scholar]
- 10. Josephson ME, Schibgilla VH. Athletes and arrhythmias: clinical considerations and perspectives. Eur Heart J. 1996;17:498-505 [DOI] [PubMed] [Google Scholar]
- 11. Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965-1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapetanopoulos A, Kluger J, Maron BJ, Thompson PD. The congenital long QT syndrome and implications for young athletes. Med Sci Sports Exerc. 2006;38:816-825 [DOI] [PubMed] [Google Scholar]
- 13. Kaplan NM, Gidding SS, Pickering TG, Wright JT. Task Force 5: systemic hypertension. J Am Coll Cardiol. 2005;45:1346-1348 [DOI] [PubMed] [Google Scholar]
- 14. Katz G, Arad M, Eldar M. Catecholaminergic polymorphic ventricular tachycardia from bedside to bench and beyond. Curr Probl Cardiol. 2009;34:9-43 [DOI] [PubMed] [Google Scholar]
- 15. Keane MG, Pyeritz RE. Medical management of Marfan syndrome. Circulation. 2008;117:2802-2813 [DOI] [PubMed] [Google Scholar]
- 16. Kennedy HL. Ventricular ectopy in athletes: don’t worry . . . more good news! J Am Coll Cardiol. 2002;40:453-456 [DOI] [PubMed] [Google Scholar]
- 17. Kim JB, Porreca GJ, Song L, et al. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science. 2007;316:1481-1484 [DOI] [PubMed] [Google Scholar]
- 18. Lampert R. Atrial fibrillation in athletes: toward more effective therapy and better understanding. J Cardiovasc Electrophysiol. 2008;19:463-465 [DOI] [PubMed] [Google Scholar]
- 19. Lampert R, Cannom D. Sports participation for athletes with implantable cardioverter-defibrillators should be an individualized risk-benefit decision. Heart Rhythm. 2008;5:861-863 [DOI] [PubMed] [Google Scholar]
- 20. Lehnart SE, Ackerman MJ, Benson DW, et al. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325-2345 [DOI] [PubMed] [Google Scholar]
- 21. Liu N, Ruan Y, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Prog Cardiovasc Dis. 2008;51:23-30 [DOI] [PubMed] [Google Scholar]
- 22. Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064-1075 [DOI] [PubMed] [Google Scholar]
- 23. Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45:1340-1345 [DOI] [PubMed] [Google Scholar]
- 24. Maron BJ, Chaitman BR, Ackerman MJ, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807-2816 [DOI] [PubMed] [Google Scholar]
- 25. Maron BJ, Zipes DP. Introduction: eligibility recommendations for competitive athletes with cardiovascular abnormalities-general considerations. J Am Coll Cardiol. 2005;45:1318-1321 [DOI] [PubMed] [Google Scholar]
- 26. Maron BJ, Zipes DP. It is not prudent to allow all athletes with implantable-cardioverter defibrillators to participate in all sports. Heart Rhythm. 2008;5:864-866 [DOI] [PubMed] [Google Scholar]
- 27. Mason PK, Mounsey JP. Common issues in sports cardiology. Clin Sports Med. 2005;24:463-476 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: classification of sports. J Am Coll Cardiol. 2005;45:1364-1367 [DOI] [PubMed] [Google Scholar]
- 29. Myerburg RJ, Estes NA, Fontaine JM, Link MS, Zipes DP. Task Force 10: automated external defibrillators. J Am Coll Cardiol. 2005;45:1369-1371 [DOI] [PubMed] [Google Scholar]
- 30. Pelliccia A, Corrado D, Bjornstad HH, et al. Recommendations for participation in competitive sport and leisure-time physical activity in individuals with cardiomyopathies, myocarditis and pericarditis. Eur J Cardiovasc Prev Rehabil. 2006;13:876-885 [DOI] [PubMed] [Google Scholar]
- 31. Pelliccia A, Fagard R, Bjornstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422-1445 [DOI] [PubMed] [Google Scholar]
- 32. Pelliccia A, Maron BJ, Di Paolo FM, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690-696 [DOI] [PubMed] [Google Scholar]
- 33. Pelliccia A, Zipes DP, Maron BJ. Bethesda Conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of US and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2008;52:1990-1996 [DOI] [PubMed] [Google Scholar]
- 34. Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol. 2000;23:1020-1028 [DOI] [PubMed] [Google Scholar]
- 35. Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circ. 2009;120:1761-1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein R, Medeiros CM, Rosito GA, Zimerman LI, Ribeiro JP. Intrinsic sinus and atrioventricular node electrophysiologic adaptations in endurance athletes. J Am Coll Cardiol. 2002;39:1033-1038 [DOI] [PubMed] [Google Scholar]
- 37. Thompson PD, Balady GJ, Chaitman BR, Clark LT, Levine BD, Myerburg RJ. Task Force 6: coronary artery disease. J Am Coll Cardiol. 2005;45:1348-1353 [DOI] [PubMed] [Google Scholar]
- 38. Vincent GM, Schwartz PJ, Denjoy I, et al. High efficacy of beta-blockers in long-QT syndrome type. 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures.” Circulation. 2009;119:215-221 [DOI] [PubMed] [Google Scholar]
- 39. Zipes DP, Ackerman MJ, Estes NA, Grant AO, Myerburg RJ, Van Hare G. Task Force 7: arrhythmias. J Am Coll Cardiol. 2005;45:1354-1363 [DOI] [PubMed] [Google Scholar]