Abstract
Converging lines of evidence suggest that the glutamatergic system may play an increasingly important role in the development of novel therapeutics for major depressive disorder (MDD), particularly agents associated with rapid antidepressant effects. Diverse glutamatergic modulators targeting N-methyl-D-aspartate receptors have shown efficacy in MDD, but their associated psychotomimetic effects presently preclude their use in larger samples. This small, randomized, double-blind, placebo-controlled, crossover pilot study evaluated the potential antidepressant efficacy and tolerability of an oral formulation of the selective N-methyl-D-aspartate NR2B antagonist MK-0657 in patients with treatment-resistant MDD (TRD). The TRD subjects underwent a 1-week drug-free period and were subsequently randomized to receive either MK-0657 monotherapy (4–8 mg/d) or placebo for 12 days. Because of recruitment challenges and the discontinuation of the compound’s development by the manufacturer, only 5 of the planned 21 patients completed both periods of the crossover administration of MK-0657 and placebo. Significant antidepressant effects were observed as early as day 5 in patients receiving MK-0657 compared with those receiving placebo, as assessed by the Hamilton Depression Rating Scale and Beck Depression Inventory; however, no improvement was noted when symptoms were assessed with the Montgomery-Asberg Depression Rating Scale, the primary efficacy measure. No serious or dissociative adverse effects were observed in patients receiving this oral formulation of MK-0657. Despite the small sample size, this pilot study suggests that an oral formulation of the NR2B antagonist MK-0657 may have antidepressant properties in TRD patients. Further studies with larger sample sizes are necessary to confirm these preliminary findings.
Keywords: depression, glutamate, NMDA, NR2B, treatment
Foremost among the limitations of current treatments for major depressive disorder (MDD) is the considerable lag of onset of action of standard antidepressants.1 This period of latency is associated with impairments in personal and family life and suicidal behavior.2,3 Thus, a critical need exists to develop therapies for MDD that act more rapidly than currently available ones. Considerable clinical and preclinical evidence suggests that drugs that modulate the glutamatergic system, especially at the N-methyl-D-aspartate (NMDA) receptor (NMDAR) complex, result in a more rapid onset of antidepressant response than existing anti-depressants.4–9 The NMDARs are widely distributed in the brain and are directly involved in the pathophysiology of MDD, as well as the mechanism of action of some novel therapeutics.10,11 For instance, controlled trials have shown that NMDA antagonists such as ketamine exert rapid antidepressant effects4,7; nevertheless, the dissociative side effects of these agents limit their clinical utility. The NMDARs are tetrameric proteins comprising NR1 and NR2 subunits; four different NR2 subunits (NR2A-D) exist in the brain. Notably, the NR2B subunit—localized primarily in the forebrain—is a prime target for the development of novel anti-depressants. For instance, a significant reduction in NR2B subunit expression was found in the prefrontal cortex of patients with MDD relative to controls.12 In addition, the selective NR2B antagonist Ro25-6981 was found to activate the mammalian target of rapamycin, a protein that has been linked to the antidepressant effects of ketamine13 and, in preclinical rodent studies, was found to have significant antidepressant-like properties.13,14
Recently, the NR2B subunit selective NMDAR antagonist CP-101,606 was tested in MDD. In this seminal double-blind, randomized, placebo-controlled, add-on trial, a single infusion of CP-101,606 showed early antidepressant effects (at day 5) in patients with treatment-resistant MDD (TRD) who had not responded to a serotonin selective reuptake inhibitor (SSRI). Dissociative effects were modest and resolved within 8 hours but, nevertheless, resulted in a reduction of the dosage and duration of the infusion.6
To our knowledge, no published data explore the use of an oral formulation of an NR2B antagonist administered chronically to patients with TRD. The present pilot study was conducted to evaluate the antidepressant efficacy, safety, and pharmacokinetics of a selective NR2B antagonist (MK-0657, 4–8 mg/d given orally) compared with placebo for 12 days in subjects with TRD. The preliminary pharmacokinetic data for a single oral MK-0657 dose indicated that MK-0657 was rapidly absorbed (within 1 hour after dose). A harmonic mean of approximately 14 to 17 and 21 to 26 hours characterized the apparent half-life of MK-0657 and its active metabolite, respectively. Area under the curve and Cmax values increased approximately linearly with dose and did not display any pronounced departures from dose proportionality. Echoing the positive results obtained by Preskorn and colleagues,6 our primary hypothesis was that MK-0657 would result in a rapid and significant antidepressant response compared with placebo in TRD patients. However, the manufacturer discontinued the program for this compound because it failed to show efficacy in early studies of Parkinson disease, which was the prioritized indication for exploration of the mechanism. Because of this, and because of slow recruitment, only 5 subjects completed the study. Thus, our results must be considered preliminary.
