Based on our earlier observation that deletion of chromosomal region 6q22-23 correlated with shorter survival of primary central nervous system lymphoma (PCNSL) patients (Cady et al, 2008) the overall goal of this study was to further refine the biomarker potential of 6q22-23. We now describe the correlation of its deletion with the overall survival (OS) of patients enrolled on three consecutive North Central Cancer Treatment Group (NCCTG) Phase II clinical trials for newly diagnosed PCNSL, 867252 (O’Neill et al, 1999), 937351 (Laack et al, in press), and 967351 (Laack et al, 2006). Formalin-fixed paraffin embedded blocks (FFPE) from patients enrolled in these three trials were retrieved from the Mayo Clinic Tumor Registry and the Biospecimen Repositories of the 30 participating trial sites (Mayo Foundation Institutional Review Board approval 08-001933). Interphase FISH was performed on thin sections of the FFPE tumour samples following our previously described method (Cady et al, 2008). Survival data were analysed for all patients, with OS being calculated from the date of tissue diagnosis to date of death or last contact. Survival curves were estimated using the Kaplan– Meier method and comparisons were made using the log rank test.
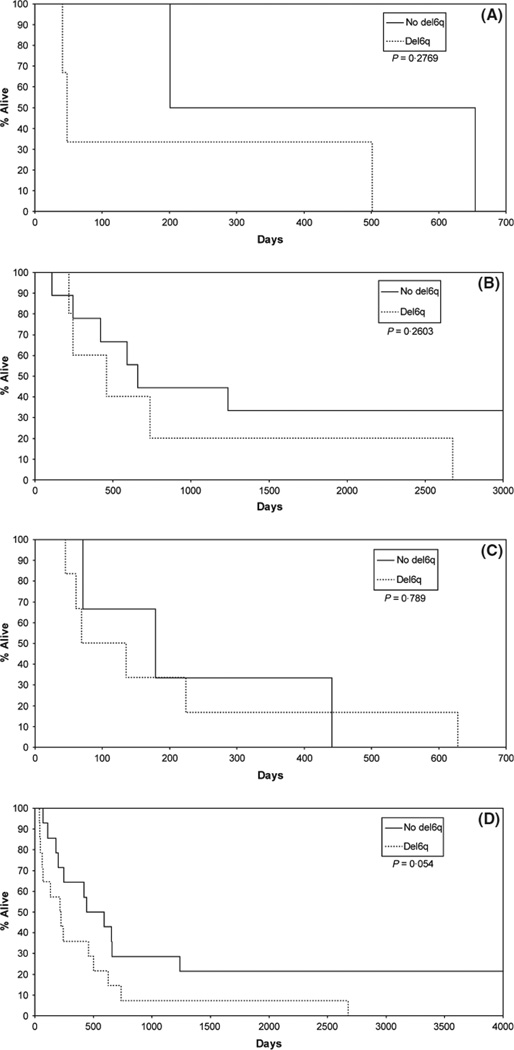
The cohort comprised 37 patients, 22 men and 15 women with a mean age of 63 years (range 34–83 years) and a median age of 65 years. The breakdown by clinical trial and fluorescent in situ hybridization (FISH) results are shown in Table I. Two patients were not evaluable because there was insufficient tissue for the proposed studies, and seven specimens failed FISH. Of the remaining 28, 14 had deletion of 6q by FISH (50%); five of the 14 had homozygous deletion by FISH (18% overall and 36% of the del6q cohort). Overall, patients with the del6q (n = 14; median survival = 221 d) survived less than half the time of the no deletion group [n = 14; median survival = 516 d (Fig 1D)], P = 0·054. Within each trial the del6q group’s survival was inferior to the group where no deletion was detected (Fig 1A–C). The Kaplan–Meier survival curve shown in Fig 1D emphasizes the more than twofold survival difference overall. Subjects with homozygous deletions (n = 5; median survival = 135 d) had worse survival than the other deleted subjects (n = 9; median survival = 244 d). The limited sample size needs to be taken into account when interpreting the results.
Table I.
Patient cohorts by NCCTG trial number.
