Abstract
Purpose
Katz et al have published a standardized scoring system of hand diagrams for carpal tunnel syndrome. The purpose of this study was to quantitatively evaluate alternative scoring of the hand diagram for detection of carpal tunnel syndrome.
Methods
In a prospective study of 1107 workers, 221 workers with hand symptoms completed hand diagrams and electrodiagnostic testing for carpal tunnel syndrome. Scoring algorithms for the hand diagrams included the Katz rating; a median nerve digit score (0–2) with a maximum of 2 symptomatic digits of thumb, index, and long; and isolated digit scores (0–1) of thumb, index, or long. Intraclass correlation coefficients quantified inter-rater reliability. Sensitivity, specificity, and logistic regression analyses evaluated scoring systems performances ability to predict abnormal median nerve conduction.
Results
One hundred ten (50%) subjects illustrated symptoms within the median nerve distribution. All scoring systems demonstrated substantial inter-rater reliability. “Classic” or “probable” Katz scores, median nerve digit score of 2, and positive long finger scores were significantly associated with abnormal median nerve distal sensory latency and median-ulnar difference. Abnormal distal motor latency was significantly associated with the median nerve digit score of 2 and positive long finger scores. Increasing Katz scores from “possible” to “probable” and “classic” were not associated with greater odds of electrodiagnostic abnormality. Positive long finger scores performed at least as well as the most rigorous scoring by Katz.
Conclusions
Symptoms diagramed within the median nerve distribution are associated with abnormal nerve conduction among workers. The median nerve digit score and the long finger score offer increased ease of use compared to the Katz method while maintaining similar performance characteristics. The long finger appears best suited for isolated digit scoring to predict abnormal median nerve conduction in a working population.
Keywords: Carpal tunnel syndrome, Hand diagram, screening, scoring
Introduction
Hand symptom diagrams are 1 method of active surveillance for detecting carpal tunnel syndrome among workers. Popularized by Katz and colleagues following publication in 1990, such diagrams have been used in clinical practice, screening of newly hired workers, and longitudinal assessment of working populations.(1–4) The diagrams allow subjects to illustrate the location and character of symptoms.
Clinicians and researchers score diagrams to predict the probability of carpal tunnel syndrome. Scoring of hand diagrams for carpal tunnel syndrome has traditionally relied on the system of Katz et al.(1,2) The Katz scoring algorithm accounts for symptoms within the median nerve distribution, with additional symptoms outside of the median-nerve-innervated digits diminishing the estimated probability of carpal tunnel syndrome. Scoring in this manner is positively associated with a clinical diagnosis of carpal tunnel syndrome in patients presenting with upper extremity paresthesias and is significantly associated (p<.05) with abnormal nerve conduction among active workers.(1,3)
The purpose of this investigation was to quantify the ability of alternative hand diagram scoring algorithms to predict positive provocative physical examination maneuvers and abnormal median nerve conduction at the wrist among active workers. Our hypothesis was that scoring based solely upon symptoms within median nerve innervated digits would perform as well as the Katz method in this working population. These alternative scoring systems were created based on the premise that presence or absence of symptoms outside of median nerve innervated digits should not affect the estimated probability of carpal tunnel syndrome when scoring hand diagrams. Symptoms outside the median nerve distribution occur commonly among individuals with carpal tunnel syndrome(1,5) and decrease the inter-rater reliability of scoring by Katz’s method.(4) Additionally, based upon literature suggesting the long finger is the most sensitive for detecting carpal tunnel syndrome, we hypothesized that the long finger score would maximize association of the diagram rating with abnormal median nerve conduction and enhance ease of use.(6)
Methods
This investigation was performed under institutional review board approval granted for the ongoing Predictors of Carpal Tunnel Syndrome (PrediCTS) Study, a prospective investigation of 1107 newly hired workers initiated in July 2004. From the local area, workers were recruited from 11 companies or organizations representing healthcare, service, managerial, and construction trade industries. Subjects were excluded for a history of carpal tunnel syndrome, peripheral neuropathy, current pregnancy, or inability to undergo nerve conduction testing. All participants provided written informed consent prior to enrollment. Baseline and 36 month assessment included a detailed demographic/work factor questionnaire, a hand symptom diagram,(1) physical examination (Tinel test over median nerve at wrist, Phalen test, and Semmes-Weinstein monofilament sensory test), and nerve conduction tests. Examinations were conducted by medically trained research team members (physicians, therapists, medical students) who received instruction and demonstrated competence in a standardized physical examination testing procedure prior to data collection. The Phalen test was performed with the wrists passively but not forcibly placed into flexion. The reverse Phalen test with wrists positioned into extension was used in the rare circumstance that full wrist flexion could not be achieved. Positive response required report of paresthesia along the nerve distal to the carpal tunnel toward a median innervated digit (thumb, index, or long). The Tinel test for the median nerve involved firm tapping over the nerve from mid-palm to the proximal margin of the carpal tunnel.
