Abstract
Evolution-driven functional changes in the primate brain are typically assessed by aligning monkey and human activation maps using cortical surface expansion models. These models use putative homologous areas as registration landmarks, assuming they are functionally correspondent. In cases where functional changes have occurred in an area, this assumption prohibits to reveal whether other areas may have assumed lost functions. Here we describe a method to examine functional correspondences across species. Without making spatial assumptions, we assess similarities in sensory-driven functional magnetic resonance imaging responses between monkey (Macaca mulatta) and human brain areas by means of temporal correlation. Using natural vision data, we reveal regions for which functional processing has shifted to topologically divergent locations during evolution. We conclude that substantial evolution-driven functional reorganizations have occurred, not always consistent with cortical expansion processes. This novel framework for evaluating changes in functional architecture is crucial to building more accurate evolutionary models.
Introduction
A basic challenge in comparative neuroscience is to develop comprehensive models explaining evolution-driven changes in brain function between primate species. Functional magnetic resonance imaging (fMRI) is currently the technique of choice for comparative sensory and cognitive experiment in monkeys and humans1–3. Interpretation of comparative fMRI often relies on spatial assumptions related to cortical expansion during evolution4. For example, cortical surface expansion models use putative homologous areas as corresponding landmarks in monkeys and humans to align the fMRI activation maps, and to identify inter-species functional similarities across the cortex5. However, the premise that homologous areas are both anatomically and functionally equivalent is not always valid. A few comparative fMRI studies have indeed shown that particular functions in an area of one species are lacking in the presumed homologous area of the other species3,6: they may be either lost or shifted to areas that do not anatomically or topographically correspond7. Recent evolutionary theories have even suggested that functional reorganization in the brain may be independent of cortical expansion8,9. In cases where evolutionary changes in function are reported for an area, the constraints inherent to cortical surface expansion models will impede to reveal whether (and which) other areas carry out the displaced functions. Thus, to make further advances, one needs complementary approaches that assess functional correspondences (analogies) without imposing topological constraints. To address this problem, we have developed a method to identify analogies across species by measuring the temporal correlation between sensory-driven fMRI responses.
In the present study we have applied the inter-species activity correlation (ISAC) method to natural vision data collected in monkeys and humans. After validating seed-based ISAC in selected visual areas for which homology and analogy are well accepted, we examined other areas that go beyond the boundaries of current knowledge. Finally, we tested for inter-species activity correlation across all the areas that were activated by the test movie in our human and monkey subjects. The ISAC method will prove crucial for defining cortical regions that are functionally but not anatomically correspondent, and for improving existing evolutionary models.
Online Methods
Subjects
Four rhesus monkeys (Macaca mulatta, three males and one female, 4–6 kg, 4–7 years old) and twenty-four right-handed young, healthy, Italian-speaking volunteers (9 males and 15 females, 20–31 years old) participated in the study. Animal care procedures met the Belgian and European guidelines, and were approved by the K.U. Leuven Medical School. Human volunteers were informed about the experimental procedures and signed a written informed consent. The study design was approved by the local Ethics Committees of both the K.U.Leuven and the Chieti University, for experiments in monkeys and humans respectively.
For the health and welfare of the animals, we followed the Belgian and EU regulations (EU directive on the protection of animals used for scientific purposes 2010/63/EU). The macaques were pair- or group-housed in the primate facility of the K.U.Leuven Medical School. The cages provide adequate space for housing multiple macaques (2 to 5) and each animal room has a large playing pen equipped with toys and enrichment tools. Access of animals to water is restricted between experiments. The monkeys are trained using operant conditioning techniques and they can drink until satiated during the experiments.
Data collection
Behavioral task
We carried out a natural-vision experiment, in which the subjects watched and listened to 30 minutes of the Italian version of the movie “the Good the Bad and the Ugly”10, from minute 16:48 to minute 46:48. The movie was divided into 3 clips of 10 minutes each. The movie clips were presented 6 times to the monkeys, and one time to the human subjects.
Experimental setup
Human volunteers lay in a supine position and watched the clips through a mirror tilted 45 degrees towards a translucent screen onto which the movie was projected at a frame rate of 60Hz. The subjects were allowed to watch the movie clips freely while keeping their gaze within the projection area (24×10.2 visual degrees, 640×272 pixels). A similar freeviewing condition was achieved in monkeys by rewarding them with juice when their gaze was kept within the 24 × 10.2 degree virtual window covering the projected movie3. Monkeys were prepared for scanning as in our previous studies3,26. Specifically, a bolus of microcrystalline-iron-oxide-nanoparticles (MION; Sinerem®, Guerbet; 6–10 mg/kg) was injected into the femoral vein of the animal prior to fMRI scanning. For both monkeys and humans, eye position was monitored using a pupil-corneal reflection system at 120 Hz (Iscan). Furthermore, MR-compatible headphones with ear-cup pad were used to deliver the acoustic stimuli associated with the movie, and to shield the ears from environmental noise.
fMRI data acquisition
Monkey fMRI was performed with a 3T MR Siemens Trio scanner in Leuven, Belgium. The functional images were collected using a gradient-echo T2-weighted echo-planar sequence (40 slices, 84 × 84 in-plane matrix, TR/TE = 2000/19 ms, flip angle = 75°, voxel size = 1.25×1.25×1.25 mm3). In addition, high-resolution, T1-weighted anatomical images (MP-RAGE sequence, TR/TE = 2200/4.06, voxel size = 0.5×0.5×0.5 mm3) were collected in separate sessions under ketamine-xylazine anesthesia to provide the anatomical reference for the functional scans.
fMRI in humans was performed with a 3T MR Philips Achieva scanner in Chieti, Italy. The functional images were obtained using T2-weighted echo-planar images (EPI) with BOLD contrast using SENSE imaging. EPIs comprised 32 axial slices acquired continuously in ascending order and covering the entire brain (32 slices, 230 × 230 in-plane matrix, TR/TE = 2000/35 ms, flip angle = 90°, voxel size = 2.875×2.875×3.5 mm3). Furthermore, a three-dimensional high-resolution T1-weighted image was collected by means of an MP-RAGE sequence (TR/TE = 8.1/3.7 ms, voxel size = 0.938×0.938×1 mm3).