| Gender | Age at diagnosis (years) |
Date of diagnosis |
Follow-up | 6q FISH results | |
|---|---|---|---|---|---|
| 86 72 52 (n = 10) | |||||
| 1 | Male | 60 | 2/18/1987 | Deceased 4/19/95 | No tumour |
| 2 | Male | 55 | 6/27/1988 | Deceased 4/12/90 | Normal |
| 3 | Male | 68 | 1/30/1990 | Deceased 6/15/91 | del6dq |
| 4 | Female | 69 | 2/7/1991 | Deceased 4/6/92 | Fail |
| 5 | Male | 56 | 4/28/1992 | Deceased 3/21/97 | Fail |
| 6 | Female | 48 | 5/19/1992 | Deceased 6/29/92 | del6q (homozygous) |
| 7 | Male | 56 | 1/13/1994 | Deceased 4/2/95 | Fail |
| 8 | Female | 47 | 7/19/1990 | Deceased 11/25/90 | Fail |
| 9 | Male | 72 | 9/14/1990 | Deceased 4/3/1991 | Normal |
| 10 | Female | 58 | 9/3/1991 | Deceased 8/15/92 | del6q (homozygous) |
| 93 73 51 (n = 16) | |||||
| 1 | Male | 34 | 12/22/1995 | Deceased 12/29/96 | No tumour |
| 2 | Male | 44 | 7/3/1997 | Alive 2/17/10 | Normal |
| 3 | Male | 62 | 6/5/2000 | Alive 3/11/10 | Normal |
| 4 | Male | 47 | 1/19/2000 | Deceased 4/20/01 | del6q (homozygous) |
| 5 | Female | 50 | 2/24/1997 | Deceased 7/15/00 | Normal |
| 6 | Male | 60 | 8/15/1995 | Deceased 3/28/97 | Normal |
| 7 | Male | 66 | 8/28/1996 | Deceased 10/22/97 | Normal |
| 8 | Male | 61 | 4/17/1998 | Deceased 8/3/98 | Normal |
| 9 | Female | 51 | 3/11/1998 | Deceased 12/30/99 | Normal |
| 10 | Male | 60 | 10/5/1998 | Deceased 6/7/99 | Normal |
| 11 | Male | 64 | 5/28/1999 | Deceased 1/27/00 | del6q |
| 12 | Female | 76 | 2/20/2003 | Deceased 9/25/03 | del6q |
| 14 | Male | 69 | 2/20/2003 | Deceased 1/10/99 | del6q |
| 14 | Female | 49 | 6/3/1999 | Alive 9/14/09 | Normal |
| 15 | Female | 69 | 8/11/1999 | Deceased 12/9/06 | del6q |
| 16 | Female | 72 | 12/2/1999 | Deceased 1/11/00 | Fail |
| 96 73 51 (n = 11) | |||||
| 1 | Female | 83 | 6/10/2002 | Deceased 2/7/05 | Fail |
| 2 | Male | 76 | 7/18/2001 | Deceased 9/27/01 | Normal |
| 3 | Male | 70 | 12/31/1999 | Deceased 3/16/01 | Normal |
| 4 | Male | 70 | 7/17/2001 | Deceased 9/16/01 | del6q |
| 5 | Male | 74 | 12/20/2001 | Deceased 2/27/02 | del6q |
| 6 | Female | 79 | 6/5/2002 | Deceased 2/23/04 | del6q |
| 7 | Male | 77 | 2/26/2000 | Deceased 4/11/00 | del6q |
| 8 | Female | 75 | 5/15/2000 | Deceased 11/10/00 | Normal |
| 9 | Male | 83 | 8/8/2000 | Deceased 9/30/00 | Fail |
| 10 | Female | 75 | 1/11/2001 | Deceased 5/26/01 | del6q (homozygous) |
| 11 | Female | 5/18/2001 | Deceased 12/28/01 | del6q (homozygous) | |
Fig. 1.
(A) Survival of patients enrolled on 867252 grouped by 6q22-23 deletion (del6q) and no deletion. (B) Survival of patients enrolled on 937351 grouped by 6q22-23 deletion (del6q) and no deletion. (C) Survival of patients enrolled to 967351 grouped by 6q22-23 deletion (del6q) and no deletion. (D) Survival of entire cohort of patients grouped by 6q22-23 deletion (del6q) and no deletion.
More than 90% of PCNSL cases are of the diffuse large B cell lymphoma variety but the histological appearance cannot predict clinical behaviour and outcome (Ferreri & Reni, 2007). Although the median survival currently hovers around 30 months there are both long term survivors and those that progress through treatment and die within months. Aside from the general prognostic factors of age and performance score there are no established prognostic and predictive markers for PCNSL (Ferreri & Reni, 2007). Patient-specific therapy is difficult to achieve because little is known regarding the molecular pathogenesis of PCNSL. This study confirmed our earlier observation that deletion of the 6q22-23 region correlated with shorter survival of PCNSL patients, suggesting a biological region of interest (Cady et al, 2008). The study reported here comprised newly diagnosed PCNSL patients enrolled in three successive phase II clinical trials conducted by the NCCTG. Each enrolled patient met strict diagnostic criteria, had standardized screening, and had uniform data collection and reporting. However, the exposure of individual patients to their trial regimen varied, because some patients progressed during treatment and some required dose modifications. Also, not all patients had archival tissue for FISH, probably because of the exhaustion of FFPE in the diagnostic workup. In others, the FISH probe failed probably because of DNA deterioration, as some specimens were over 20 years old. This is the reality of clinical trials; these experiences are documented in the publication of each trial (O’Neill et al, 1999; Laack et al, 2006; Laack et al, in press).
