Abstract
Ethanol extract of Rhodomyrtus tomentosa (Aiton) Hassk. leaf was evaluated for antibacterial activity against 47 clinical isolates of Streptococcus pyogenes. The extract exhibited good anti-S. pyogenes activity against all the tested isolates with similar minimum inhibitory concentration (MIC, 3.91–62.5 μg mL−1) and minimum bactericidal concentration (MBC, 3.91–62.5 μg mL−1) ranges. No surviving cells were detected at 16 h after treatment with 8 × MIC of the extract. The extract-treated cells demonstrated no lysis and cytoplasmic leakage through the bacterial membrane. Electron micrographs further revealed that the extract did not cause any dramatic changes on the treated cells. Rhodomyrtone, an isolated compound, exhibited good anti-S. pyogenes activity (14 isolates), expressed very low MIC (0.39–1.56 μg mL−1) and MBC (0.39-1.56 μg mL−1) values. Rhodomyrtus tomentosa leaf extract and rhodomyrtone displayed promising antibacterial activity against clinical isolates of S. pyogenes.
1. Introduction
Streptococcus pyogenes, also known as group A streptococcus, is a major upper respiratory tract bacterial pathogen that causes a wide variety of diseases from common and mostly uncomplicated cases of pharyngitis and impetigo to severe invasive infections [1]. It is the most common cause of bacterial pharyngitis in children and may lead to nonsuppurative complications, such as rheumatic fever and glomerulonephritis. Penicillin remains the treatment of choice for S. pyogenes infections based on its narrow antibacterial spectrum, good efficacy, safety profile, and low cost [2]. However, increasing failure rates (20–30%) of penicillin therapy for S. pyogenes infections have been reported by many controlled studies [3–8]. Some studies have even indicated failure rates of 40% to 80% with the second course of the treatment [4, 5]. Because of these increasing failure rates, questions arise whether or not penicillin still should be considered standard therapy [9]. Erythromycin and related macrolide antibiotics, which are used in patients with a known or suspected allergy to penicillin, are considered as alternative drugs [2, 10, 11]. Unfortunately, an increasing incidence of erythromycin resistance has been reported in several parts of the world in recent years [12–15]. Moreover, no vaccines are now available to prevent streptococcal infections and their sequelae. Streptococcus pyogenes vaccines are currently in development [16, 17]. Therefore, the discovery of potential new drugs might be helpful for the treatment of S. pyogenes infections in the near future.
Many medicinal plants have been studied and some have a strong activity and good potential to be developed into an effective drug. Downy rose myrtle, Rhodomyrtus tomentosa (Aiton) Hassk., is a Thai medicinal plant used to treat oral, gastrointestinal, urinary tract infections, and used as an antiseptic wash for wounds. Our preliminary antibacterial screening data from a number of plants found that R. tomentosa leaf extract was very effective against many Gram-positive bacteria [18]. Therefore, the aims of this study were to evaluate this effective plant against various clinical isolates of S. pyogenes and examine its mechanisms of action.
2. Materials and Methods
2.1. Plant Extraction
Classified reference voucher specimen of R. tomentosa (NPRC0057) was deposited at Faculty of Traditional Thai Medicine, Prince of Songkla University, Thailand. The crude ethanol extract of R. tomentosa leaf and rhodomyrtone were prepared according to the previously published methodology [18]. The extract and compound were checked for the same qualitative and quantitative profiles that were comparable with recently published data [19]. They were dissolved in 100% dimethyl sulfoxide (DMSO, Merck, Germany) before use (10 g L−1for the crude extract and 1 g L−1 for rhodomyrtone).
2.2. Bacterial Strains and Culture Conditions
Forty-seven clinical isolates of S. pyogenes (NPRC 101-147) were obtained from patients admitted at Prince of Songkla Hospital with tonsillitis or pharyngitis. A throat swab of each patient was individually plated onto Columbia blood agar base (Oxoid, UK) containing 5% sheep red blood cells (BA). Betahaemolytic streptococcallike colonies were subjected to appropriate biochemical testing as described previously [20]. A reference strain, S. pyogenes DSM 11728, was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). The bacterial cultures were stored in brain heart infusion (BHI) broth (Difco, France) containing 20% glycerol at −70°C until use. All isolates were cultured on BA plates incubated with 5% CO2 at 37°C for 24 h.
