Abstract
In the abdominal aortocaval (AV) fistula model of heart failure, we have shown that the acute doubling of cardiac mature mast cell (MC) density involved the maturation, but not proliferation, of a resident population of immature cardiac MCs. An increase in stem cell factor (SCF) may be responsible for this MC maturation process. Thus, the purpose of this study was to determine if: 1) myocardial SCF levels are increased following the initiation of cardiac volume overload; 2) the incubation of left ventricular (LV) tissue slices with SCF results in an increase in mature MC density; and 3) chemical degranulation of mature cardiac MCs in LV tissue slices results in an increase in SCF and mature MC density via MC chymase. Male rats with either sham or AV fistula surgery were studied at 6 hrs and 1 and 3 days post-surgery. LV slices from normal male rat hearts were incubated for 16 hrs with media alone or media containing one of the following: 1) recombinant rat SCF (20 ng/ml) to determine the effects of SCF on MC maturation; 2) the MC secretagogue compound 48/80 (20 μg/ml) to determine the effects of MC degranulation on SCF levels and mature MC density; 3) media containing compound 48/80 and anti-SCF (5μg/ml) to block the effects of SCF; 4) chymase (100 nM) to determine the effects of chymase on SCF; and 5) compound 48/80 and chymostatin (chymase inhibitor, 10 μM) to block the effects of MC chymase. In AV fistula animals, myocardial SCF was significantly elevated above that in the sham group at 6 hrs and 1 day post fistula by 2 and 1.8 fold, respectively, and then returned to normal by 3 days; this increase slightly preceded significant increases in MC density. Incubation of LV slices with SCF resulted in a doubling of mature MC density and this was concomitant with a significant decrease in the number of immature mast cells. Incubation of LV slices with compound 48/80 increased media SCF levels and mature MC density, anti-SCF and chymostatin prevented these compound 48/80-induced increases. Incubation with chymase increased media SCF levels and mature MC density. These findings indicate that activated mature cardiac mast cells are responsible, in a paracrine fashion, for the increase in mature MC density post AV fistula by rapidly increasing SCF levels via the release of chymase.
Keywords: Stem cell factor, Mast cell maturation, Left ventricular tissue slice culture, Abdominal aortocaval fistula, Compound 48/80, Chymase
1. INTRODUCTION
Mast cells (MC) are derived from multipotent hematopoietic progenitor cells circulating in the blood. Once peripherally located in connective or mucosal tissues, they differentiate to a final phenotype under the influence of the local microenvironment [1]. Mast cells are capable of producing and releasing a wide variety of cytokines, chemokines and growth factors that have the potential to mediate tissue remodeling. In fact, cardiac MC have been shown to play a causal role in the pathogenesis of adverse myocardial remodeling secondary to sustained elevations in myocardial stress [2–7]. As part of this response, cardiac MC density increases in a variety of cardiac pathologies including hypertension [8, 9], myocardial infarction [10, 11], volume overload [4, 12] and heart failure [13]. In the abdominal aortocaval (AV) fistula model of heart failure, we have shown that the acute increase in cardiac MC density was associated with maturation, but not proliferation, of a resident population of immature cardiac MC [14]. One possible mechanism driving the cardiac MC maturation is stem cell factor (SCF). This is supported by studies demonstrating the expansion of cardiac and other organ MC populations following a 2 to 3 week administration of exogenous recombinant SCF to mice, rats and primates [15–17]. These results, together with our findings, suggest that perturbations to the heart elicited by volume overload may regulate local SCF production and/or release, thereby promoting maturation and differentiation of resident immature cardiac MC. Thus, the purpose of this study was to determine if: 1) myocardial SCF levels are increased following the initiation of cardiac volume overload; 2) the incubation of left ventricular tissue slices with SCF results in an increase in mature MC density; and 3) chemical degranulation of cardiac mast cells in LV tissue slices results in an increase in SCF and mature MC density via MC chymase.