The 6q22-23 region of the human chromosome comprises multiple genes including six potentially significant genes, namely the BCL2-associated transcription factor 1 (BCLAF1); HS Transcription Factor 2 (HSF2), tumour protein D52-Like1 (TPD52L1); the ‘absent in melanoma’ 1 (AIM1) gene; cyclin C (CCNC); and, protein tyrosine phosphatase, receptor type kappa (PTPRK). Of these, PTPRK appears to be the most relevant (Nakamura et al, 2003).
PTPRK belongs to the protein tyrosine phosphatase (PTP) superfamily of enzymes (Forrest et al, 2006). These proteins are key regulators of cell signalling pathways such as PTEN (MMAC1), which is mutated in a multitude of human cancers including GBM and PTPN13 (SHP1), which is down regulated in leukaemia and lymphoma by promoter methylation (Jacob & Motiwala, 2005). PTPRK appears to play a key role in inhibiting cell survival and growth by interfering with EGFR-mediated signalling (Xu et al, 2005). Although the functional significance of PTPRK in lymphocytes and lymphoid neoplasms is not known, a similar PTP, PTPR-Ot, has been recently shown to regulate SYK phosphorylation and thus control B-cell receptor signalling and cellular proliferation in B-cell lymphoma cell lines (Chen et al, 2006). It is likely that PTPRK plays a similar role.
A sizable number of PCNSL patients have losses on chromosome 6q and these are likely to be biologically important in tumourigenesis. Specific regions, such as 6q22-23, may harbour tumour suppressor genes that play an accelerant role. As newer techniques become applicable to FFPE interrogation, this should accelerate discovery of other genetic regions of interest and further clarify the importance of the 6q region in PCNSL tumourigenesis.
Acknowledgements
The work reported here was supported in part by ‘Steve’s Run’; the Iowa/Mayo Clinic Lymphoma SPORE (P50CA97274; PI: Weiner G); and the Mayo SPORE in Brain Cancer (P50CA108961; PI: O’Neill BP), including SPORE Supplement P50CA108961-03S1 to Dr O’Neill.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Cady FM, O’Neill BP, Law ME, Decker PA, Kurtz DM, Giannini C, Porter AB, Kurtin PJ, Johnston PB, Dogan A, Remstein ED. Del(6)(q22) and BCL6 rearrangements in primary central nervous system lymphoma (PCNSL) are indicators of an aggressive clinical course. Journal of Clinical Oncology. 2008;26:4814–4819. doi: 10.1200/JCO.2008.16.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Juszczynski P, Takeyama K, Aguiar RCT, Shipp MA. Protein tyrosine phosphatase receptor–type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell–receptor signaling, and cellular proliferation. Blood. 2006;108:3428–3433. doi: 10.1182/blood-2006-03-013821. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Reni M. Primary central nervous system lymphoma. Critical Reviews in Oncology/Hematology. 2007;63:257–268. doi: 10.1016/j.critrevonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Forrest AR, Taylor DF, Crowe ML, Chalk AM, Waddell NJ, Kolle G, Faulkner GJ, Kodzius R, Katayama S, Wells C, Kai C, Kawai J, Carninci P, Hayashizaki Y, Grimmond SM. Genome-wide review of transcriptional complexity in mouse protein kinases and phosphatases. Genome Biology. 2006;7:R5. doi: 10.1186/gb-2006-7-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SA, Motiwala T. Epigenetic regulation of protein tyrosine phosphatases: potential molecular targets for cancer therapy. Cancer Gene Therapy. 2005;12:665–672. doi: 10.1038/sj.cgt.7700828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laack N, Ballman KV, Brown PB, O’Neill BP Cancer North Central Group, Treatment. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: results of North Central Cancer Treatment Group (NCCTG) 96-73-51. International Journal of Radiation Oncology, Biology, and Physics. 65:1429–1439. doi: 10.1016/j.ijrobp.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Laack N, Ballman KV, O’Neill BP. CHOD/BVAM chemotherapy and whole brain radiotherapy for newly diagnosed primary central nervous system lymphoma. [26 Aug 2010];International Journal of Radiation Oncology, Biology, and Physics. doi: 10.1016/j.ijrobp.2010.06.002. (in press) [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, Shimada K, Ishida E, Konishi N. Novel tumor suppressor loci on 6q22-23 in primary central nervous system lymphomas. Cancer Research. 2003;63:737–741. [PubMed] [Google Scholar]
- O’Neill BP, Wang CH, O’Fallon JR, Colgan JD, Earle JD, Krigel RL, Brown LD, McGinnis WL. Primary central nervous system nonhodgkin’s lymphoma (PCNSL): survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86-72-52. International Journal of Radiation Oncology, Biology, and Physics. 1999;43:559–563. doi: 10.1016/s0360-3016(98)00450-7. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tan LJ, Grachtchouk V, Voorhees JJ, Fisher GJ. Receptor-type protein-tyrosine phosphatase-kappa regulates epidermal growth factor receptor function. Journal of Biology Chemistry. 2005;280:42694–42700. doi: 10.1074/jbc.M507722200. [DOI] [PubMed] [Google Scholar]