2.3. Antibacterial Activities
Broth microdilution method was carried out according to Clinical and Laboratory Standards Institute Guidelines [21]. Minimum inhibitory concentration (MIC) was recorded as the lowest concentration that produced a complete suppression of visible growth. An aliquot (20 μL) from the broth (200 μL) with no growth were pipetted and dropped onto BA plates and incubated with 5% CO2 at 37°C for 24 h. The minimum bactericidal concentration (MBC) was defined as the lowest concentration of the extract completely preventing bacterial growth. Penicillin G and erythromycin (Sigma, France) were used as reference antibiotics. 1% DMSO was used as a negative control. All tests were performed in triplicate independent experiments.
2.4. Time-Kill Assay
The bactericidal activity of the extract was studied using a time-kill assay [22]. A representative isolate S. pyogenes NPRC 101 was used in this study. Suspension of S. pyogenes in 0.85% normal saline solution (NSS) at the stationary phase of growth was prepared from the culture on BHI agar. The bacterial suspension was added to BHI broth containing the extract at 1/2 × MIC, MIC, 2 × MIC, 4 × MIC, and 8 × MIC and incubated with 5% CO2 at 37°C. The final cell concentration was 5 × 105 CFU mL−1. The samples were collected at 2 h intervals over 24 h period, and the surviving bacteria were cultured on BA. 1% DMSO was used as a negative control. The assay was carried out in duplicate.
2.5. Bacteriolysis
A modified method from Carson et al. [23] was used in this experiment. Briefly, suspensions of S. pyogenes NPRC 101 in NSS were prepared from the culture on BHI agar. The suspensions were supplemented with the plant extract at 1/2 × MIC, MIC, 2 × MIC, and 4 × MIC and mixed with a vortex mixer. The final cell concentration was 1.5 × 108 CFU mL−1. Optical density at 620 nm (OD620) was measured at 2 h intervals until 24 h to detect cell lysis as indicated by a decrease in OD620. Corresponding dilutions of test agents were used as blank, and 1% DMSO was used as a negative control. The assay was carried out in triplicate.
The results were expressed in percent as the ratio of OD620 at each time interval versus OD620 at 0 min.
2.6. Loss of 260-nm-Absorbing Materials
A modified method from Carson et al. [23] was used in this assay. Suspension of S. pyogenes was prepared from the culture on BHI agar. The bacterial cells were washed twice with NSS and resuspended in NSS. The extract was added at final concentrations equivalent to 1/2 × MIC, MIC, 2 × MIC, and 4 × MIC. The final cell concentration was 1.5 × 108 CFU mL−1. 1% DMSO was used as a negative control. Samples were removed at 0, 2, 4, 6, 8, 10, 12, and 24 h, diluted 1 in 100, filtered through a 0.2 μm pore-size filter, and OD260 was determined. Filtrates of appropriate dilution of each agent were prepared and used as blank. OD260 at each time point was expressed as a proportion of initial OD260. The assay was carried out in triplicate. Mean ratios for each treatment extract and time were calculated and compared to the means for the corresponding untreated samples. A Dunnett-ANOVA test was used to compare between the tests and control at each time point.
2.7. Transmission Electron Microscopy
The bacterial suspension of S. pyogenes in NSS (1.5 × 109 CFU mL−1) was prepared from the culture on BHI agar. Then, 1 mL of suspension was added into 9 mL BHI broth supplemented with the extract at its MBC. The suspensions were incubated with 5% CO2 at 37°C for 14 h according to the time before the cells were killed with the extract. The bacterial cells were collected and prepared for transmission electron microscopy [24].