MATERIALS AND METHODS
Patient Selection
Male and female patients, aged 18 to 55 years, were eligible to participate; all patients were diagnosed with MDD, currently depressed without psychotic features, as assessed using the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Disorders–Patient Version (SCID-P).15 Patients with a history of antidepressant- or substance-induced hypomania or mania were excluded. All subjects were studied at the National Institute of Mental Health (NIMH) Clinical Research Center in Bethesda, Maryland. Subjects were required to have a score of 22 or higher on the Montgomery-Asberg Depression Rating Scale (MADRS) at screening and at baseline (day of first dose of study medication). In addition, patients had to have previously failed at least 2 adequate antidepressant trials in the current episode (adequacy of antidepressant trials was determined using the Antidepressant Treatment History Form modified16).
All subjects were in good physical health, as determined by medical history, physical examination, blood laboratory tests, ECG, chest radiography, urinalysis, and toxicology screen. Subjects were free of comorbid substance abuse or dependence (excluding nicotine or caffeine) for at least 3 months and had a negative urine toxic screen on admission. Comorbid Axis I Anxiety Disorder diagnoses were permitted if they were not the primary focus of treatment within 12 months before screening. Other exclusion criteria included a diagnosis of bipolar disorder, psychotic features, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis II diagnosis, suicidal ideation, serious unstable medical disorder or condition, previous use of ketamine or phencyclidine, and concomitant treatment with psychotropic medications in the 2 weeks (5 weeks for fluoxetine) before the study or electroconvulsive therapy in the 3 months before the study; female subjects could not be pregnant or nursing.
The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health (NIH). All subjects provided written informed consent before entering the study.
Study Design
This was a single-center, double-blind, placebo-controlled, randomized, crossover study. Following a 1-week drug-free period, 5 subjects with TRD currently experiencing a major depressive episode without psychotic features were randomized in a double-blind manner to receive either 4 to 8 mg/d of MK-0657 (provided by Merck & Co, Inc) or placebo for 12 days. At day 12, the study drug was discontinued; subjects remained drug-free for 14 days and then crossed over to the other treatment condition. Dosage in the second experimental treatment condition (days 27 through 38) was identical to the first crossover phase. A double-blind dummy design was used throughout the study; each capsule contained 1 mg of MK-0657 or placebo. Patients were required to take 8 capsules a day in the morning from days 1 through 12 and 27 through 38. Initial doses were 4 mg/d of MK-0657 (ie, 4 capsules of MK-0657 and 4 capsules of placebo) on day 1 or 27 (the first day of the second crossover phase). The dose of study medication was increased in a blinded fashion every four days by 2 mg/day until the appearance of treatment-limiting side effects or completion of the study. A dose of 8 mg/day was the treatment target for all participants unless tolerability issues ensued. Dose reductions were permitted under double-blind conditions by 2 capsules (2 mg) in case of side effects. Subjects who were unable to tolerate the lowest dose of MK-0657 allowable (4 mg/d) were discontinued from the study. Treatment compliance was monitored by capsule counts and by the nurses who administered the study medication. No additional medications that primarily affect the central nervous system were allowed during the study.
Patients were hospitalized at the Clinical Research Center of the NIMH-NIH for the duration of the study. Vital signs were obtained daily. Hematology, blood chemistry, and urinalysis were performed at baseline and on days 6, 12, 32, 38, 42, and 52 or study exit. The ECG was performed at screening, baseline, day 12, day 38, and study exit.