Data analysis
Eye gaze analysis
We analyzed eye-movement trajectories during fMRI scanning. Eye traces were converted to visual degrees by a four-point spatial projection calibration. Next, the variability in eye-positions along the x- and y-axes was quantified by standard deviation. To statistically assess differences between humans and monkeys with regard to eye-position variability, an unpaired t-test was calculated. Furthermore, we measured eye movements by computing speeds in the x- and y-directions in the eye traces and calculating the square root of the sum of their squares. We then measured temporal correlations between eye movements across subjects13. This was done for each monkey, within monkey and human groups, and finally between the two groups. An unpaired t-test between monkey and human inter-subject correlations was calculated as well.
fMRI preprocessing
fMRI data preprocessing was performed with the SPM5.0 software package27 (Wellcome Trust Centre for Neuroimaging, London, UK). We preprocessed functional time-series to compensate for slice-dependent time shifts, head motion and linear trends. We spatially warped the monkey and human data to F99 and MNI atlas spaces, respectively. The final spatial resolution was 1 and 3 mm isotropic, respectively, for the two species. To reduce the contributions of artifactual sources, we removed signals from a ventricular region of interest and a region centered in the white matter11 using a regression technique. Next, we spatially smoothed the data with a Gaussian kernel at 1.5 and 4.5 mm FWHM, for monkeys and humans respectively.
We further applied temporal preprocessing to the fMRI data to minimize signal differences arising from the different hemodynamic response functions (HRFs). The deconvolution of the fMRI time-series28 is typically used to correct for different HRFs, particularly when the timing of the experimental events is available. Since we intended that the ISAC method should not rely on this information, we used an alternative approach. We convolved the monkey and human fMRI time-courses with a canonical human and monkey HRF26,29,30, respectively (Supplementary Fig. 1). In this manner, we could make allowance for different hemodynamic peak delays and spectral contents (see Supplementary Fig. 2 and Supplementary Note). To avoid any border effects due to signal convolution, we removed the first 20 and the last ten functional volumes from each run. Finally, we converted the time-courses related to the three consecutive movie blocks to z-scores, and then concatenated them. As a result, each dataset representing a single movie repetition was composed of 810 functional volumes. For each selection of datasets, an average dataset was constructed by averaging the time-courses in corresponding voxels. This procedure allowed us to maximize the relative contribution of stimulus-evoked responses exceeding spontaneous activity in our analysis.
Conversion from volumes to surfaces
The conversion from volumes to surfaces was performed with Caret 5.61 software5. The surface maps were visualized on a flattened cortex, together with the borders of anatomically- and/or functionally-defined areas. Anatomical areas in the monkey were defined based upon the cortical parcellation of Felleman and Van Essen16, included in Caret, whereas functional areas were designated based on results from our previous studies3,4,6,20. Human anatomical areas were defined on the basis of the cytoarchitectonical maps available in the SPM Anatomy Toolbox, whereas human functional areas were defined from our localizers10,17 and the visuotopic maps included in Caret5.
Analysis of fMRI response reliability within a species
To estimate the relative contribution of stimulus-driven activity to the fMRI data, we calculated voxel-by-voxel temporal correlations across subjects, or inter-subject correlation within a species10. This analysis was performed independently for each individual monkey, and for monkeys and humans at the group level. Following Bartlett’s theory to account for autocorrelation in an fMRI signal11, the degrees of freedom were defined as the total number of timepoints used to calculate the correlation (810 in our datasets) divided by a correction factor c, defined as the time integral of the square of the lagged autocorrelation function. The latter was computed by Fast Fourier Transform (FFT)11 for an estimation across all monkey and human brain voxels. The distribution of correction factors across gray matter voxels was computed for the average monkey (deformed to the human space to equalize the number of voxels) and human datasets (Supplementary Fig. 3).
We estimated an autocorrelation-based correction factor of 6.76, i.e. the mean value of the joint monkey/human distribution. Based on the degrees of freedom defined as 810 / 6.76 −2 = 117.82, we converted the correlations to probability values, and applied the false discovery rate (FDR) method to account for multiple comparisons. Accordingly, we thresholded the monkey and human inter-subject correlation maps at q < 0.05. Finally, we defined a monkey and a human common signal as the average of the fMRI signals showing inter-subject correlation.
Inter-species activity correlation (ISAC)
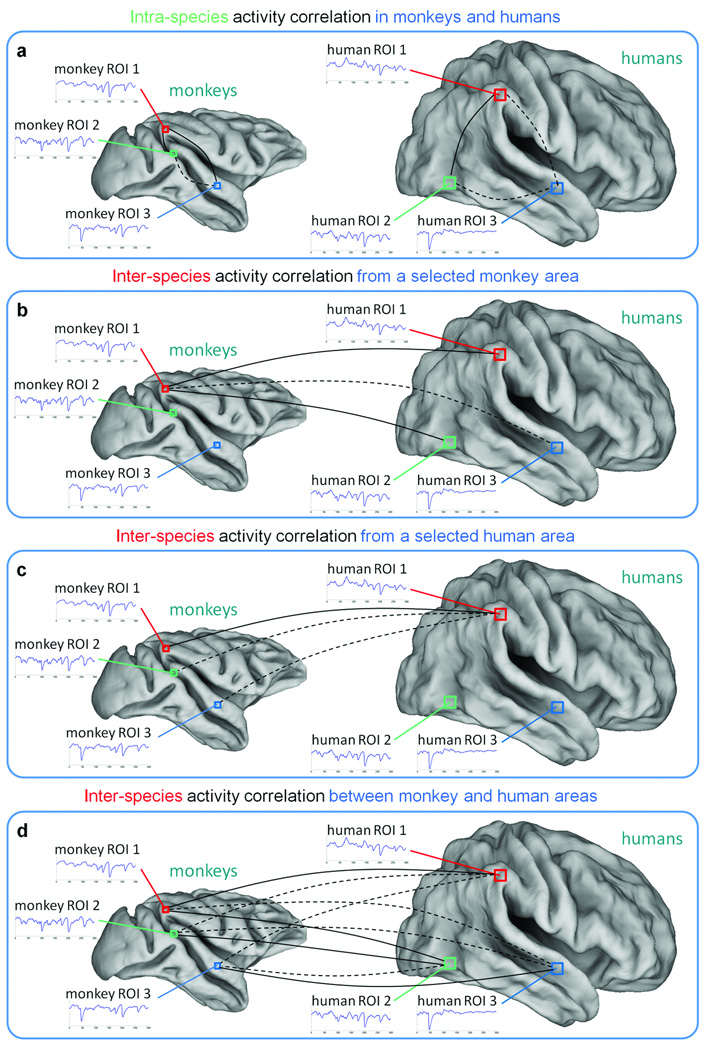
We used regression analysis11 to attenuate any common signals in the fMRI data (as defined in the intra-species reliability analysis), thus removing any effect these might have on similarities detected between pairs of time-courses. To detect similar functional processing based on similar fMRI responses, we used temporal correlation to compare time-courses extracted from the respective areas of the two species. We calculated intra-species and inter-species activity correlation maps, by correlating the seed time-course with all the voxel timecourses in the brains of the same (Fig. 1a) and the other species (Fig. 1b–c). To identify brain areas with responses similar to that in the seed, we thresholded the maps at q < 0.001. In addition, we performed pair-wise comparisons between selected monkey and human areas, so that we formed an ISAC matrix (Fig. 1d). Again, we thresholded the ISAC matrix at q < 0.001 to detect significant inter-species similarities.