Nerve conduction testing was performed with an automated device (NC-Stat, NEUROMetrix, Inc, Waltham, MA). Reliability and criterion validity of this device are previously established as comparable to other methods of nerve conduction testing.(7,8) Abnormal median nerve conduction findings were defined in this study as a distal sensory latency (DSL) of >3.5 ms, distal motor latency (DML) of >4.5ms, or paired transcarpal median-ulnar sensory difference (MUD) of >0.5ms. Transcarpal DSL measurements were recorded in the long finger. The NC-stat sensor recorded temperature 3 times during the testing, and the temperature value was used to normalize the latencies. As part of the study questionnaire, subjects completed hand symptom diagrams (Figure 1). The hand diagram was only completed if the subject reported the presence of hand symptoms defined as a positive response to the question: “In the past year, have you had recurring (repeated) symptoms in your hands, wrists, or fingers more than 3 times or lasting more than 1 week?”. Subjects completed hand diagrams if they have symptoms of burning, pain, tingling, or numbness. The instructions ask subjects to shade in the area of the problem but not to try to represent the type of their symptoms on the diagram. A total of 221 subjects with completed hand diagrams were available for analysis: 141 subjects who reported hand symptoms at baseline and a separate group (n=80) who reported hand symptoms at 36 months.
Figure 1.
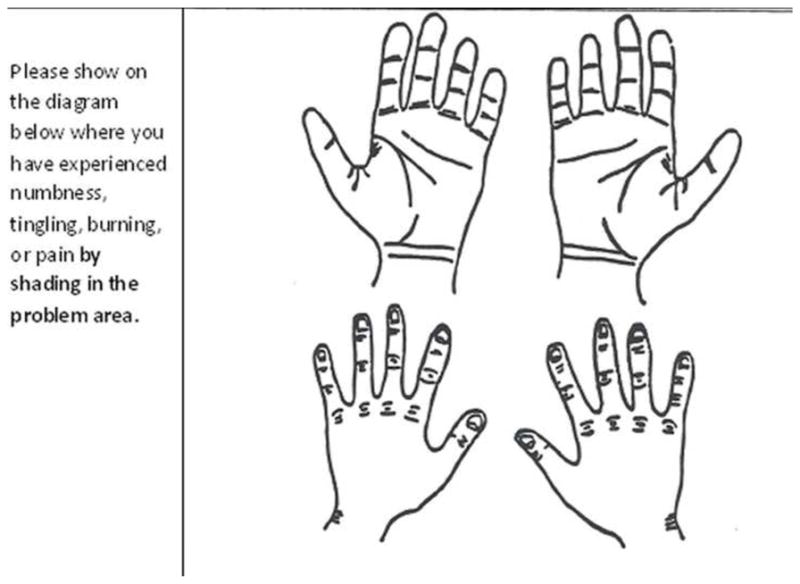
Hand diagram and questions from survey.