Outcome Measures
Rating scales included the MADRS,17 which was the primary outcome measure. Secondary outcome measures were the 17-item Hamilton Rating Scale for Depression (HAM-D),18 the self-administered Beck Depression Inventory (BDI),19 the visual analog scale for depression (VAS-depression),20 the Hamilton Anxiety Rating Scale (HAM-A),21 the Brief Psychiatric Rating Scale-positive symptoms (BPRS),22 the Clinician Administered Dissociative States Scale (CADSS),23 the Young Mania Rating Scale (YMRS),24 and the Snaith Hamilton Pleasure Scale-Modified Scoring System (SHAPS-M).25 The MADRS, HAM-D, BDI, VAS, HAM-A, CADSS, BPRS, YMRS, and SHAPS-M ratings were obtained daily on days 1 to 12 and 27 to 38.
Pharmacokinetic, Pharmacodynamic, and Safety Evaluation
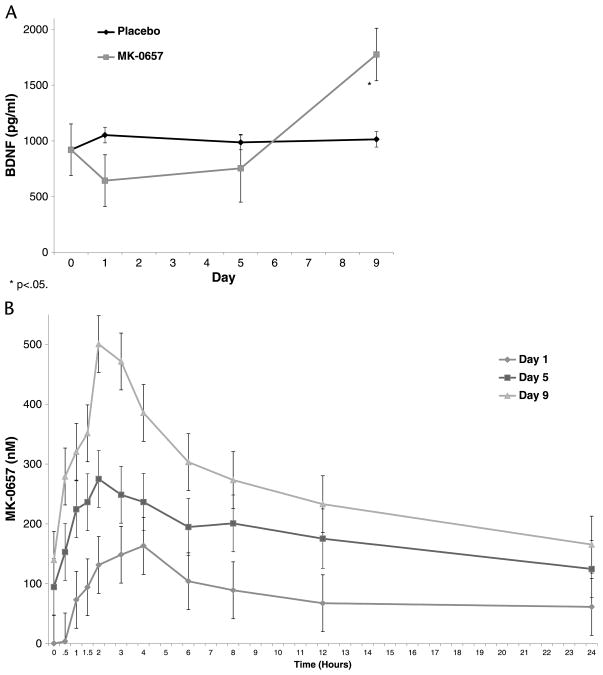
Blood (2 mL) was drawn into a heparin-containing tube. Samples were processed by centrifugation (3000 rpm at 4°C for 10 minutes), and plasma was transferred and stored at −70°C until MK-0657 concentrations were determined. Pharmacokinetic and pharmacodynamic assays were performed as previously described.26 Pharmacokinetic measures for MK-0657 were obtained at predose, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, and 24 hours after dose and on days 1, 5, 9, 27, 31, and 35 (Fig. 1).
FIGURE 1.
Changes in plasma BDNF (A) and MK-0657 levels (B) during 9 days of treatment with MK-0657. The BDNF levels were evaluated at baseline and on days 1, 5, and 9 of treatment in the active drug compared with placebo. Blood samples for MK-0657 pharmacokinetics were collected predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, and 24 hours after dose on days 1, 5, 9. *P < 0.05.
Brain-Derived Neurotrophic Factor Levels
Blood samples for brain-derived neurotrophic factor (BDNF) analysis were collected as putative biomarkers of antidepressant response. Samples were collected at baseline and on days 1, 5, and 9 of each of the 2 study phases (Fig. 1) and processed as previously described.27 Experiments were carried out in duplicate blind to clinical information.
Statistical Analyses
The primary aim of the study was to access the efficacy of 12 days of MK-0657 versus placebo in improving overall depressive symptomatology in TRD patients. A linear mixed model with restricted maximum likelihood estimation was used to examine the effects of treatment (MK-0657 and placebo) over time (treatment day) with the baseline of each phase as a covariate. A main effect for study phase was also included. Schwarz Bayesian criterion was used to determine the best-fitting variance-covariance structure, an autoregressive moving average model. Bonferroni-adjusted simple effect tests were used to evaluate the significance of omnibus effects. Analyses were conducted in the same manner for the MADRS, HAM-D, BDI, VAS-depression, HAM-A, BPRS, CADSS, YMRS, and SHAPS-M. The MADRS was the primary outcome measure. Significance was evaluated at P < 0.05, 2-tailed. Analysis of secondary outcomes were Bonferroni corrected, so that significance was evaluated at P < 0.006, 2-tailed. Further analyses included measuring plasma levels of the active compound, BDNF levels, and side effects. Cohen d is shown for comparisons of MK-0657 and placebo at 12 days. Cohen d is an effect size showing the standardized magnitude of change, where 0.8 is considered a large effect, 0.5 is moderate, and 0.3 is small.28
A linear mixed model with restricted maximum likelihood estimation of plasma NR2B levels was used to examine the change in levels over the course of trial day (days 1, 5, and 9) and time of day (hours 0 to 24) as well as the interaction between day and time. An autoregressive moving average variance-covariance structure was used. A similar model was used for BDNF levels, but no time of day factor was included, and a compound symmetry variance-covariance structure was used.