3. Results
3.1. Antibacterial Activities
The antibacterial activities of the ethanol extract of R. tomentosa and reference antibiotics against 47 clinical isolates of S. pyogenes were expressed as MIC and MBC (Table 1). All strains tested with penicillin (MIC ≤ 0.12 μg mL−1) and erythromycin (MIC ≤ 0.25 μg mL−1) were sensitive to both antibiotics. The extract of R. tomentosa showed significant activity against all 47 clinical isolates with similar MIC (3.91–62.5 μg mL−1) and MBC (3.91–62.5 μg mL−1) ranges. The MIC50 and MIC90 of the extract on S. pyogenes were 7.81 and 15.62 μg mL−1, respectively. The antibacterial activity of rhodomyrtone was evaluated against 14 clinical isolates of S. pyogenes. It exhibited good anti-S. pyogenes activity, with very low MIC (0.39–1.56 μg mL−1) and MBC (0.39–1.56 μg mL−1) values. The MIC50 and MIC90 were 0.78 and 1.56 μg mL−1, respectively.
Table 1.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of antibiotics, ethanol extract of Rhodomyrtus tomentosa, and rhodomyrtone against clinical Streptococcus pyogenes isolates.
| Antibacterial agents | Antibacterial activity (μg mL−1) | Resistance (%) | |||||
|---|---|---|---|---|---|---|---|
| *MIC50 | †MIC90 | MIC range | ‡MBC50 | §MBC90 | MBC range | ||
| Antibiotics (n = 47) | |||||||
| Erythromycin | <0.015 | 0.031 | <0.015–0.125 | 0.031 | 0.031 | <0.015–0.125 | 0 |
| Penicillin G | <0.015 | <0.015 | <0.015–0.062 | <0.015 | 0.031 | <0.015–0.062 | 0 |
| Plant extract/compound | |||||||
| Ethanol extract (n = 47) | 7.81 | 15.62 | 3.91–62.50 | 15.62 | 15.62 | 3.91–62.50 | |
| Rhodomyrtone (n = 14) | 0.78 | 1.56 | 0.39–1.56 | 1.56 | 1.56 | 0.39–1.56 | |
*MIC at which 50% of the isolates were inhibited (MIC50).
†MIC at which 90% of the isolates were inhibited (MIC90).
‡MBC at which 50% of the isolates were killed (MBC50).
§MBC at which 90% of the isolates were killed (MBC90).
n: Number of test isolate.
3.2. Time-Kill Assay
Time-kill curves of S. pyogenes after treatment with the plant extract are demonstrated in Figure 1. Rhodomyrtus tomentosa extract exhibited killing effect at 8 × MIC (MBC) after 16 h, while the concentration at 4 × MIC and 2 × MIC inhibited growth of the organisms. At 1/2 × MIC, the extract inhibited the bacterial growth until 10 h, reached log phase and stationary phase after 10 and 14 h, respectively. The growth level at the stationary phase was 2-3 log CFU mL−1 lower than the control until 24 h.
Figure 1.
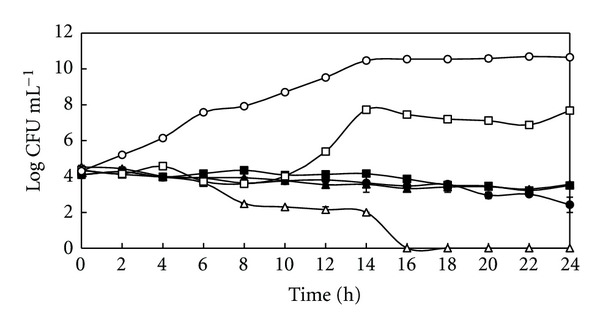
Time-kill curves of Streptococcus pyogenes NPRC 101 after treatment with the ethanol extract of Rhodomyrtus tomentosa leaf at 1/2 × MIC (□), MIC (■), 2 × MIC (▲), 4 × MIC (●), and 8 × MIC (∆). 1% DMSO was used as a control (○). Each symbol indicates the mean ± SD.
3.3. Bacteriolysis
The bacteriolytic activity of the extract on S. pyogenes is presented in Figure 2. Treatment of S. pyogens cells with the extract at all concentrations produced no effect on bacterial cell lysis within 24 h (% relative absorbance at OD620 range from 80.2–99.7%).