2. Methods
The study protocol was approved by the Institution’s Animal Care and Use Committee and conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For all experiments, adult male Sprague-Dawley rats, seven to eight wks of age, were purchased from Harlan Laboratories. The animals were housed under standard environmental conditions and maintained on a normal rodent diet and tap water ad libitum. Anesthesia for non-terminal surgical procedures was achieved using inhaled isoflurane (4%), with post-operative analgesia maintained by administration of buprenorphine hydrochloride (0.025 mg/kg). Euthanasia was accomplished by removal of the heart after the rats were deeply anesthetized with an intra-peritoneal injection of pentobarbital sodium (70 mg/kg).
2.1. Volume Overload
An infrarenal AV fistula was created in rats to induce a sustained cardiac volume overload as previously described [4, 5, 18]. Briefly, a ventral abdominal laparotomy was performed to expose the abdominal aorta and the caudal vena cava distal to the renal arteries. These vessels were then temporally occluded proximal and distal to the intended puncture site. The fistula was created by inserting an 18-gauge needle into the aorta and advancing it through the medial wall into the vena cava. After withdrawing the needle, the aortic puncture site was sealed with cyanocrylate and blood flow restored. A successful fistula was verified by the presence of oxygenated, pulsatile blood flow into the vena cava. A surgical sham control group was also created for each time point whereby an identical surgical procedure was performed with the exception of creating the fistula.
Groups of rats were studied at 6 hrs and 1 and 3 days post-fistula (n=3 – 6 per time period). Sham-operated rats (n=3 – 6 per time period) were used as controls. At each end-point, fistula patency was verified, the animals were sacrificed by excision of the heart, and the left ventricle (LV) plus septum was weighed. Complete mid-ventricular LV cross sections were placed in 4% paraformaldehyde and the remainder of the LV tissue was snap frozen and stored at −80°C.
2.2 LV Tissue Slice Culture
Following anesthesia with isoflurane, a median sternotomy was performed aseptically and the heart removed and placed in cold sterile saline. The LV plus septum was separated from the rest of the heart and filled with 2.5% agarose and then placed into a metal cylinder containing agarose. The LV was sliced perpendicular to the long axis (250 to 300 μm in thickness) using a Brendel/Vitron Tissue Slicer (Vitron Organ Slicing Tech., Tuscon, AZ). To determine the effect of SCF on cardiac MC density and maturation, 15 to 20 LV slices from each heart were randomly divided into three groups with five or more slices per well: 1) culture media alone (RPMI 1640 and 10% FBS); 2) culture media with recombinant rat SCF (20 ng/ml) [15–17] (Peprotech, Rocky Hill, NJ); and 3) culture media with SCF and anti-SCF antibody (5μg/ml, Millipore, Temecula, CA). To determine the effects of the MC degranulation on SCF levels, LV slices from each heart were randomly separated into three groups with five or more slices per well: 1) culture media alone; 2) culture media with the MC secretagogue, compound 48/80 (20 μg/ml; Sigma, St Louis, MO); 3) culture media with compound 48/80 and anti-SCF; and 4) culture media with compound 48/80 and chymostatin (10 μM, MP Biomedicals, Solon, OH). To determine the effects of chymase on SCF levels and MC maturation, LV slices from each heart were randomly separated into three groups with five or more slices per well: 1) culture media alone; 2) culture media with chymase (100 nM, Sigma, St Louis, MO) [19]. After 16 hrs of incubation (37°C, 95% O2, and 5% CO2), the slices from each culture well were first weighed and then two of the slices were fixed with 10% buffered formalin and the remaining slices and culture media were snap frozen and stored at −80°C.
2.3. Cardiac Mast Cell Density
Mature cardiac MC density from AV fistula and sham-operated animals or cultured LV tissue slices was determined by staining 5 μm thick LV cross-sections with toluidine blue, which stains mast cell heparin and histamine granules [20]. Since immature MC do not contain histamine and heparin [21], toluidine blue stained mast cells are considered to be mature mast cells. For each heart, cardiac mature mast cell density was determined in 4 sections from the 2 formalin-fixed slices in each group, with the observer (JL) being blinded as to the source of the tissue, by counting the total number of MC present in each LV cross-section and dividing this number by the area of the section.