RESULTS
The study originally intended to recruit 21 patients. Of the first 24 patients screened, 19 were excluded because they either did not meet study criteria (n = 13) or refused to participate (n = 6). Five subjects were randomized and completed both phases of the study before it was terminated. Three of five patients received MK-0657 in the first phase.
Efficacy
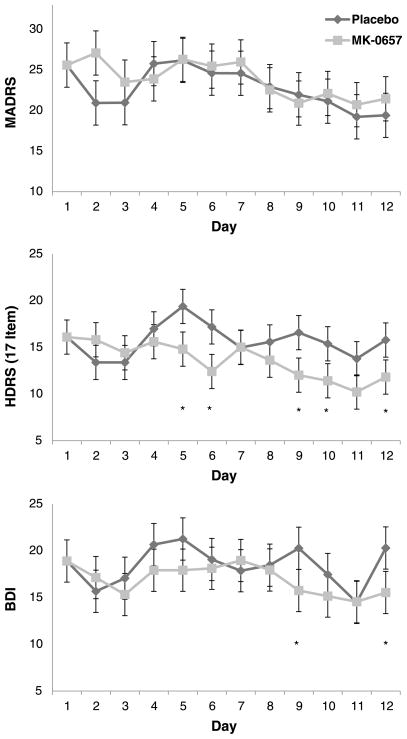
Linear mixed models indicated no significant improvement in patients receiving MK-0657 compared with those receiving placebo, as assessed via the primary outcome measure (MADRS, P = 0.27). However, linear mixed models showed that MK-0657 improved depressive symptoms when the HAM-D and BDI were used to assess improvement (HAM-D, P = 0.001, d = 0.48; BDI, P = 0.01, d = 0.52) (Fig. 2). The YMRS ratings were also significantly lower in patients receiving MK-0657 versus placebo (P = 0.002, d = 0.57). However, no such improvement was seen when the CADSS, BPRS, VAS-depression, HAM-A, or SHAPS-M were used to assess improvement (MADRS, P = 0.27; CADSS, P = 0.13; BPRS, P = 0.47; SHAPS-M, P = 0.77). After correction for multiple comparisons, the effects for the HAM-D and YMRS remained significant.
FIGURE 2.
Change in the 17-item Hamilton Depression Rating Scale (HAM-D) scores, Beck Depression Inventory (BDI) scores, and Montgomery-Asberg Depression Rating Scale (MADRS) scores in drug-free TRD subjects followed receiving 12 days of MK-0657 compared with placebo. *P < 0.05.
Given this discrepancy in depression rating scale scores, we examined changes in individual symptoms for the two depression scales (HAM-D and MADRS). Drug comparisons were made between the main effects of MK-0657 versus placebo; drug × time interactions indicated differences between the drugs at specific time points. For the HAM-D, significant improvement was noted on the following symptoms: guilt (drug, P = 0.01; drug × time [D× T], P = 0.29), suicidal ideation (drug, P = 0.24; D× T, P = 0.045), early insomnia (drug: P = 0.002; D× T, P = 0.69), middle insomnia (drug, P = 0.008; D× T, P = 0.19), work and interests (drug, P = 0.005; D× T, P = 0.12), and psychic anxiety (drug, P < 0.001; D× T, P = 0.75). General somatic symptoms were significantly higher in patients receiving MK-0657 (drug, P = 0.002; D× T, P = 0.40). On the MADRS, only 1 symptom (inner tension) was significantly improved with MK-0657 (drug, P < 0.001; D× T, P = 0.41). Another symptom (pessimistic thoughts) was significantly worse but only on day 7 (drug, P = 0.93; D× T, P = 0.02). After Bonferroni corrections were applied, only psychic anxiety on the HAM-D and inner tension on the MADRS remained significantly improved. Further analyses examined individual YMRS items to identify where changes occurred. Patients receiving MK-0657 had significantly less motor activity (drug, P = 0.008; D× T, P = 0.37), irritability (drug, P < 0.001; D× T, P = 0.70), language or thought disorder (Drug: P = 0.006, D× T: P = 0.80), and poor appearance (drug, P = 0.046; D× T, P = 0.24).