Figure 2.
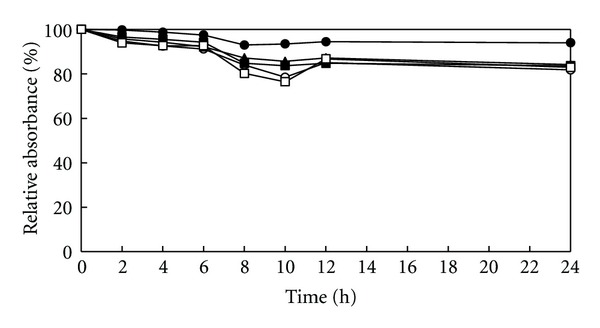
Bacteriolytic activity of the ethanol extract of Rhodomyrtus tomentosa leaf against Streptococcus pyogenes NPRC 101 at 1/2 × MIC (□), MIC (■), 2 × MIC (▲), 4 × MIC (○), and 1% DMSO (●). The results were expressed in percent as the ratio of OD620 at each time interval versus OD620 at 0 min (relative absorbance). The values are from triplicate experiments.
3.4. Loss of 260-nm-Absorbing Materials
Leakage through bacterial cytoplasmic membrane was analysed by determining of the absorbance reading at 260 nm (Figure 3). The results demonstrated that only the filtrate from 4 × MIC of the treatment with R. tomentosa extract was significantly different from the control after 2 h (P < 0.05). Significant increase in the OD260 s occurred at 2 × MIC and 4 × MIC during the treatment at 4–24 h (P < 0.05). However, the patterns were inconsistent and no remarkable increase in OD260s was observed in relation to the exposure time.
Figure 3.
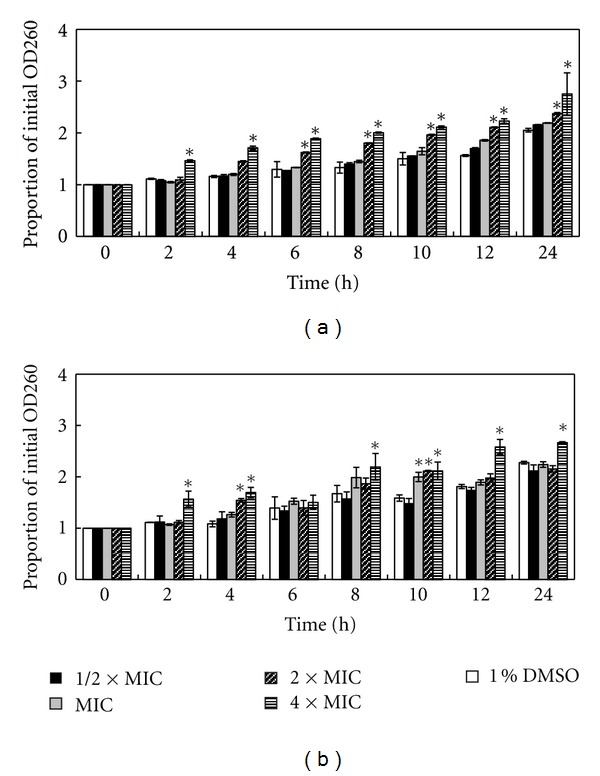
Measuring absorbance of the cell material contents at 260 nm releasing from Streptococcus pyogenes NPRC 101 (a) and S. pyogenes NPRC 109 (b) after treatment with the ethanol extract of Rhodomyrtus tomentosa leaf at 1/2 × MIC, MIC, 2 × MIC, 4 × MIC, and 1% DMSO. Mean values of triplicate independent experiments and SDs are shown. Dunnett test demonstrates significant difference between the tests and the control at each time point (*P < 0.05).
3.5. Transmission Electron Microscopy
Transmission electron microscopy was carried out to observe any morphological changes in S. pyogenes cells caused by the plant extract at its MBC value (Figure 4). The bacterial cells were collected after 14 h before they were killed according to the time-kill assay (Figure 1). The extract of R. tomentosa did not cause any dramatic changes on the bacterial cells before they were killed. However, a few treated cells seem to have some disorders during cell division process (Figures 4(a) and 4(b)). The treated cells demonstrated irregular shape with different sizes during binary fission.