Hand diagrams were reviewed by 3 researchers (2 physicians, 1 occupational therapist) independently. Each reviewer scored all diagrams twice, at least 1 month apart. Diagrams were randomized for each review. Scoring was performed according to the recommendations of Katz and Stirrat(1) with modification including additional explicit clarification(3) to maximize inter-rater agreement.(4) Diagrams scored by this modified Katz system were scored as “unlikely (0)”, “possible (1)”, “probable (2)”, or “classic (3)” for carpal tunnel syndrome (Table 1). Alternative scoring systems included the median nerve digit score (MNDS) in which diagrams were scored based upon the number of median innervated digits (thumb, index, and long) with distal volar shading. A final score of 2 indicated positive results for 2 or more digits. In this MNDS the presence of any palm, wrist, or dorsal symptoms was inconsequential. Each digit was also scored separately (thumb rating, index rating, and long rating) as positive or negative based solely on the presence or absence of distal volar shading in the finger. Any shading outside of that location did not impact the score. In all alternative scoring systems, distal volar shading was defined as shading of the volar surface of the distal phalanx and/or greater than half of the volar surface of the middle phalanx. For thumb, this required volar shading over the distal phalanx (Figure 2). Study group characteristics are shown in Table 2.
Table 1.
Detailed Katz scoring algorithm
| Classic (3) | Tingling, numbness, burning or pain in at least 2 of the digits (thumb, index, and long). Excluded if symptoms in the palm and dorsum of hand; small finger symptoms, wrist pain, or radiation proximal to the wrist allowed. *For index and long digits, must include shading >1/2 of the volar surface over middle phalanx and/or some of the distal phalanx. For thumb, must include volar shading over distal phalanx |
| Probable (2) | Same shading as Classic but allowed to extend into palm volarly unless confined to ulnar side of palm |
| Possible (1) | Tingling, numbness, burning, or pain in at least one of thumb, index, and long. May include dorsum of hand |
| Unlikely (0) | No shading of volar thumb, index, and long |
Figure 2.
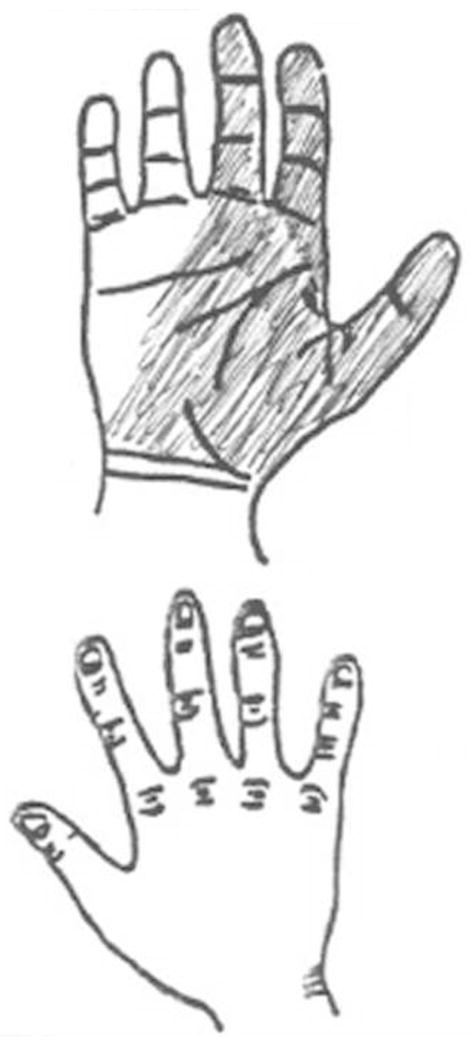
Example of a shaded hand diagram. This diagram was rated as follows: Katz “probable”, median nerve digit score 2, positive long finger rating, positive index finger rating, and positive thumb rating.
Table 2.
Characteristics of study group at time of nerve conduction testing (n=221)
| Mean (SD) | |
|---|---|
| Age | 31.8 (10.6) |
| BMI | 29.7 (6.3) |
|
| |
| n (%) | |
|
| |
| Hand Dominance (right) | 193 (87) |
| Gender (male) | 156 (71) |
| Race | |
| Caucasian | 164 (74) |
| Diabetic | 8 (4) |
| Positive Provocative Testing | |
| Phalen | 47 (21) |
| Tinel | 69 (31) |
| Abnormal Nerve Conduction | |
| DSL (>3.5ms) | 81 (37) |
| DML (>4.5ms) | 51 (23) |
| MUD (>.05ms) | 73 (33) |
Statistical Analysis
Descriptive statistics were produced to summarize subject demographic data as well as the prevalence of positive provocative physical examination maneuvers and abnormal nerve conduction. Only right hand diagrams were chosen for analysis. We excluded left hand diagrams based upon previously documented significant correlation (p<.001) between bilateral electrodiagnostic results that prohibit analysis as independent data points in a single data set. (3) For some analyses, hand diagram scores for the Katz system were dichotomized as positive (“classic” or “probable”) and negative (“possible” or “unlikely”). For the MNDS algorithm, a score of 2 (2 or more digits) was considered positive and scores of 0 or 1 digit were considered negative.