Because MK-0657 and ketamine may have similar mechanisms of action, effect sizes were calculated for 17 individual HAM-D items from the present study and from our previously published crossover study of ketamine in patients with MDD7 (Table 1). Specifically, the ketamine data were reanalyzed using the same model used in the current study. Because baseline was used as a covariate, effect sizes reflect the main effect of drug versus placebo, not interactions with time. The HAM-D symptoms showing moderate or better (d ≥ 0.50) improvement in both studies were: psychic anxiety, work and interests, early insomnia, and guilt. Only MK-0657 appeared to moderately improve middle insomnia, and only ketamine appeared to moderately improve depressed mood, suicidal ideation, late insomnia, and hypochondriasis. MK-0657 moderately worsened, and ketamine largely improved, general somatic symptoms. One of the patients receiving MK-0657 achieved a 50% response, but none met response criteria on placebo. No patient met remission criteria (MADRS, < 10).
TABLE 1.
Contrast of Change in Hamilton Depression Rating Scale Items Between the Selective NR2B Antagonist MK-0657 and the Broad NMDA Antagonist Ketamine*
| Item | HAM-D Symptom | MK-0657
|
Ketamine
|
|---|---|---|---|
| d | d | ||
| 10 | Psychic Anxiety | 1.19 | 0.84 |
| 7 | Work and Interests | 0.80 | 1.29 |
| 5 | Insomnia, Middle | 0.71 | 0.27 |
| 4 | Insomnia, Early | 0.71 | 0.60 |
| 2 | Guilt | 0.64 | 1.14 |
| 9 | Agitation | 0.47 | 0.24 |
| 11 | Somatic Anxiety | 0.38 | 0.37 |
| 3 | Suicidal Ideation | 0.25 | 0.68 |
| 15 | Hypochondriasis | 0.10 | 0.50 |
| 12 | Somatic Symptoms, GI | 0.05 | 0.05 |
| 14 | Genital Symptoms | 0.04 | 0.56 |
| 6 | Insomnia, Late | −0.16 | 0.50 |
| 16 | Weight Loss | −0.19 | 0.23 |
| 8 | Retardation | −0.27 | 0.39 |
| 1 | Depressed Mood | −0.30 | 1.20 |
| 13 | Somatic Symptoms, General | −0.76 | 1.13 |
| d ≥ .8 | |||
| d ≥ .5 and < .8 | |||
| d ≤ − .5 | |||
Positive effects sizes indicate lower levels (improvement) NR2B and negative ones indicate higher levels (worsening) on NR2B.
From Zarate et al.7
Plasma BDNF and MK-0657 Levels
By day 9, plasma BDNF levels were significantly higher in patients receiving MK-0657 than in those receiving placebo (P = 0.03). In the linear mixed model of NR2B plasma levels, a significant main effect was observed for time of day (P < 0.001) and day of treatment (P < 0.001); no significant interaction was noted between the two (P = 0.60). MK-0657 levels increased significantly from days 1 to 5 (P = 0.004) and days 5 to 9 (P = 0.005). Within a given day, levels increased significantly from baseline at 1 hour (P = 0.002) through 8 hours (P = 0.04). Levels were not significantly higher than baseline after a half hour (P = 0.06), at 12 hours (P = 0.25), or at 24 hours (P = 1.0).
Adverse Events
MK-0657 was well tolerated; no serious adverse events were observed throughout the study. No significant difference was noted in the emergence of dissociative or psychotomimetic side effects between groups nor were significant changes from baseline noted for laboratory tests or weight. In addition, no significant changes were observed between treatment groups in systolic or diastolic blood pressure or heart rate (supine or standing).