Figure 4.
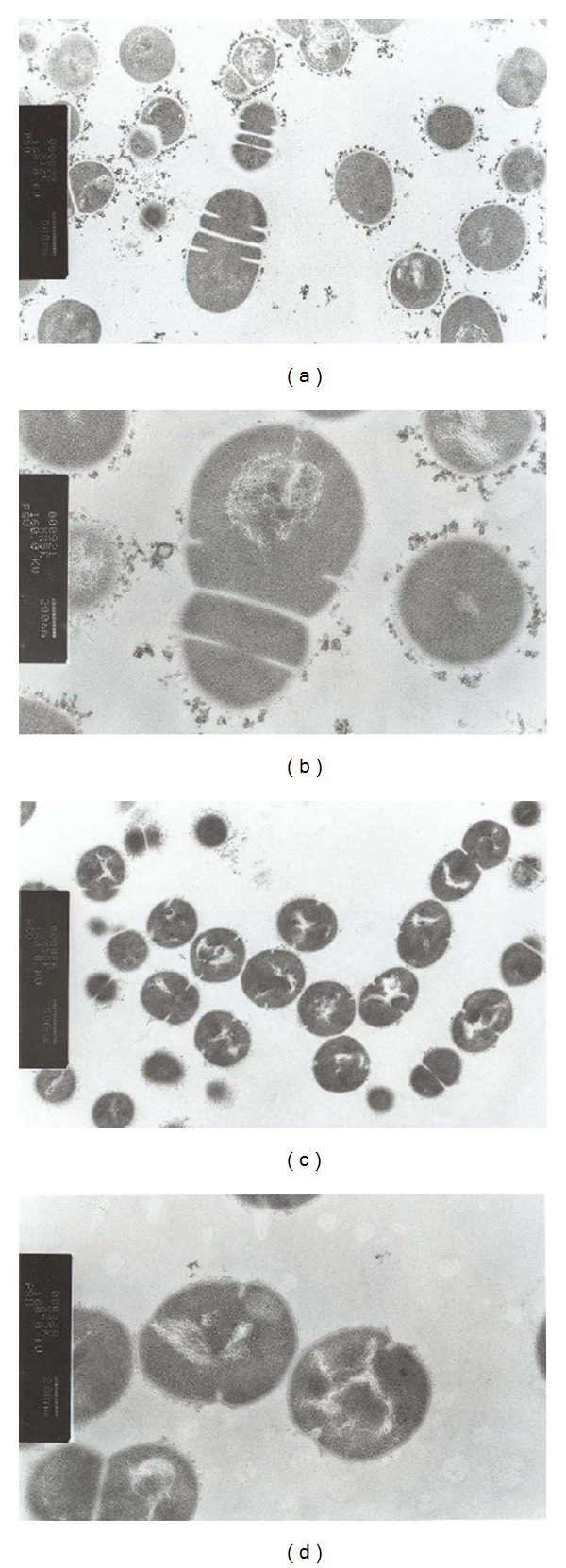
Transmission electron micrographs of the ethanol extract of Rhodomyrtus tomentosa treated Streptococcus pyogenes NPRC 101 cells at MBC concentration for 14 h according to the time before the cells were killed with the extract ((a) and (b)). 1% DMSO was used as control ((c) and (d)). Magnification: ×12000 ((a) and (c)) and ×25000 ((b) and (d)).