Inter-rater reliability among the 3 raters was assessed by intraclass correlation coefficient (ICC) using a 2-way mixed model for absolute agreement. Kappa values quantified intra-rater reliability, in other words, assessing consistency in scoring for each rater between repeated reviews.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to show each scoring system’s (Katz, MNDS, long finger, index finger, thumb) dichotomous performance compared to abnormal median nerve test results defined as median DSL >3.5ms, median DML >4.5ms, or MUD >0.5ms. Chi square tests were then conducted to compare all 3 scoring symptoms performance for each of these 3 measures. Additional chi-square tests were conducted for each scoring system separately to determine the association between patient age (dichotomized at >30 years), body mass index (dichotomized at >30), race (dichotomized Caucasian versus other), gender, positive Phalen test, and positive Tinel test over the carpal tunnel.
Logistic regression analysis determined the predictive value of each scoring system for abnormal nerve conduction parameters using the pre-determined cut-points. We specifically evaluated the predictive ability of each Katz score to test our hypothesis that the presence of symptoms outside of the median innervated digits does not impact the probability of median nerve dysfunction.
For all analyses, significance values less than 0.05 were accepted as statistically significant. Less than 10 values were missing for each nerve conduction parameter. These missing data were excluded from analyses affected.
Results
One hundred ten (50%) subjects completing hand diagrams illustrated symptoms within the median nerve distribution and 94 of these subjects (43%) had at least 1 abnormal nerve conduction parameter. Table 3 presents the distribution of hand diagram ratings for each of the scoring systems. The prevalence of symptoms meeting the definition of CTS was smallest for the Katz hand diagram (27%), and largest for the long finger definition (43%).
Table 3.
Distribution of hand diagram scores according to scoring method (n=221)
| Hand Diagram Score+ | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Katz | 111 (50) | 51 (23) | 24 (11) | 35 (16) |
| MNDS* | 111 (50) | 30 (14) | 80 (36) | - |
| Long finger** | 126 (57) | 95 (43) | - | - |
| Index finger** | 137 (62) | 84 (38) | - | - |
| Thumb** | 164 (74) | 57 (26) | - | - |
Values listed as number (%)
Median Nerve Digit Score
maximum score of 2
maximum score of 1
Intraclass correlation coefficients demonstrated substantial inter-rater reliability for all scoring systems (range: 0.87–0.98). Inter-rater reliability slightly improved when using the long finger score (ICC: 0.98; 95%CI: 0.97–0.98) or MNDS (ICC: 0.96; 95%CI: 0.95–0.97) versus the Katz scoring (ICC: 0.87; 95%CI: 0.84–0.90). Mean kappa values of intra-rater reliability were 0.86 for Katz scoring, 0.97 for the MNDS system, and 0.97 for long finger scores. Inter-rater and intra-rater reliability were substantial for all scoring systems and all reviewers. Scores from the first review by 1 physician were used for subsequent analyses.
Table 4 presents the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of positive scores for each rating system relative to abnormal nerve conduction results. The potential range of values for Table 4 is from 0 to 1. If a scoring system for a hand diagram had perfect sensitivity (1.0), then every patient with an abnormal nerve conduction parameter would have a positive hand diagram, i.e. no false negatives. Perfect specificity (1.0) would mean that all patients with normal nerve conductions would have had negative hand diagram rating, i.e. no false positives. The PPV refers to the proportion of positively scored hand diagrams that actually had an abnormal nerve conduction parameter. The NPV refers to the proportion of subjects with negatively scored hand diagrams who actually had normal nerve conduction parameters. Notably, positive and negative predictive values depend on the prevalence of the outcome (in this case abnormal nerve conduction values) in a given study population. Chi square tests revealed greater sensitivity for all abnormal nerve conduction parameters using the long finger score (p<0.01) compared to all other hand diagram scoring systems. Smaller and statistically non-significant differences (p>0.05) were demonstrated between the 3 scoring systems and specificity, PPV, and NPV of abnormal nerve conduction results. All scoring systems were significantly associated with a positive Phalen test (p<0.05), but not with age, BMI, gender, race, or positive Tinel test.