2.4. Determining Mast Cell Maturation Stages
To differentiate between immature and mature MC in the cultured LV tissue slices, the alcian blue/safranin staining technique was used with modification [14]. Briefly, the cultured LV tissue slices were fixed in 10% buffered formalin, embedded in paraffin and sectioned to a 5μm thickness. The slices were then deparafinized, rehydrated and stained with 0.5% alcian blue (in PBS, pH 1.0) at room temperature for 45 mins. After washing in PBS (pH 1.0), the sections were stained with 0.5% safranin (in PBS, pH 1.0) at room temperature for 45 mins. The slides were again washed in PBS, dehydrated and coverslipped. In each heart, the percentages of mature (violet-orange and orange in color) and immature (blue in color) MC were determined in the 4 sections from the 2 formalin-fixed slices from each group by the observer (JL) in a blinded fashion.
2.5. ELISA Analysis of SCF
Approximately 100 mg of LV tissue from the infrarenal AV fistula experiments and three or more LV slices from each culture well were homogenized in PBS buffer containing protease inhibitor cocktail (Sigma, St Louis, MO) to extract total protein for the SCF assay. LV tissue slice culture media from each culture group was assayed directly. Levels of SCF were determined using a commercially available ELISA kit (Abcam, Cambridge, MA). All samples were run in duplicate.
2.6. Determining Mast Cell Proliferation
To determine whether SCF-induced MC proliferation contributed to the increase in mature MC density, LV tissue slices were cultured in the following three groups: 1) culture media alone, 2) culture media and bromodeoxyuridine (BrdU, 10 μM) (Sigma, St Louis, MO), 3) culture media and BrdU plus SCF (20 ng/ml). After 16 hrs of culture, three slices from each group were examined by immunostaining with mouse monoclonal anti-BrdU antibody (2 μg/ml; Sigma, St Louis, MO).
2.7. Statistical Analysis
Results are presented as mean ± SEM. For the comparison between groups, one-way analysis of variance (ANOVA) was performed, followed by the Tukey post test. Statistical significance was set at a P value < 0.05.
3. RESULTS
The postoperative course was well tolerated for all animals. A patent AV fistula was visually confirmed by the presence of a pulsatile flow of oxygenated blood into the vena cava at the study endpoints. Prior to surgery and at completion of the study, there were no fistula/sham group differences in body, lung and left ventricular weights.
3.1. Myocardial SCF Level and Mature Mast Cell Density in Response to an Acute Sustained Volume Overload
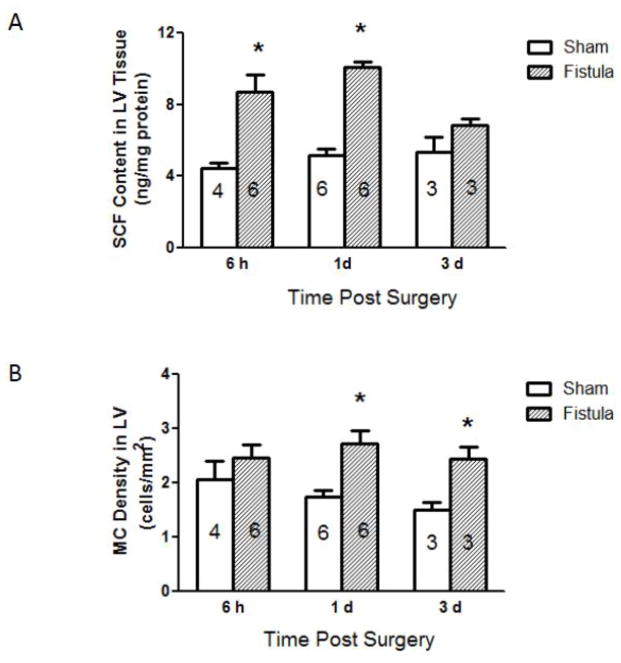
Myocardial SCF protein levels measured at each sham and post fistula time-point are presented in Figure 1A. As can be seen, tissue SCF was significantly elevated above that in the sham group at 6 hrs and 1 day post fistula by 2 and 1.8 fold, respectively. By day 3 post fistula the fistula group SCF level had returned to that in the sham group. The cardiac mature MC densities in the fistula groups were greater by 56 and 63% than their respective sham groups at days 1 and 3 post surgery with the peak value occurring at 1 day (Figure 1B).