Fig. 1. Detection of activity correlations between monkeys and humans.
Similarities in the fMRI time-courses across species are assessed by temporal correlation, which may be significant (continuous line) or not significant (dashed line). (a) Intra-species activity correlation is measured by comparing the time-course of a selected area with the voxel time-courses in the same brain. (b–c) Inter-species activity correlation is measured by comparing the time-courses of a monkey and human area respectively, with the voxel time-courses in the other species. (d) Inter-species activity correlations can be also computed between time-courses in multiple monkey and human brain areas. ROI: region of interest.
Analysis of ISAC reliability
To assess the reproducibility of the ISAC results, we first focused on early visual areas in monkeys and humans. Since the monkeys viewed the movie multiple times, we calculated the ISAC values between the whole human dataset and subsets of the monkey data corresponding to single movie repetitions. To test for the presence of differences in these correlations across movie repetitions, we performed a one-way Kruskal-Wallis analysis of variance on them.
In addition, we conducted a reliability analysis on the ISAC mapping. We selected monkey area MT and human area MT+ as seeds, and then mapped the intra- and inter-species correlations on the whole monkey and human datasets using the seed time-courses derived from monkeys 01–02 and 03–04, and humans 01–12 and 13–24, respectively. We assessed the correspondence of ISAC maps from either monkey or human seeds by spatial correlation.
Finally, we tested the reliability of the ISAC matrix by comparing the results obtained from halves of the monkey (monkeys 01–02 and 03–04) or the human datasets (humans 01–12 and 13–24). To assess the correspondence between the resulting ISAC matrices, we again used spatial correlations.
Results
Description of the ISAC method
The ISAC method neither relies on information about stimulation protocols nor on prior knowledge about corresponding non-human and human brain areas. It requires specific pre-processing techniques and statistical analyses to detect similar activity profiles between different species, under the accepted assumption that fMRI activity in a given brain area reflects a specific type of functional processing. As it compares evoked responses to the same task or sensory stimulation, the data need to be collected in the different species under the same experimental protocol, particularly with regard to the order and timing of the events.
Following general preprocessing steps and conversion of the functional volumes into standard coordinate systems, the fMRI data are convolved with a canonical hemodynamic response function (HRF) from the other species to account for differences in HRF between species (see Online Methods and Supplementary Figs. 1 and 2; see also Supplementary Note). Next, non-neuronal signal components measured in the white matter and cerebro-spinal fluid are removed from the data by linear regression. Non-selective signal components shared across multiple brain areas10 are similarly removed in order to increase the sensitivity during subsequent analysis steps. In addition, multiple datasets of subjects from the same species are averaged to preserve the stimulus-evoked signals within a species while reducing spontaneous, stimulus-independent activity (see Online Methods).
To assess the functional similarities of brain areas in the two species, we calculate the temporal correlations between their stimulus-related responses. We first correlate the average time-course of a specific seed ROI with the time-courses from all conspecific voxels to examine functional relationships with other regions within the same species11 (Fig. 1a). Subsequently, we correlate the time-course of the same seed ROI with those from all non-conspecific voxels (Fig. 1b–c). Finally, we assess large-scale functional similarities of multiple cortical regions showing stimulus-evoked responses. In particular, we calculate correlations between average time-courses of all activated areas in the two species to create an ISAC matrix (Fig. 1d). In all analyses, statistics are corrected for autocorrelation in the fMRI time-series and for multiple comparisons (see Online Methods and Supplementary Fig. 3).
ISAC on natural vision data
We validated the ISAC method using natural-vision fMRI data collected in monkeys (n = 4) and humans (n = 24). We sought to avoid any task-paradigm modeling and to examine inter-species functional similarities across multiple brain regions in a manner that would have been challenging with experimentally-controlled stimulation paradigms. Indeed, natural vision conditions evoke activity in large portions of the cortex and minimize correlations between responses to different stimuli10,12. Monkey and human fMRI acquisitions were conducted with cerebral blood volume (CBV)–weighted and blood-oxygen level dependent (BOLD) techniques, respectively. All participants freely watched and listened (through headphones) to 30 minutes of the film “The Good, the Bad and the Ugly” by Sergio Leone. The movie clips were presented six times to the monkeys, and one time to the humans. Eye-movement behavior was monitored during scanning. The related data showed significantly greater variability in the eye traces of humans (t = 2.32, P = 0.028) compared to monkeys (Supplementary Fig. 4a), likely due to the extensive passive fixation training that the animals received prior to the current experiment. However, eye-movement signals were significantly correlated (P < 0.001) across monkeys (r = 0.36), across humans (r = 0.25) and between species (r = 0.22, Supplementary Fig. 4b), in line with previous reports13.