4. Discussion
An increase in antibiotic-resistant S. pyogenes and failure rates of antibiotic therapy for S. pyogenes infections has been described [3, 12–15, 25]. A number of medicinal plants have been reported to have anti-S. pyogenes activities [26]. Our results revealed that R. tomentosa extract produced very good antibacterial activity against all 47 clinical S. pyogenes isolates. Nevertheless, little is known about the antibacterial mechanism and pharmacological effects of this effective plant and its active compound. Determination of their mechanisms of action will provide a starting point for the discovery of new medicines. A previous study of 73 essential oils against Streptococcus pneumoniae reported that the essential oils from oregano, rosewood, and thyme caused membrane damage and induced cell lysis [27]. Melaleuca alternifolia (tea tree) oil and its components have been reported to induce delayed lysis in Staphylococcus aureus by releasing membrane-bound cell wall autolytic enzymes when the bacterial cells were treated for several hours [23]. In contrast, R. tomentosa extract did not cause any delayed effect, and the action of this extract does not appear to involve cell wall damage or activation of autolytic enzymes. Leakage in the cytoplasmic membrane was analysed by determining of the absorbance reading at 260 nm to detect the release of cell materials including nucleic acid, metabolites, and ions [28]. Several antibacterial agents and plant extracts such as chlorhexidine [29], hexachlorophene [30], phenethyl alcohol [31], lemongrass oil [32], tea tree oil [23], and Eleutherine americana extract [33] have shown an effect on bacterial cytoplasmic membranes through inducing a loss of 260-nm-absorbing materials. Our results showed slight loss of 260-nm-absorbing materials, suggesting loss of negligible amount of nucleic acids through a damaged cytoplasmic membrane. This leakage could also be caused by other reasons such as weakening of the cell wall or disruption of cell membrane by osmotic pressure when the bacterial cells were kept for several hours. As revealed in the ultrastructural analysis of the treated cells, this extract did not reveal any dramatic changes on the bacterial cells. However, a few of treated cells demonstrated irregular shape with different sizes during binary fission.
Rhodomyrtone, an isolated active compound from R. tomentosa, exhibited strong antibacterial activity against S. pyogenes. Rhodomyrtone is a newly reported novel antibacterial compound and there have been very brief reports on its activity. Significant antibacterial activities of rhodomyrtone against many Gram-positive bacteria have been documented [18, 34]. Although the previous researches have attempted to explain the actions of rhodomyrtone [35–37], its antibacterial mechanisms of action are still unclear. Therefore, the development of this compound as a new lead drug needs more information and researches on its modes of action.
The results obtained from this study shed light on the antibacterial mechanisms of R. tomentosa leaf extract. Rhodomyrtone has powerful in vitro activity against S. pyogenes. This active compound may be used as a therapeutic drug candidate for the control of streptococcal infections. Further research that examines it's in vitro and in vivo mechanisms of action, toxicity, and therapeutic effect is necessary.
Acknowledgments
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and the Thailand Research Fund through the Royal Golden Jubilee, Ph.D. Program (PHD/0029/2548). The authors gratefully acknowledge the support by DAAD (German Academic Exchange Service).
References
- 1.Cunningham MW. Pathogenesis of group a streptococcal infections. Clinical Microbiology Reviews. 2000;13(3):470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clinical Infectious Diseases. 2002;35(2):113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 3.Pichichero ME, Casey JR, Mayes T, et al. Penicillin failure in streptococcal tonsillopharyngitis: causes and remedies. Pediatric Infectious Disease Journal. 2000;19(9):917–923. doi: 10.1097/00006454-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 4.Gastanaduy AS, Huwe BB, Kaplan EL. Failure of penicillin to eradicate group A streptococci during an outbreak of pharyngitis. The Lancet. 1980;2(8193):498–502. doi: 10.1016/s0140-6736(80)91832-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith TD, Huskins WC, Kim KS, Kaplan EL. Efficacy of β-lactamase-resistant penicillin and influence of penicillin tolerance in eradicating streptococci from the pharynx after failure of penicillin therapy for group A streptococcal pharyngitis. The Journal of Pediatrics. 1987;110(5):777–782. doi: 10.1016/s0022-3476(87)80023-9. [DOI] [PubMed] [Google Scholar]