DISCUSSION
Despite the preliminary nature of the findings, this small, pilot, double-blind, placebo-controlled study of the oral formulation of a selective NR2B antagonist (MK-0657) in TRD patients detected an antidepressant effect for MK-0657, as reflected by improvement on a number of items that constitute 2 of 3 standard scales used in assessing antidepressant response. Notably, MK-0657 significantly improved depressive symptoms, as assessed by the clinician-administered HAM-D and the self-rated BDI; these antidepressant effects were seen within 5 days. However, no such antidepressant effect was observed when the MADRS was used to assess improvement. Another key finding is that at the oral doses used, MK-0657 caused no dissociative or other adverse effects, an issue that has been particularly problematic in the development of novel antidepressant agents from this class of drugs. Finally, MK-0657 significantly increased plasma BDNF levels compared with placebo after 9 days of treatment, demonstrating a biological effect typically observed with most other antidepressants.29 Nevertheless, because of the small sample size of this pilot study, these results must be considered preliminary.
These findings support preclinical work13,14 and complement the results of a recent clinical study by Preskorn and colleagues,6 suggesting that selective NR2B antagonists have antidepressant properties. In that double-blind clinical trial, 30 patients with MDD who had not responded to a prospective trial of an SSRI were randomized to receive a single infusion of the NR2B antagonist CP-101,606 or saline solution. An anti-depressant response to CP-101,606 was observed as early as day 5; 78% of patients maintained response status for at least 1 week after infusion. Although the small sample size of the present study precludes our ability to make definitive comparisons with the study by Preskorn and colleagues,6 it is noteworthy that we observed a similar onset of antidepressant effects (by day 5).
It is also important to note that the different findings obtained here when different rating scales were used could reflect a difference in symptom profile in the patient population, could be due to the small sample size, or could reflect differences in the rating scales themselves. Differential sensitivity to drug effects between the HAM-D and the MADRS has been observed in large controlled clinical trials; for instance, the HAM-D was noted to separate SSRI response from placebo better than the MADRS, which, in turn, better separated tricyclic antidepressants from placebo.30 On 1 level, this should not be surprising, given that the MADRS was developed in the context of finding a scale more sensitive to the effects of tricyclic antidepressants. Furthermore, far more research has been conducted into the sensitivity of various HAM-D items to drug effects, with reasonable consensus on those that are “core” to both tricyclic antidepressants and SSRIs.31 In the current study, 3 items (guilt, work/interests, and psychic anxiety) overlapped with the 6 most replicated set of core HAM-D items for drug response.30
To address the first of these issues, we compared the symptom profiles of response to MK-0657 in this study with response to the broad subunit NMDA antagonist ketamine from a previous study7; notably, we found an overlap in the improvement of 5 HAM-D symptoms–psychic anxiety, work interests, middle insomnia, early insomnia, and guilt–in the moderate to large effect size range (Table 1). Contrasting broader spectrum agents such as ketamine with more selective ones (eg, MK-0657) may help tease apart the symptom profile most likely to improve with more subunit-selective NMDA antagonists. It is noteworthy that these include only 3–guilt, work interests, and psychic anxiety–of the 6 “core” symptoms that analyses typically suggest the greatest improvement in response to both tricyclic and SSRI antidepressants.32,33 It would not be surprising that a different spectrum of symptoms is altered in patients who did not respond to SSRIs, particularly if this response is mediated through a different primary pharmacological mechanism.
One significant difference between the present study and that of Preskorn and colleagues6 is that no subjects in this study experienced dissociative adverse events during the follow-up period (2 weeks). In contrast, the prior study found that the first few subjects exposed to the NR2B antagonist did experience dissociative side effects, which led to a reduction in the dose of study medication; following this adjustment, no additional cases were reported. Although it is possible that such adverse events would have appeared in the present study had patients received MK-0657 for longer than 12 days, this report is nevertheless the first to demonstrate that an oral formulation of an NR2B antagonist exerted antidepressant effects within 5 days with no evidence of dissociative or psychotomimetic effects. In addition, at the dose tested, there was no evidence of elevated blood pressure, a side effect associated with early-generation NR2B antagonists. These results add another piece of evidence to support the possibility that NR2B antagonists are viable therapeutic targets for the development of novel antidepressants. Indeed, other brain-penetrant NR2B antagonists are currently being developed.34,35
The key strength of this study is the placebo-controlled crossover design, which minimized several confounding factors. Its main limitation is the small sample size. We did not succeed in randomizing enough patients to have sufficient power to detect small to moderate differences between the active drug and placebo. Nevertheless, the present study is consistent with a previous report6 that selective NR2B subunit antagonism may produce relatively rapid antidepressant effects in TRD patients. Furthermore, it is the first such study to demonstrate that these antidepressant effects can be obtained with an oral formulation without inducing dissociative side effects. Larger controlled studies are warranted to extend these preliminary findings and to further characterize the ability of oral formulations of NR2B antagonists to exert antidepressant effects.