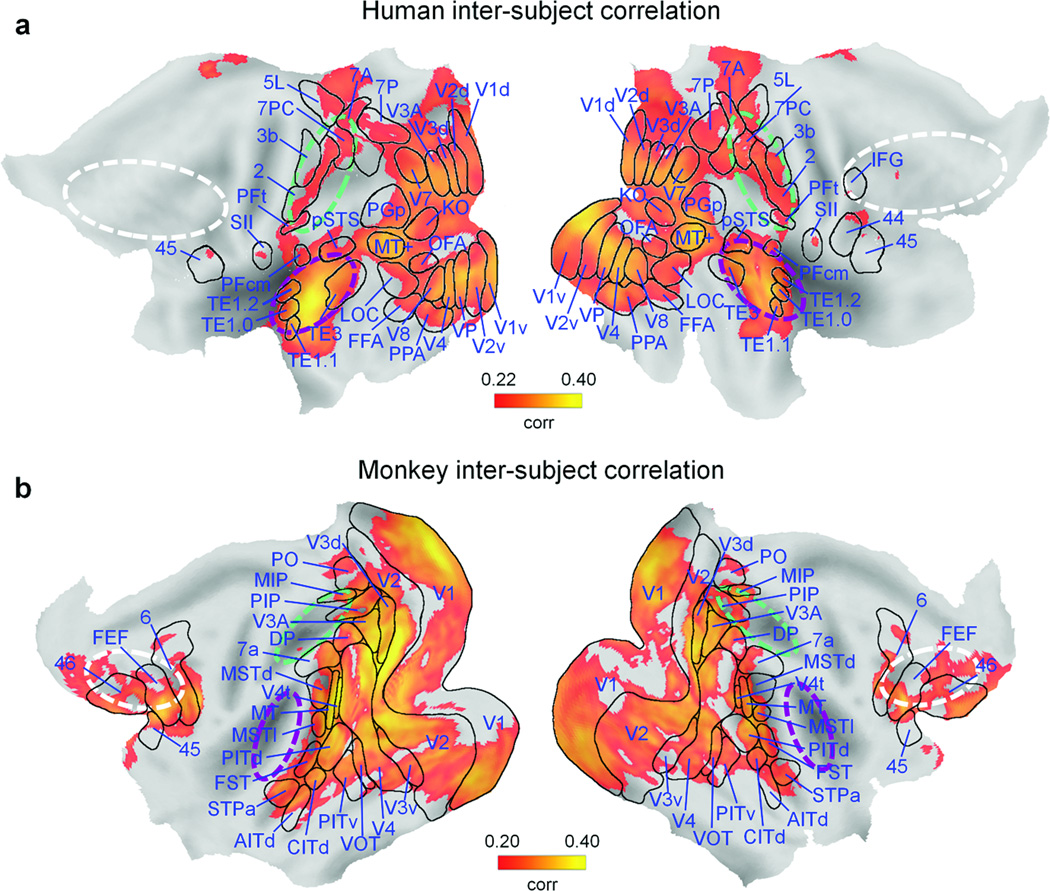
To detect brain regions with consistent stimulus-evoked fMRI activity, we first calculated inter-subject correlation maps10 for each of the two species (see Online Methods). We found significant correlations (false discovery rate, FDR of q < 0.05) in 30.5% and 29.8% of the monkey and human cortical surfaces, respectively (Fig. 2). In humans, the spatial maps encompassed visual, parietal and temporal areas, mostly those involved in lower and higher-level visual and auditory processing (Fig. 2a). In monkeys, striate and extrastriate visual cortex contributed much more to the correlation pattern than did auditory and parietal cortex (Fig. 2b). In contrast to humans, significant inter-subject correlations were also observed in prefrontal macaque areas. The observed differences between monkey and human inter-subject correlation maps largely match results from previous comparative fMRI studies4,6,14. The definition of brain regions with consistent stimulus-evoked fMRI activity is critical for the selection of seed areas to be used in ISAC analyses.
Fig. 2. Inter-subject correlation of brain activity during natural vision.
Spatial maps of correlated brain activity (FDR of q < 0.05) across participants of the same species. (a) Brain areas with significantly correlated responses across 24 human volunteers (human inter-subject correlation), plotted on a flattened cortex. (b) Brain areas with significantly correlated responses across 4 monkeys (monkey inter-subject correlation), plotted on a flattened cortex. Boundaries of identified areas are superimposed onto the cortex. The approximate location of parietal, auditory and frontal regions is indicated by green, purple and white dashed lines, respectively. AITd: dorsal anterior inferotemporal area; CITd: dorsal central inferotemporal area; CITv: ventral central inferotemporal area; DP: dorsal prelunate area; FEF: frontal eye fields; FFA: fusiform face area; FST: fundal superior temporal area; IFG: inferior frontal gyrus; KO: kinetic occipital region; LOC: lateral occipital complex; MIP: medial intraparietal area; MSTd: dorsal medial superior temporal area; MSTl: lateral medial superior temporal area; MT: middle temporal area; MT+: middle temporal complex; OFA: occipital face area; PIP: posterior intraparietal area; PITd: dorsal posterior inferotemporal area; PITv: ventral posterior inferotemporal area; PO: parieto-occipital area; PPA: parahippocampal place area; pSTS: posterior superior temporal sulcus; SII: secondary somatosensory area; STPa: anterior superior temporal polysensory area; V1d: visual area V1, dorsal subdivision; V1v: visual area V1, ventral subdivision; V2d: visual area V2, dorsal subdivision; V2v: visual area V2, ventral subdivision; V3d: visual area V3, dorsal subdivision; V3v: visual area V3, ventral subdivision; V4t: transitional visual area V4; VIP: ventral intraparietal area; VOT: ventral occipitotemporal area; VP: ventroposterior visual area.