- 6.Stromberg A, Schwan A, Cars O. Five versus ten days treatment of group A streptococcal pharyngotonsillitis: a randomized controlled clinical trial with phenoxymethylpenicillin and cefadroxil. Scandinavian Journal of Infectious Diseases. 1988;20(1):37–46. doi: 10.3109/00365548809117215. [DOI] [PubMed] [Google Scholar]
- 7.Roos K, Larsson P. Loracarbef versus phenoxymethylpenicillin in the treatment of recurrent streptococcal pharyngotonsillitis. Scandinavian Journal of Infectious Diseases. 1997;29(2):141–145. doi: 10.3109/00365549709035874. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Johnson DR. Unexplained reduced microbiological efficacy of intramuscular benzathine penicillin G and of oral penicillin V in eradication of group A streptococci from children with acute pharyngitis. Pediatrics. 2001;108(5):1180–1186. doi: 10.1542/peds.108.5.1180. [DOI] [PubMed] [Google Scholar]
- 9.Baker AS, Behlau I, Tierney MR. Infections of the pharynx, larynx, epiglottis, trachea, and thyroid. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious Diseases. Philadelphia, Pa, USA: W. B. Saunders Company; 1998. pp. 540–548. [Google Scholar]
- 10.Stein GE, Christensen S, Mummaw N. Comparative study of clarithromycin and penicillin V in the treatment of streptococcal pharyngitis. European Journal of Clinical Microbiology and Infectious Diseases. 1991;10(11):949–953. doi: 10.1007/BF02005450. [DOI] [PubMed] [Google Scholar]
- 11.Adam D, Scholz H, Aspe C, et al. Five days of erythromycin estolate versus ten days of penicillin V in the treatment of group A streptococcal tonsillopharyngitis in children. European Journal of Clinical Microbiology and Infectious Diseases. 1996;15(9):712–717. doi: 10.1007/BF01691957. [DOI] [PubMed] [Google Scholar]
- 12.Feng L, Shen X. PP-011 High level macrolide resistant Streptococcus pyogenes Isolated from Chinese children and the relationship with Tn6002. International Journal of Infectious Diseases. 2009;13(supplement 1):p. S53. [Google Scholar]
- 13.Gracia M, Díaz C, Coronel P, et al. Antimicrobial susceptibility of Streptococcus pyogenes in Central, Eastern, and Baltic European Countries, 2005 to 2006: the cefditoren surveillance program. Diagnostic Microbiology and Infectious Disease. 2009;64(1):52–56. doi: 10.1016/j.diagmicrobio.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Michos AG, Bakoula CG, Braoudaki M, et al. Macrolide resistance in Streptococcus pyogenes: prevalence, resistance determinants, and emm types. Diagnostic Microbiology and Infectious Disease. 2009;64(3):295–299. doi: 10.1016/j.diagmicrobio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Malli E, Tatsidou E, Damani A, et al. Macrolide-resistant Streptococcus pyogenes in Central Greece: prevalence; mechanism and molecular identification. International Journal of Antimicrobial Agents. 2010;35(6):614–615. doi: 10.1016/j.ijantimicag.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Bauer MJ, Georgousakis MM, Vu T, et al. Evaluation of novel Streptococcus pyogenes vaccine candidates incorporating multiple conserved sequences from the C-repeat region of the M-protein. Vaccine. 2012;12(12):2197–2205. doi: 10.1016/j.vaccine.2011.12.115. [DOI] [PubMed] [Google Scholar]
- 17.Zaman M, Abdel-Aal AB, Fujita Y. Immunological evaluation of lipopeptide group A streptococcus (GAS) vaccine: structure-activity relationship. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0030146.e30146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limsuwan S, Trip EN, Kouwen TRHM, et al. Rhodomyrtone: a new candidate as natural antibacterial drug from Rhodomyrtus tomentosa . Phytomedicine. 2009;16(6-7):645–651. doi: 10.1016/j.phymed.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Hiranrat A, Mahabusarakam W. New acylphloroglucinols from the leaves of Rhodomyrtus tomentosa . Tetrahedron. 2008;64(49):11193–11197. [Google Scholar]