Figure 1.
Left ventricular stem cell factor (SCF) concentration (A) and mature mast cell (MC) density (B) in male rat left ventricular sections at several time points post sham or fistula surgery. Number within each bar indicates the number of rat hearts in the group. All values are mean ± SEM. * p < 0.05 vs. corresponding group shams.
3.2. Cardiac Mast Cell Maturation in Response to SCF in Cultured LV Tissue Slices
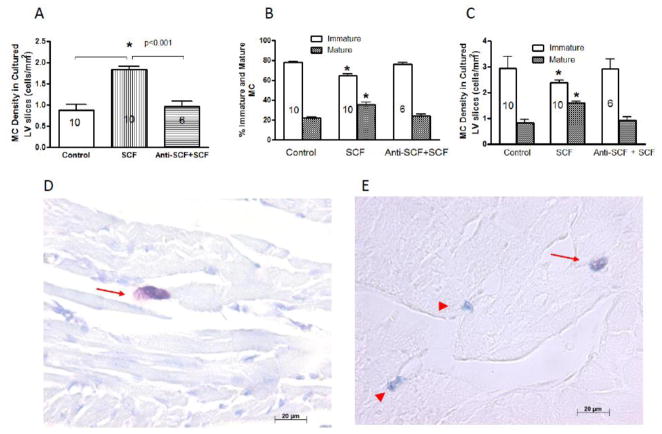
The ability of SCF to induce maturation of resident cardiac MC was assessed by culturing LV tissue slices with and without SCF. As can be seen in Figure 2, incubation with SCF for 16 hrs resulted in a doubling in the density of toluidine blue stained MC and anti-SCF antibody blocked this SCF-induced increase (2A). This was concomitant with a significant decrease in the percent and density of alcian blue-stained (immature) MC and a significant increase in percent and density of alcian blue/safranin-stained (mature) MC (2B and 2C). Also depicted in Figure 2 are representative images of MC stained with toluidine blue (2D) and with alcian blue/safranin staining (2E) showing mature MC (arrow) and immature MC (arrow head).
Figure 2.
Left ventricular tissue slice mature mast cell (MC) density (2A) and percent (%) and density of immature and mature MCs (2B and 2C, respectively) that were incubated with media alone (control), stem cell factor (SCF, 20 ng/ml) alone, or SCF plus anti-SCF antibody (Anti-SCF, 5 μg/ml) for 16 hrs. Number within a bar represents the number of rat hearts in the group. All values are mean ± SEM. * p < 0.01 vs. control and Anti-SCF+SCF groups. Representative images of MC stained with toluidine blue (2D) and with alcian blue/safranin staining (2E) showing mature MC (arrow) and immature MC (arrow head).
3.3. SCF Release and Mast Cell Density Following Mast Cell Degranulation in Cultured LV Tissue Slices
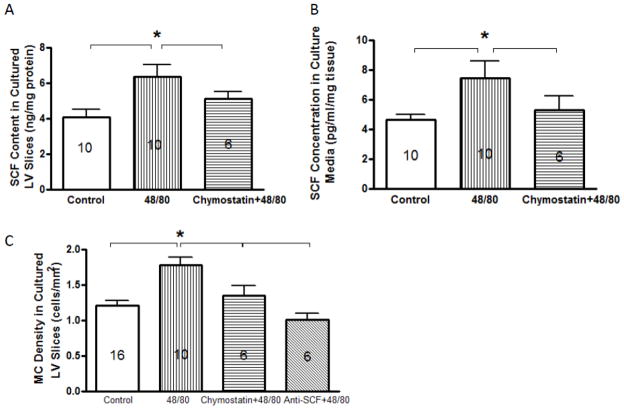
To determine whether MC degranulation can contribute to the increases in SCF level and mature MC density in the heart, LV tissue slices were incubated with the MC secretagogue, compound 48/80 with and without anti-SCF and the chymase inhibitor, chymostatin. SCF was measured in the LV slice and culture media. Compound 48/80 caused a statistically significant increase in SCF levels in both the LV slices and culture media (Figure 3A and 3B). Compound 48/80 also caused an increase in mature MC density in cultured LV slices, which was prevented by anti-SCF (figure 3C). Chymostatin prevented the compound 48/80-induced increases in SCF in both the LV slice and culture media as well as the increase in mature MC density (Figure 3A, B and C).