Functional correspondence in early visual areas
We first applied our ISAC analysis to the dorsal and ventral subdivisions of visual areas V1 and V2 (V1d, V1v, V2d, V2v), for which anatomical and functional correspondences between monkey and human counterparts are well accepted15. We observed high and significant correlations (r ≥ 0.58, P < 0.001) between the corresponding areas of the two species (Supplementary Table 1). However, such correlations can be induced partially by non-selective, stimulus-related components common to many areas10,12. To minimize contributions by non-selective components, we extracted a common, stimulus-related response for each species by averaging the fMRI signals from all voxels with significant inter-subject correlation (Fig. 2). The non-selective components correlated significantly between monkeys and humans (r = 0.56, P < 0.001) and were removed from the data by linear regression. This additional step reduced the intra-species activity correlations, yet increased the specificity of the ISAC procedure (Supplementary Figs. 5–6). When we repeated the ISAC analysis on the early visual areas, we found reduced, though still significant, inter-species correlations (r ≥ 0.37, P < 0.001) (Supplementary Table 1).
Since the monkeys viewed the movie multiple times, we tested the robustness of the results across movie repetitions. We measured ISAC using the entire human dataset and subsets of monkey data corresponding to single repetitions (Supplementary Table 1). All correlation values were still significant (r ≥ 0.30, P ≤ 0.001), and we observed no differences across movie repetitions (one-way Kruskal-Wallis analysis of variance, χ2 = 8.11, P = 0.150). This suggests that habituation effects in the monkeys, if present, affected the ISAC results only minimally.
As it is well established that cortical functions depend on networks rather than individual areas, we attempted to detect correspondences between functional networks across species by using the seed-based ISAC mapping. We first selected early human visual areas as seeds, and we examined the resulting intra-species (Fig. 1a) and inter-species activity correlation maps (Fig. 1c). For all seeds, we observed an intra-species correlation pattern that clearly extends over a large network of visual areas. This generally resulted in the detection of more than one functionally-related visual area in the other species. By seeding in right human V1d, we obtained a strong ISAC focus in right monkey V1d, as well as left V1d (Supplementary Fig. 7). Similarly, other early visual regions of the human were significantly correlated with anatomically correspondent areas of the monkey.
Functional correspondence in the middle temporal region
The monkey middle temporal region (MT or V5) is an extrastriate visual area for which strong anatomical and functional evidence exists that it is homologous to human MT (or V5), the largest component of the human MT complex (MT+). Accordingly, we used the ISAC mapping to visualize all conspecific and non-conspecific voxels showing significant temporal correlations with the time-course of a seed region in bilateral monkey MT16 (Fig. 1a–b). To assess the reproducibility of the ISAC mapping, we extracted seed time-courses either from monkeys 01–02 or from monkeys 03–04, and we calculated the intra- and inter-species correlations in the complete monkey and human datasets, respectively (Supplementary Fig. 8a–b). Significantly, the predicted functional correspondence between monkey MT and human MT+ was found for both selections, and with a high degree of reliability (spatial correlation between maps: r = 0.818, P < 0.001). Since MT is typically co-activated together with other motion-sensitive areas, we obtained an inter-species activity correlation pattern that included not only MT+ but also a network of areas comprising visual areas V3, V3A, and V4 in humans. The same analysis using human MT+ as the seed ROI also revealed its monkey counterpart in a reliable manner (spatial correlation between maps: r = 0.797, P < 0.001) (Supplementary Fig. 8c–d). To summarize, in addition to early visual regions, we also demonstrated convergence between anatomical and functional correspondence in extrastriate visual areas such as MT.
Does anatomical correspondence imply analogy?
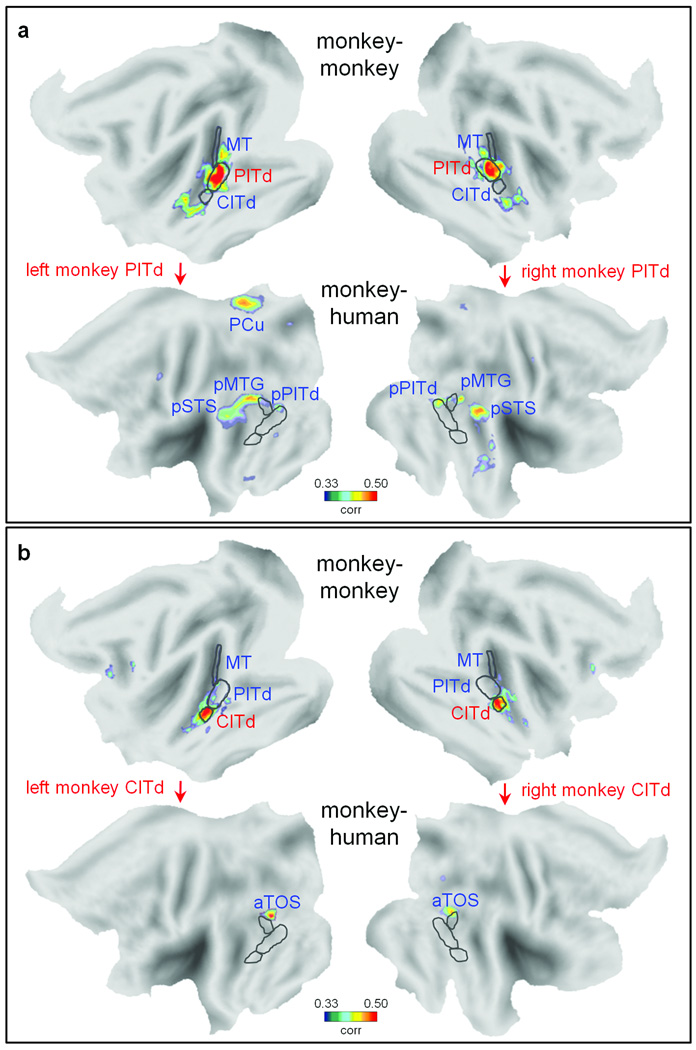
Recent evolutionary theories have suggested that functional reorganization does not always adhere to cortical surface expansion models8,9. To test for proposed functional reorganizations in ventral stream regions17,18, we carried out an ISAC analysis on two neighboring inferotemporal areas (PITd and CITd) located rostral to area MT in the monkey16. Our analyses showed distinct intra- and inter-species correlation maps for the two regions (Fig. 3). The ISAC map for PITd revealed a network including posterior PITd (pPITd), the posterior middle temporal gyrus (pMTG), the posterior superior temporal sulcus (pSTS) and the precuneus (PCu) (Fig. 3a). With the exception of the latter area, these data are consistent with a simple cortical surface expansion model17. In contrast, the ISAC map for neighboring monkey area CITd (Fig. 3b), located ventro-rostrally with respect to MT and PITd, showed the human anterior transverse occipital sulcus (aTOS), located dorso-caudally to human MT+19. Therefore, activations of adjacent areas (PITd, CITd) in the monkey brain seem to be functionally related to response patterns in human pSTS, pMTG, pPITd and aTOS that are, topographically speaking, sharply divergent. This pattern does not fit with the systematic topographical shift and expansion predicted by cortical surface expansion models. Instead, the results are more consistent with an evolution-driven functional reorganization of parts of the ventral stream.