- 20.Forbes BA, Sahm DF, Weissfeld AS. Bailey & Scott’s Diagnostic Microbiology. St. Louis, Mo, USA: Mosby; 2002. [Google Scholar]
- 21.CLSI: Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute Document M07-A8. 8th edition. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. [Google Scholar]
- 22.Chusri S, Voravuthikunchai SP. Quercus infectoria: a candidate for the control of methicillin-resistant Staphylococcus aureus infections. Phytotherapy Research. 2008;22(4):560–562. doi: 10.1002/ptr.2377. [DOI] [PubMed] [Google Scholar]
- 23.Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrobial Agents and Chemotherapy. 2002;46(6):1914–1920. doi: 10.1128/AAC.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suwalak S, Voravuthikunchai SP. Morphological and ultrastructural changes in the cell structure of enterohaemorrhagic Escherichia coli O157:H7 following treatment with Quercus infectoria nut galls. Journal of Electron Microscopy. 2009;58(5):315–320. doi: 10.1093/jmicro/dfp024. [DOI] [PubMed] [Google Scholar]
- 25.Pichichero ME, Casey JR. Systematic review of factors contributing to penicillin treatment failure in Streptococcus pyogenes pharyngitis. Otolaryngology. 2007;137(6):851–857. doi: 10.1016/j.otohns.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Limsuwan S, Subhadhirasakul S, Voravuthikunchai SP. Medicinal plants with significant activity against important pathogenic bacteria. Pharmaceutical Biology. 2009;47(8):683–689. [Google Scholar]
- 27.Horne D, Holm M, Oberg C, Chao S, Young DG. Antimicrobial effects of essential oils on Streptococcus pneumoniae . Journal of Essential Oil Research. 2001;13(5):387–392. [Google Scholar]
- 28.Oonmetta-aree J, Suzuki T, Gasaluck P, Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus . LWT-Food Science and Technology. 2006;39(10):1214–1220. [Google Scholar]
- 29.Hugo WB, Longworth AR. Some aspects of the mode of action of chlorhexidine. The Journal of Pharmacy and Pharmacology. 1964;16(10):655–662. doi: 10.1111/j.2042-7158.1964.tb07384.x. [DOI] [PubMed] [Google Scholar]
- 30.Joswick HL, Corner TR, Silvernale JN, Gerhardt P. Antimicrobial actions of hexachlorophene: release of cytoplasmic materials. Journal of Bacteriology. 1971;108(1):492–500. doi: 10.1128/jb.108.1.492-500.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver S, Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. Journal of Bacteriology. 1967;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onawunmi GO. In vitro studies on the antibacterial activity of phenoxyethanol in combination with lemon grass oil. Pharmazie. 1988;43(1):42–44. [PubMed] [Google Scholar]
- 33.Ifesan BOT, Joycharat N, Voravuthikunchai SP. The mode of antistaphylococcal action of Eleutherine americana . FEMS Immunology and Medical Microbiology. 2009;57(2):193–201. doi: 10.1111/j.1574-695X.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- 34.Saising J, Hiranrat A, Mahabusarakam W, Ongsakul M, Voravuthikunchai SP. Rhodomyrtone from Rhodomyrtus tomentosa (Aiton) Hassk. as a natural antibiotic for staphylococcal cutaneous infections. Journal of Health Science. 2008;54(5):589–595. [Google Scholar]
- 35.Limsuwan S, Hesseling-Meinders A, Voravuthikunchai SP, van Dijl JM, Kayser O. Potential antibiotic and anti-infective effects of rhodomyrtone from Rhodomyrtus tomentosa (Aiton) Hassk. on Streptococcus pyogenes as revealed by proteomics. Phytomedicine. 2011;18(11):934–940. doi: 10.1016/j.phymed.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Sianglum W, Srimanote P, Wonglumsom W, Kittiniyom K, Voravuthikunchai SP. Proteome analyses of cellular proteins in methicillin-resistant Staphylococcus aureus treated with rhodomyrtone, a novel antibiotic candidate. PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0016628.e16628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visutthi M, Srimanote P, Voravuthikunchai SP. Responses in the expression of extracellular proteins in methicillin-resistant Staphylococcus aureus treated with rhodomyrtone. The Journal of Microbiology. 2011;49(6):956–964. doi: 10.1007/s12275-011-1115-0. [DOI] [PubMed] [Google Scholar]