Figure 3.
Stem cell factor (SCF) concentration in left ventricular (LV) tissue slices (3A) and the conditioned medium (3B) and mature mast cell (MC) density (3C) that were incubated with media alone (control), compound 48/80 (20 μg/ml), or 48/80 plus chymostatin for 16 hrs. SCF levels in LV tissue slices and culture media were normalized with total protein and tissue slice weight, respectively, and are presented as mean ± SEM. Number within bars represents the number of rat hearts in the group. * p < 0.01 vs. control and chymostatin groups.
3.4. Effects of Chymase on SCF Levels and Mature Mast Cell Density
To further investigate whether MC degranulation increases cardiac SCF levels via the release of chymase, LV tissue slices were incubated with chymase. Chymase caused statistically significant increases in SCF levels in the culture media (Figure 4A) and in mature MC density in the cultured LV slice (Figure 4B).
Figure 4.
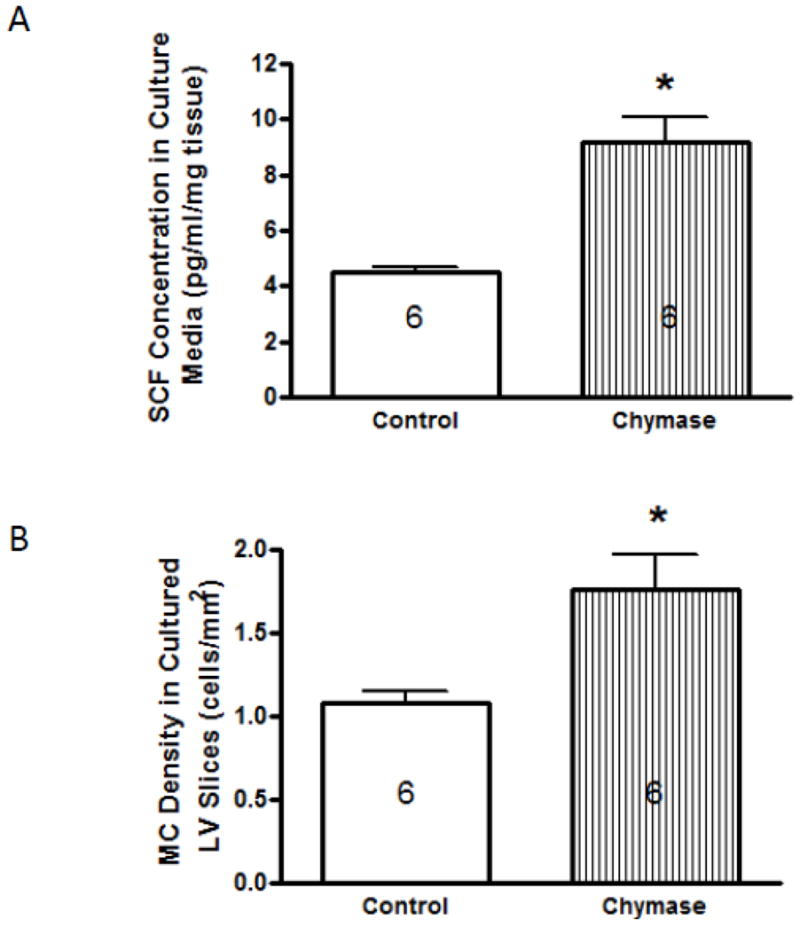
Stem cell factor (SCF) concentration in culture medium (4A) and mature mast cell (MC) density in cultured left ventricular (LV) tissue slices (4B) that were incubated with media alone (control) or chymase (100 nM) for 16 hrs. SCF levels in culture media were normalized with total tissue slice weight, and are presented as mean ± SEM. Number within bars represents the number of rat hearts in the group. * p < 0.01 vs. control.