Fig. 3. Intra- and inter-species activity correlation from monkey areas PITd and CITd.
Intra- and inter-species activity correlation maps (FDR of q < 0.001) from both left and right monkey (a) PITd and (b) CITd. The correlation maps are shown only for the same hemisphere in which the seed area is positioned. The borders of monkey areas MT, PITd, CITd are drawn over the monkey flat map. The same borders after monkey-to-human cortical surface expansion are drawn over the human flat map. aTOS: anterior transverse occipital sulcus; PCu: precuneus; pMTG: posterior middle temporal gyrus; pPITd: human posterior area PITd.
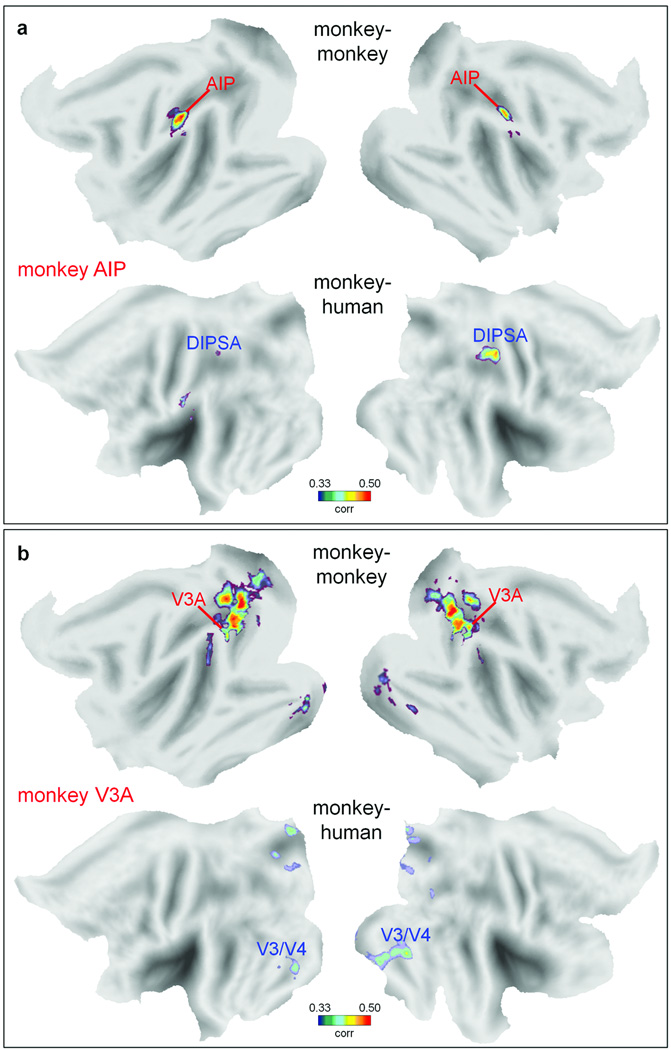
Next, we tested the hypothesis that stronger functional reorganizations tend to occur in regions with greater degrees of anatomical expansion7, by applying the ISAC analysis on the anterior intraparietal area (AIP) and area V3A of the monkey. These two higher-level areas are located in regions respectively with higher and lower degrees of cortical expansion (about 20-fold and three-fold) as compared to the mean (ten-fold) across the cortex5. When seeding in monkey AIP20, we found significantly similar responses in the anterior dorsal intraparietal sulcus area (DIPSA), most likely its human homologue6,21 (Fig. 4a). AIP and DIPSA are both activated by the observation of hand movement10,22, and belong to the monkey and human mirror neuron system, respectively. Conversely, seeding in monkey V3A3 revealed no significant functional correspondence with human V3A, whereas the largest inter-species correlation was unexpectedly located in the human ventral occipital areas including V4 (Fig. 4b). Hence, the degree of anatomical cortical expansion does not necessarily predict the degree of functional reorganization in individual areas within these regions.
Fig. 4. Intra- and inter-species activity correlation from monkey areas AIP and V3A.
Intra- and inter-species activity correlation maps (FDR of q < 0.001) from functionally-defined monkey areas are illustrated. (a) Monkey and human areas showing activity correlated with that in monkey AIP. (b) Monkey and human areas showing activity correlated with that in monkey V3A. AIP: anterior intraparietal area; DIPSA: anterior dorsal intraparietal sulcus area.