Table 4.
Test characteristics of hand diagram rating systems relative to abnormal nerve conduction values
| DSL (n=216) | DML (n=219) | MUD (n=213) | |
|---|---|---|---|
| Sensitivity (95% CI) | |||
|
| |||
| Katz* | .38 (.28–.50) | .33 (.20–.46) | .40 (.28–.52) |
| MNDS** | .54 (.43–.65) | .55 (.40–.69) | .58 (.45–.69) |
| Long Finger | .67 (.55–.77) | .67 (.52–.79) | .67 (.55–.78) |
| Index Finger | .54 (.43–.65) | .55 (.40–.69) | .59 (.47–.70) |
| Thumb | .31 (.21–.42) | .35 (.22–.50) | .33 (.22–.45) |
|
| |||
| Specificity (95% CI) | |||
|
| |||
| Katz* | .81 (.73–.87) | .76 (.69–.82) | .80 (.72–.87) |
| MNDS** | .76 (.68–.83) | .70 (.62–.76) | .75 (.67–.82) |
| Long Finger | .73 (.64–.80) | .65 (.57–.72) | .70 (.62–.77) |
| Index Finger | .73 (.64–.80) | .67 (.60–.74) | .73 (.65–.80) |
| Thumb | .79 (.71–.85) | .77 (.70–.83) | .79 (.71–.85) |
|
| |||
| Positive Predictive Value (95% CI) | |||
|
| |||
| Katz* | .54 (.41–.67) | .29 (.18–.41) | .51 (.37–.64) |
| MNDS** | .57 (.45–.68) | .35 (.25–.47) | .55 (.43–.66) |
| Long Finger | .59 (.49–.70) | .37 (.27–.47) | .54 (.43–.64) |
| Index Finger | .54 (.43–.65) | .34 (.24–.45) | .53 (.42–.64) |
| Thumb | .46 (.33–.60) | .32 (.20–.45) | .44 (.31–.59) |
|
| |||
| Negative Predictive Value (95% CI) | |||
|
| |||
| Katz* | .69 (.61–.76) | .79 (.72–.85) | .70 (.62–.77) |
| MNDS** | .74 (.65–.81) | .84 (.76–.89) | .77 (.69–.84) |
| Long Finger | .78 (.70–.85) | .87 (.79–.92) | .80 (.72–.87) |
| Index Finger | .73 (.64–.80) | .83 (.76–.89) | .77 (.69–.84) |
| Thumb | .65 (.58–.73) | .80 (.73–.86) | .69 (.61–.76) |
Classic or Probable rating
MNDS rating of 2
The odds ratio (OR) of abnormal nerve conduction values based on hand diagram scores are presented in Table 5. All scoring system parameters, except thumb, were found to be significant predictors of abnormal nerve conduction with the long finger providing the highest OR of 5.3 (95% CI: 2.9–9.7). An OR of 2 means that individuals with the hand diagram score listed are twice as likely to have abnormal median nerve conduction parameters as a subject with a negative hand diagram. A positive hand diagram score would have an odds ratio of 1 if it was no more likely to predict abnormal nerve conduction than a negative hand diagram score.
Table 5.
Odds ratio of abnormal nerve conduction values according to hand diagram score. Reference is negative diagram for each scoring system
| DSL OR (95% CI) |
DML OR (95% CI) |
MUD OR (95% CI) |
|
|---|---|---|---|
| Katz | |||
| 1 | 4.3 (2.1–9.0) | 5.5 (2.5–12.5) | 5.1 (2.4–11.0) |
| 2 | 4.4 (1.7–11.5) | 3.3 (1.1–9.4) | 4.7 (1.8–12.6) |
| 3 | 4.3 (1.9–9.8) | 3.0 (1.2–7.7) | 5.0 (2.2–11.5) |
| MNDS | |||
| 1 | 3.1 (1.3–7.5) | 4.0 (1.5–10.4) | 3.5 (1.4–8.7) |
| 2 | 4.8 (2.6–9.3) | 4.1 (2.0–8.9) | 5.6 (2.9–11.2) |
| Long Finger | 5.3 (2.9–9.7) | 3.7 (1.9–7.3) | 4.8 (2.6–8.9) |
| Index Finger | 3.1 (1.8–5.7) | 2.5 (1.3–4.7) | 3.8 (2.1–7.1) |
| Thumb | 1.6 (0.9–3.1) | 1.8 (0.9–3.5) | 1.8 (0.9–3.4) |
Increasing Katz scores from “possible” to “probable” or “classic” were not associated with greater odds of abnormal nerve conduction.