Acknowledgments
Funding for this work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, and Department of Health & Human Services (IRP-NIMH-NIH-DHHS).
The authors acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, and Department of Health & Human Services (IRP-NIMH-NIH-DHHS). The authors thank the 7SE research unit and research staff for their support, Ioline Henter for her outstanding editorial assistance in the preparation of this manuscript, and Kerry Budd for her assistance in materials transfer to help support the project.
Footnotes
AUTHOR DISCLOSURE INFORMATION
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression. Dr Zarate has assigned his patent rights on ketamine and its metabolites to the US government but will share a percentage of any royalties that may be received by the government. Dr Herring is an employee of Merck Sharp and Dohme Corp. and holds stock and stock options in the company. This work was initiated when Dr Potter was an employee of Merck Sharp and Dohme Corp; he has since retired but continues to hold stock in the company.
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Machado-Vieira R, Salvadore G, Luckenbaugh DA, et al. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69(6):946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 4.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 5.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preskorn SH, Baker B, Kolluri S, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Valentine GW, Mason GF, Gomez R, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191(2):122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Sanacora G, Zarate CA, Krystal JH, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist. 2009;15(5):525–539. doi: 10.1177/1073858409336093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feyissa AM, Chandran A, Stockmeier CA, et al. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 16.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 17.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 20.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62(10):989–993. [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 23.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 24.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 25.Snaith RP, Hamilton M, Morley S, et al. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Addy C, Assaid C, Hreniuk D, et al. Single-dose administration of MK-0657, an NR2B-selective NMDA antagonist, does not result in clinically meaningful improvement in motor function in patients with moderate Parkinson’s disease. J Clin Pharmacol. 2009;49(7):856–864. doi: 10.1177/0091270009336735. [DOI] [PubMed] [Google Scholar]
- 27.Machado-Vieira R, Yuan P, Brutsche N, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70(12):1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Max MB. Small clinical trials. In: Gallin JI, editor. Principles and Practice of Clinical Research. New York, NY: Academic Press; 2002. pp. 207–224. [Google Scholar]
- 29.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faries D, Herrera J, Rayamajhi J, et al. The responsiveness of the Hamilton Depression Rating Scale. J Psychiatr Res. 2000;34:3–10. doi: 10.1016/s0022-3956(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 31.Mallinckrodt CH, Meyers AL, Prakash A, et al. Simple options for improving signal detection in antidepressant clinical trials. Psychopharmacol Bull. 2007;40:101–114. [PubMed] [Google Scholar]
- 32.Bech P, Allerup P, Gram LF, et al. The Hamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatr Scand. 1981;63:290–299. doi: 10.1111/j.1600-0447.1981.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 33.Bech P, Gram LF, Dein E, et al. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51:161–170. doi: 10.1111/j.1600-0447.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 34.Borza I, Bozo E, Barta-Szalai G, et al. Selective NR1/2B N-methyl-D-aspartate receptor antagonists among indole-2-carboxamides and benzimidazole-2-carboxamides. J Med Chem. 2007;50(5):901–914. doi: 10.1021/jm060420k. [DOI] [PubMed] [Google Scholar]
- 35.Suetake-Koga S, Shimazaki T, Takamori K, et al. In vitro and antinociceptive profile of HON0001, an orally active NMDA receptor NR2B subunit antagonist. Pharmacol Biochem Behav. 2006;84(1):134–141. doi: 10.1016/j.pbb.2006.04.018. [DOI] [PubMed] [Google Scholar]