3.5. Determination of Mast Cell Proliferation in LV Slices Cultured with SCF
Immunohistochemical staining indicated that mature MC in LV slices cultured with SCF for 16 hrs did not incorporate BrdU, indicating that the SCF-induced increase in mature MC density was not the result of MC proliferation. This observation is consistent with our previous finding that less than 1% of cardiac MC isolated from volume overloaded hearts were labeled positive for BrdU [14].
4. DISCUSSION
The first report linking mast cells with the diseased heart was in 1964 where an increase in mature mast cell number was observed in hearts from patients with endomyocardial fibrosis [22]. Since then, increased cardiac mature MC number and density has been reported in rodents, canine and/or humans in: hypertension [8, 9], myocardial infarction [10, 11], volume overload [4, 12], myocarditis [23], explanted human hearts [23], idiopathic cardiomyopathy [25, 26], and heart failure [13]. While a mature MC causal role for myocardial and ventricular remodeling has been established in a number of these cardiac pathologies [2–6, 12, 27], the question as to the source of the increased number of mature MC remained. In this study, we have demonstrated the maturation of resident immature MC brought on by an increase in myocardial SCF to be a source.
Galli and his colleagues [15–17] have demonstrated that daily intravenous injections of SCF over a two to three week period increased mature MC populations with phenotypic characteristics of connective tissue MC in all organs and tissue that they studied including skin, lung, liver, heart, spleen, glandular stomach, and ileum. We demonstrate in the current manuscript that myocardial levels of SCF were increased at the 6 hr and 1 day time-points, following volume overload. This slightly preceded the increases in mature MC density, which occurred at 1 and 3 days, indicating that increased SCF results in an increased numbers of mature MC in the volume overloaded heart. Previously we have reported mature MC density to have returned to normal levels at 5 days post fistula [4]. This rapid decline could be explained by the observation in this study that SCF had returned to that of the sham group by 3 days post fistula. Galli et al., in addition to showing that the long term administration of SCF increased mature MC density throughout the body, found that discontinuation of SCF treatment for 15 days resulted in a decline in mature MC numbers to pre-SCF administration control levels [17]. While their results are supportive of our findings, our novel results highlight the rapidity of the immature and mature MC density responses to SCF increases and subsequent decreases.
Three possibilities exist to explain this increase in mature MC density: 1) proliferation of existing MC; 2) recruitment of MC precursors; and 3) maturation of resident immature MC. Subcutaneous injection of SCF has been demonstrated to increase dermal MC number by increasing maturation of immature MC, proliferation of MC, and recruitment of precursor cells [15, 16]. However, we have previously ruled out proliferation as a source of increased mature MC in the heart following volume overload since less than 1% of cardiac MC isolated from volume overloaded hearts labeled positive for BrdU by flow cytometry [14]. Also, in the present study, we incubated LV tissue slices with BrdU and SCF for 16 hours. MCs in the cultured LV tissue slices did not incorporate BrdU, indicating that the SCF-induced increase in mature MC was not due to MC proliferation. Mast cells are derived from multipotent hematopoietic progenitor cells from bone marrow which do not develop into MC until reaching the tissue or organ in which they become resident [28]. Several possible progenitor cells have been identified, including Thy-1loc-Kithi progenitor mastocytes, Lin−Kit+Sca-1−Ly6c−FcεRIα−CD27−β7+T1/ST2+, Lin−Kit+FcγRII/IIIhiβ7hi, CD45+Lin−CD34+−β7hiFcεRIαlo cells in mice [28–31], while in humans MC progenitors circulate in the blood as mononuclear leucocytes without granules, and express the surface markers CD13, CD33, CD38, CD34 and Kit [28]. However, recruitment of MC precursors is unlikely to account for the rapid increase in cardiac mature MC following volume overload since the recruitment process and subsequent development into a mature MC in the tissue requires a significant amount of time. This was demonstrated in a study where MCs were depleted from the peritoneal cavity in rats. It took 6 days before mature MC numbers began to approach control levels and a full 20 days for full restoration to be achieved [32]. In the days prior to this point, the MC present were mostly immature. Because of this protracted timeline, recruitment is not considered to be the source for the increase in mature MC seen in the heart by days 1 to 3 following the initiation of cardiac volume overload.