Discussion
Our findings indicate that the scoring of hand diagrams solely based on symptoms of 1 or 2 digits within the median nerve distribution in a population of working subjects can yield results that are similar to results obtained using the approach of Katz et al.(1) Such scoring maximizes inter- and intra-rater reliability, minimizes time required for scoring, and predicts abnormal nerve conduction parameters when screening for carpal tunnel syndrome similar to Katz’s scoring method.
The original scoring system of the self-administered hand diagram for the diagnosis of carpal tunnel syndrome by Katz and Stirrat(1) considers symptoms within and outside of the median nerve distribution. The presence of symptoms outside of the thumb, index, and long digits downgrades the estimated probability of carpal tunnel syndrome. In Katz’s series of patients evaluated for upper extremity paresthesia (88% prevalence of clinically diagnosed carpal tunnel syndrome), 42 of 75 patients with carpal tunnel syndrome were rated as “probable” or “possible” compared to 32 patients with “classic” diagrams (1 patient had “unlikely” diagram). This suggests that the predominance of carpal tunnel syndrome patients presented with extra-median nerve symptoms and is consistent with the 55% prevalence reported by Stevens et al.(9) The series by Katz and Stirrat as well as another by Elfar et al(5) document that patients with clinically diagnosed carpal tunnel syndrome almost always diagram symptoms in the median-nerve-innervated digits along with a preponderance of extra-median symptoms. In a similar fashion, our study found that symptoms outside of the median nerve distribution were common among workers with abnormal median nerve conduction values. The odds of having abnormal median nerve conduction remained nearly identical for hand diagrams scored as “possible”, “probable”, and “classic” according to Katz criteria. Thus, in our population of active workers, scoring based on the presence of extra-median symptoms (used to differentiate these ratings) failed to improve the diagram’s performance in predicting nerve conduction abnormalities in this population.
The sensitivity and specificity of hand diagram scores in predicting median nerve conduction abnormalities in a cohort of workers was lower than that reported by Katz (sensitivity: 80%, specificity: 90%).(1) This discrepancy is likely attributable to the populations from which study cohorts were drawn. Existing literature demonstrates greater predictive ability of hand diagrams for the clinical diagnosis of carpal tunnel syndrome and nerve conduction abnormalities in clinic-based studies.(1,10,11) It is likely that those seeking medical care are different from active workers who are symptomatic yet may not be seeking treatment. Those presenting to medical professionals with upper extremity paresthesias might be more likely to have a higher prevalence of electrodiagnostic abnormalities and more advanced degrees of nerve compression. Differences in the prevalence of electrodiagnostic abnormalities do not directly affect estimates of sensitivity and specificity but do alter the positive predictive values which would require normalization of disease prevalence for comparison between studies. Alternatively, the act of seeking care may bias physicians scoring the hand diagrams.(4) Potentially the result of several factors, hand diagrams have demonstrated poorer predictive performance in population studies.(12–14)
There is no consensus digit identified as the most sensitive for detecting carpal tunnel syndrome. Although the index finger is commonly used to record median sensory data, different investigators have supported isolated testing of the thumb,(15) long,(6,16,17) and ring fingers(18,19) as the most sensitive digital location. Comparing the predictive ability of single-digit scoring on the hand diagram in our study showed that the long finger out performed the index and thumb in its association with abnormal nerve conduction measurements. The specificity of these digits was similar but the long finger demonstrated superior sensitivity. This is in accord with the findings of Elfar et al(6) who found that the long finger was subjectively the “worst” digit among patients with clinical and electrodiagnostically diagnosed carpal tunnel syndrome. Although our population cannot be assumed to have carpal tunnel syndrome, it appears that symptomatic workers with abnormal median nerve conduction parameters were more likely to diagram symptoms in the long finger than in the index or thumb. Thus, the long finger with greater sensitivity appears particularly well suited to serve as a single digit for evaluation during a first stage screening for carpal tunnel syndrome in population studies. This association may have been enhanced by measuring DSL in the long finger for this investigation, although the long finger performed well when evaluating against DML as well.