Large-scale analysis of inter-species correspondences
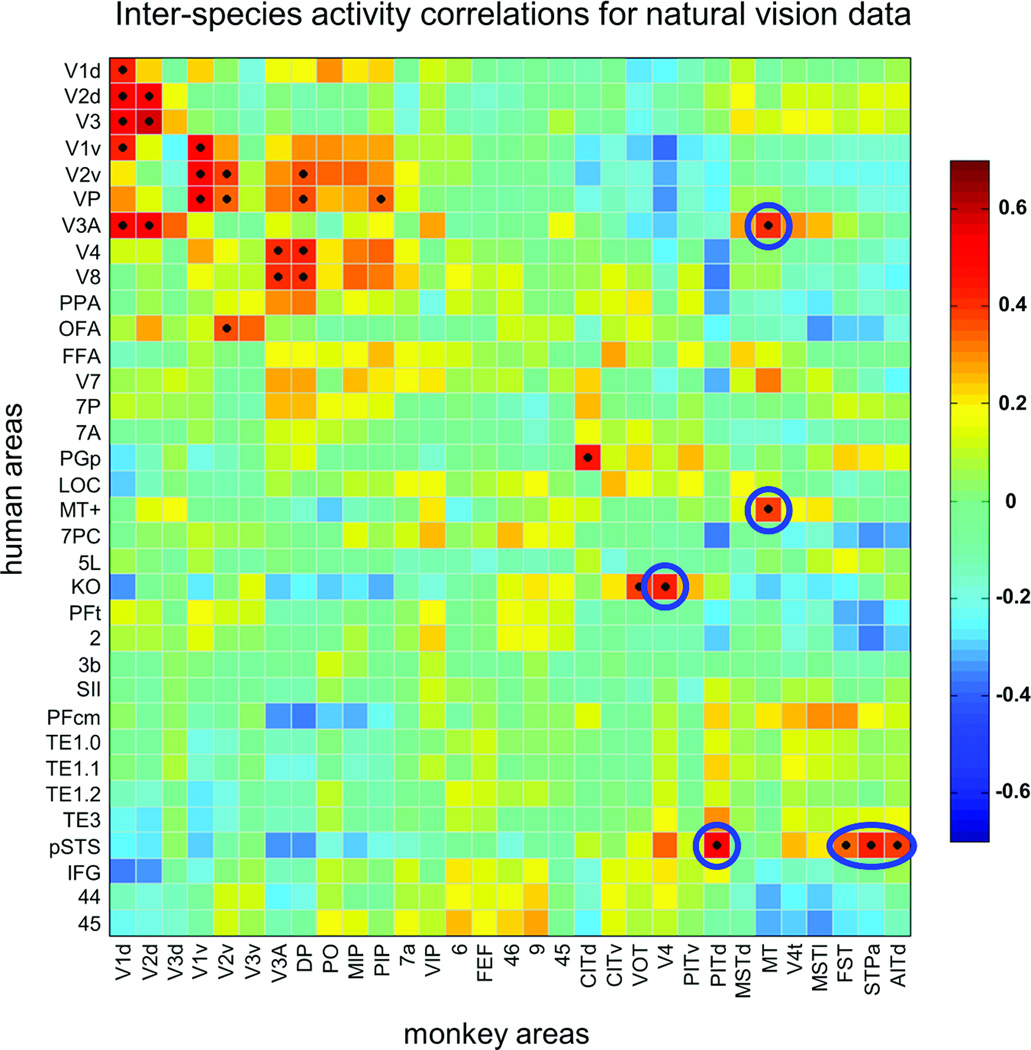
Our ISAC results clearly indicate a number of putative functional similarities between areas of the monkey and the human cortex whose anatomical locations do not correspond (Figs. 3b and 4b). To allow larger-scale inferences, we performed an ISAC analysis across all cortical regions that were activated by the stimuli (Fig. 1d). Accordingly, we defined 31 monkey and 34 human areas showing significant intra-species correlation (Fig. 2), and then compared the stimulus-related responses of these sets of areas by means of temporal correlation. First, we analyzed the reliability of this analysis by assessing the similarity of the ISAC matrices obtained from each of the two halves of either the monkey or human data (Supplementary Fig. 9). Their structures largely corresponded (spatial correlation between matrices: r = 0.886, P < 0.001 and r = 0.864, P < 0.001, for halves of monkey and human data, respectively). Next, we examined the significant correlations (FDR of q < 0.001) in the ISAC matrix calculated using the complete data sets (Fig. 5). In general, the matrix showed results consistent with those found through the seed-based ISAC mapping. For instance, we confirmed monkey-human functional correspondences for the early visual areas (Supplementary Fig. 7), and between monkey MT and human MT+ (Supplementary Fig. 8). As shown previously by ISAC mapping, significant correlations were also present between areas that do not anatomically correspond (Fig. 5). As an example, monkey MT showed similar responses not only to human MT+ (r = 0.41, P < 0.001), but also to human V3A (r = 0.37, P < 0.001); conversely, responses in human MT+ and monkey V3A were substantially unrelated (r = −0.10, P = 0.858). This result is consistent with studies suggesting functional differences between monkey and human V3A, particularly with regard to their motion sensitivities3,6. Close inspection of the ISAC matrix revealed additional significant inter-species similarities (FDR of q < 0.001), which provide a more complete picture of putative evolution-driven functional reorganizations (Fig. 5). For instance, fMRI signals of human pSTS were significantly correlated with those of monkey PITd (r = 0.54), but also with signals in monkey FST (r = 0.35), STPa (r = 0.43), and AITd (r = 0.39). As a further example, we found similar functional responses in human KO and monkey V4 (r = 0.42), areas for which neuroimaging and electrophysiological studies suggest a sensitivity to kinetic motion boundaries23,24. This finding shows the potential of large-scale ISAC analyses to provide specific targets for new functional investigations between species. Reverse correlation analyses may be used to probe whether patterns of specific stimuli evoke consistent fMRI responses in selected brain areas10.
Figure 5. Inter-species activity correlations between monkey and human areas.
The ISAC matrix, calculated on 31 monkey and 34 human areas with consistent fMRI responses, is sorted so that areas with the strongest intra-species correlations are neighboring. Significant functional correspondences, defined on the basis of the Pearson’s correlation test (FDR of q < 0.001), are marked with a black dot. Inter-species correspondences that are commented in the text are marked with either circular or oval-shaped borders.
Discussion
During recent decades, the non-human primate visual system has become a model for the human visual system4,25, on the presumption that similar functions and thus computations are carried out by anatomically corresponding cortical circuitries (the principle of homology or cortical proximity)7. However, the same functions may have shifted during evolution to different or new cortical areas, or functions may have been re-organized on the basis of different principles8,9. An unbiased assessment of cortical evolution and organization requires assumption-free methods to compare functional response patterns across species. In this study we have presented a novel experimental approach to this problem. In particular, we have investigated inter-species functional correspondences based on fMRI activity profiles, without constraints on the cortical topology. Our results with natural vision data confirmed that anatomically corresponding early visual areas are functionally correspondent. However, as we ascended within the cortical hierarchy, we observed both similarities and discrepancies between anatomically and functionally correspondent regions. For example, responses in monkey PITd and AIP are related to topographically corresponding areas in humans. On the other hand, responses in monkey CITd and V3A correlated with regions located respectively more dorso-caudally and ventrally in the human than predicted by cortical surface expansion models. Importantly, these findings suggest that functional reorganization is not strictly related to cortical expansion processes, and may result from mechanisms whereby neuronal circuitries are adapted and recycled to enable more complex cognitive functions8,9.
Our study with natural vision data has a number of limitations. First, the interpretation of our results depends on the assumption that individuals in the two species engage the same processes. During observation of the movie, the monkeys’ understanding of spoken language, actions and plot cannot be compared to that of human subjects. Still large parts of the brain are engaged by the powerful multi-modal sensory stimuli, as shown by our findings (Fig. 2). Controlled stimuli are needed to compare functional similarities and differences in higher-order functions. Second, correlations across visual and other stimulus properties can occur during natural vision, potentially leading to false positive results. Again, this may be minimized using well-controlled stimuli and experimental designs. Third, the ISAC method relies on the definition of seed areas to reveal analogies across the cortex. To overcome this disadvantage, we are working on data-driven approaches to define functional correspondences independently of seed definitions.
We suggest that the ISAC approach will permit comprehensive studies of functional correspondences between higher-level areas in the primate brain, using methods devoid of spatial constraints on the cortical surface. When applied to fMRI data obtained from monkeys and humans performing specific sensory and cognitive tasks, the ISAC method may clarify whether specific functions are preserved in areas that anatomically correspond, are absent in one of the two species, or are shifted to other cortical locations. This novel approach will be critical for shedding light on evolution-driven changes in the functional architecture of the primate brain, and ultimately, for clarifying how human-specific cognitive abilities emerged.
Supplementary Material
Acknowledgments
We thank C. Fransen, C. Van Eupen and A. Coeman for animal training and care; J.T. Arsenault, K. Nelissen, O. Joly, H. Kolster, W. Depuydt, G. Meulemans, P. Kayenbergh, M. De Paep, M. Docx, and I. Puttemans for technical assistance; and S. Raiguel for his comments on the manuscript. This work received support from European Union Seventh Framework Programme FWP-200728, Inter-University Attraction Pole 6/29, Programme Financing PFV/10/008, Geconcerteerde Onderzoeks Actie 10/19, Impulsfinanciering Zware Apparatuur and Hercules funding of the Katholieke Universiteit Leuven, Fonds Wetenschappelijk Onderzoek–Vlaanderen G062208N10, G083111N10 and G043912N, National Science Foundation BCS-0745436 and Geneeskundige Stichting Koningin Elisabeth prize “Janine en Jacques Delaruelle”. D.M. is postdoctoral fellow of the Fonds Wetenschappelijk Onderzoek–Vlaanderen. The Martinos Center for Biomedical Imaging is supported by National Center for Research Resources grant P41RR14075.
Footnotes
Author Contributions
W.V., M.C., G.L.R., D.M. and G.A.O. designed the research; D.M., V.B. and M.G.P. collected the data; D.M. analyzed the data under the supervision of U.H. and W.V.; D.M. and W.V. wrote the first draft of the manuscript, which was revised and approved by all authors.
Competing Interests Statement
The authors declare no competing financial interests.
References
- 1.Tootell RB, Tsao D, Vanduffel W. Neuroimaging weighs in: humans meet macaques in "primate" visual cortex. J. Neurosci. 2003;23:3981–3989. doi: 10.1523/JNEUROSCI.23-10-03981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 3.Vanduffel W, et al. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- 4.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Orban GA, et al. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Striedter GF. Brain homology and function: an uneasy alliance. Brain Res. Bull. 2002;57:239–242. doi: 10.1016/s0361-9230(01)00692-x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson ML. Neural reuse: a fundamental organizational principle of the brain. Behav. Brain Sci. 2010;33:245–266. doi: 10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- 9.Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 12.Hasson U, Malach R, Heeger DJ. Reliability of cortical activity during natural stimulation. Trends Cogn Sci. 2010;14:40–48. doi: 10.1016/j.tics.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd SV, Steckenfinger SA, Hasson U, Ghazanfar AA. Human-monkey gaze correlations reveal convergent and divergent patterns of movie viewing. Curr. Biol. 2010;20:649–656. doi: 10.1016/j.cub.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denys K, et al. Visual activation in prefrontal cortex is stronger in monkeys than in humans. J. Cogn Neurosci. 2004;16:1505–1516. doi: 10.1162/0898929042568505. [DOI] [PubMed] [Google Scholar]
- 15.Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35:89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- 16.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 17.Jastorff J, Orban GA. Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. J. Neurosci. 2009;29:7315–7329. doi: 10.1523/JNEUROSCI.4870-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 20.Durand JB, et al. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron. 2007;55:493–505. doi: 10.1016/j.neuron.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr. Opin. Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 22.Peeters R, et al. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mysore SG, Vogels R, Raiguel SE, Orban GA. Processing of kinetic boundaries in macaque V4. J. Neurophysiol. 2006;95:1864–1880. doi: 10.1152/jn.00627.2005. [DOI] [PubMed] [Google Scholar]
- 24.Van Oostende S, Sunaert S, Van Hecke P, Marchal G, Orban GA. The kinetic occipital (KO) region in man: an fMRI study. Cereb. Cortex. 1997;7:690–701. doi: 10.1093/cercor/7.7.690. [DOI] [PubMed] [Google Scholar]
- 25.Sereno MI, Tootell RB. From monkeys to humans: what do we now know about brain homologies? Curr. Opin. Neurobiol. 2005;15:135–144. doi: 10.1016/j.conb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Vanduffel W, et al. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 27.Friston KJ, et al. statistical parametric mapping: the analysis of functional brain images. 1 ed. Academic Press, Oxford; 2007. [Google Scholar]
- 28.Penny W, Ghahramani Z, Friston K. Bilinear dynamical systems. Philos. Trans. R. Soc. Lond B Biol. Sci. 2005;360:983–993. doi: 10.1098/rstb.2005.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leite FP, et al. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- 30.Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat. Rev. Neurosci. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.