The high inter- and intra-rater reliabilities documented in this study are consistent with several prior investigations.(1,3,4) This suggests that the scoring of hand diagrams in subsequent investigations can be reasonably performed once by a single experienced investigator. Multiple blinded ratings and duplicative ratings by additional investigators are expected to minimally impact hand diagram scores. Dale et al suggested that rater disagreement was likely related to consideration of extra-median nerve symptoms.(4) Our data demonstrated improved reliability when only scoring symptoms within the thumb, index, and long fingers. However, reliability measures are also expected to improve when classification or grading algorithms are simpler with fewer numbers of potential categories.
There are several limitations inherent to this study. Only active workers without medically diagnosed nerve compression were enrolled in this study. Therefore, hand diagrams could not be evaluated against a true standard of subjects clinically diagnosed with, and subsequently successfully treated for, carpal tunnel syndrome. We instead have inferred accuracy in screening for carpal tunnel syndrome based upon comparisons to nerve conduction testing. Despite not using formal office based nerve conduction testing, the NC-stat device employed in this study has demonstrated criterion validity and yields comparable data in the research setting.(8,20) To this point, the prevalence of abnormal nerve conduction testing in asymptomatic individuals within this study compares favorably to that using formal nerve conduction in a population based study.(21) Atroshi et al identified abnormal median-ulnar differential (>0.8) in 18% of otherwise normal asymptomatic individuals while 16% of asymptomatic workers in our study had a MUD >0.5. Therefore, it is accepted that some patients will have nerve conduction results that fail to coincide with their clinical presentation. We do not presume that our results can be generalized to either patients with carpal tunnel syndrome or to those seeking medical care for upper extremity nerve complaints. When determining inter-rater reliability among individuals who routinely score such diagrams, we expect that our results represent a best case scenario and that there may be more discordant scores produced when raters are less experienced.
Hand diagram scores suggestive of carpal tunnel syndrome are associated with abnormal median nerve conduction velocities among active workers. Scoring of hand diagrams in the general population without consideration of symptoms outside of the median nerve distribution maintains the performance characteristics of the Katz scoring system while increasing the ease of use. We believe that the use of simpler scoring algorithms will aid in epidemiologic studies where notable time and effort are required to collect and score hand diagrams.
Acknowledgments
Grant Support:
2 R01 OH008017-06 Evanoff (PI)National Institute for Occupational Safety and Health Post-Offer Screening and Risk Factors for CTS
Calfee Support: This publication was made possible by Grant Number UL1 RR024992 from the NIH-National Center for Research Resources (NCRR).
Footnotes
Work completed at Washington University in St. Louis
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg. 1990;15A:360–363. doi: 10.1016/0363-5023(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495–1498. [PubMed] [Google Scholar]
- 3.Franzblau A, Werner R, Albers J, Grant C, Olinski D, Johnston E. Workplace surveillance for carpal tunnel syndrome using hand diagrams. Journal of Occupational Rehabilitation. 1994;4:185–198. doi: 10.1007/BF02331615. [DOI] [PubMed] [Google Scholar]
- 4.Dale AM, Strickland J, Symanzik J, Franzblau A, Evanoff B. Reliability of hand diagrams for the epidemiologic case definition of carpal tunnel syndrome. J Occup Rehabil. 2008;18:233–248. doi: 10.1007/s10926-008-9139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elfar JC, Calfee RP, Stern PJ. Topographical assessment of symptom resolution following open carpal tunnel release. J Hand Surg. 2009;34A:1188–1192. doi: 10.1016/j.jhsa.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elfar JC, Yaseen Z, Stern PJ, Kiefhaber TR. Individual finger sensibility in carpal tunnel syndrome. J Hand Surg. 2010;35A:1807–1812. doi: 10.1016/j.jhsa.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong X, Gozani SN, Hayes MT, Weinberg DH. NC-stat sensory nerve conduction studies in the median and ulnar nerves of symptomatic patients. Clin Neurophysiol. 2006;117:405–413. doi: 10.1016/j.clinph.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong TN, Dale AM, Al-Lozi MT, Franzblau A, Evanoff BA. Median and ulnar nerve conduction studies at the wrist: criterion validity of the NC-stat automated device. J Occup Environ Med. 2008;50:758–764. doi: 10.1097/JOM.0b013e3181645425. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JC, Smith BE, Weaver AL, Bosch EP, Deen HG, Jr, Wilkens JA. Symptoms of 100 patients with electromyographically verified carpal tunnel syndrome. Muscle Nerve. 1999;22:1448–1456. doi: 10.1002/(sici)1097-4598(199910)22:10<1448::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Szabo RM, Slater RR, Jr, Farver TB, Stanton DB, Sharman WK. The value of diagnostic testing in carpal tunnel syndrome. J Hand Surg. 1999;24A:704–714. doi: 10.1053/jhsu.1999.0704. [DOI] [PubMed] [Google Scholar]
- 11.Katz JN, Lew RA, Bessette L, Punnett L, Fossel AH, Mooney N, et al. Prevalence and predictors of long-term work disability due to carpal tunnel syndrome. Am J Ind Med. 1998;33:543–550. doi: 10.1002/(sici)1097-0274(199806)33:6<543::aid-ajim4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Homan MM, Franzblau A, Werner RA, Albers JW, Armstrong TJ, Bromberg MB. Agreement between symptom surveys, physical examination procedures and electrodiagnostic findings for the carpal tunnel syndrome. Scand J Work Environ Health. 1999;25:115–124. doi: 10.5271/sjweh.413. [DOI] [PubMed] [Google Scholar]
- 13.Ferry S, Silman AJ, Pritchard T, Keenan J, Croft P. The association between different patterns of hand symptoms and objective evidence of median nerve compression: a community-based survey. Arthritis Rheum. 1998;41:720–724. doi: 10.1002/1529-0131(199804)41:4<720::AID-ART20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Walker-Bone KE, Palmer KT, Reading I, Cooper C. Criteria for assessing pain and nonarticular soft-tissue rheumatic disorders of the neck and upper limb. Semin Arthritis Rheum. 2003;33:168–184. doi: 10.1016/s0049-0172(03)00129-x. [DOI] [PubMed] [Google Scholar]
- 15.Kothari MJ, Rutkove SB, Caress JB, Hinchey J, Logigian EL, Preston DC. Comparison of digital sensory studies in patients with carpal tunnel syndrome. Muscle Nerve. 1995;18:1272–1276. doi: 10.1002/mus.880181109. [DOI] [PubMed] [Google Scholar]
- 16.Padua L, Lo Monaco M, Valente EM, Tonali PA. A useful electrophysiologic parameter for diagnosis of carpal tunnel syndrome. Muscle Nerve. 1996;19:48–53. doi: 10.1002/(SICI)1097-4598(199601)19:1<48::AID-MUS6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Macdonell RA, Schwartz MS, Swash M. Carpal tunnel syndrome: which finger should be tested? An analysis of sensory conduction in digital branches of the median nerve. Muscle Nerve. 1990;13:601–606. doi: 10.1002/mus.880130707. [DOI] [PubMed] [Google Scholar]
- 18.Terzis S, Paschalis C, Metallinos IC, Papapetropoulos T. Early diagnosis of carpal tunnel syndrome: comparison of sensory conduction studies of four fingers. Muscle Nerve. 1998;21:1543–1545. doi: 10.1002/(sici)1097-4598(199811)21:11<1543::aid-mus28>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Valls J, Llanas JM. Orthodromic study of the sensory fibers innervating the fourth finger. Muscle Nerve. 1988;11:546–552. doi: 10.1002/mus.880110605. [DOI] [PubMed] [Google Scholar]
- 20.Leffler CT, Gozani SN, Cros D. Median neuropathy at the wrist: diagnostic utility of clinical findings and an automated electrodiagnostic device. J Occup Environ Med. 2000;42:398–409. doi: 10.1097/00043764-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]