To determine whether SCF could induce an increase in mature MC number in the heart, and whether this could occur in the time-frame similar to that occurring in cardiac volume overload, we incubated slices of LV tissue with SCF for 16 hours. This resulted in significant increases in mature MC density as well as the percent of mature MC. Neutralization of SCF by anti-SCF antibody blocked the SCF-induced increases in mature MC density and the percent of mature MC. This straightforward experiment demonstrates that mature MC numbers in the heart can increase simply by maturation of immature resident MC. That is, this increase could not be the result of blood-borne precursor cell recruitment because the LV slices were treated in dishes containing only cell culture media. The 16 hour time period is also very representative of what we see following the initiation of cardiac volume overload. This demonstrates that a SCF driven maturation alone can account for the rapid increase in mature MC density that occurs following cardiac volume overload. Thus, while Tsai et al. [16] had treated mice for 3 weeks with SCF before assessing its effect on mature MC numbers, our results highlight the rapid nature of MC maturation following exposure to SCF.
Both the soluble and membrane-bound forms of SCF can be derived from several sources including fibroblasts, endothelial cells and smooth muscle cells; even MC themselves are capable of producing SCF [33]. In our previous studies we had also noted that prevention of MC degranulation with the MC stabilizing compounds, cromolyn and nedocromil, had the effect of preventing or reducing cardiac mature MC density in fistula and sham animals, respectively [4, 34]. These findings indicate that MC degranulation is required for mature MC density to increase. To determine whether MC degranulation results in increases in myocardial SCF levels, we treated cultured LV slices with the MC secretagogue, compound 48/80, and found that SCF levels were significantly increased in both the LV slice itself and the culture media indicating increased SCF synthesis as well as secretion. While we did not determine specifically whether soluble or membrane-bound SCF increased, the fact that SCF in the culture media increased would indicate at least an increase in released, soluble SCF. Non-cardiac MC chymase has been shown to cleave membrane-bound SCF releasing a soluble form [35]. In line with this observation, incubation of LV slices with chymase caused the increases in SCF levels in the culture media, compound 48/80 increased SCF release from cultured LV slice, and chymostatin, a non-specific inhibitor of chymase, prevented the compound 48/80-induced increases in SCF levels in both LV tissue slices and culture media indicating membrane-bound SCF synthesis and cleavage is responsible for MC degranulation-caused SCF increases. At this stage we cannot discount the possibility that a portion of the increased SCF was from cardiac MC in addition to other cells in response to MC degranulation. Accordingly, the activated MC induced-increase in SCF may very well be the result of an autocrine- and paracrine-mediated process. Concomitant with the increase and decrease of SCF following MC degranulation and inhibition of chymase, respectively, was an increase and decrease in mature MC density in LV slices.
In summary, the tissue slice results clearly indicate that SCF can rapidly increase cardiac mature mast cell density by stimulating resident immature MCs to mature. In addition, compelling evidence is presented to indicate that the rapid increase in cardiac MC density following the initiation of a sustained cardiac volume overload is mostly the result of a paracrine-mediated increase in myocardial SCF secondary to mast cell activation.
Highlights.
Mast cell degranulation increases cardiac stem cell factor via the release of chymase.
Stem cell factor induces maturation of cardiac resident immature mast cells.
Volume overload increases cardiac stem cell factor and mature mast cell density.
Acknowledgments
We would like to thank Will Spencer for his excellent technical assistance. This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health (R21-HL-089483 to J.S.J.)
Footnotes
Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Crivellato E, Nico B, Ribatti D. The history of the controversial relationship between mast cells and basophils. Immunol Lett. 2011;141:10–17. doi: 10.1016/j.imlet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, Nakatani K, et al. Evidence for a Role of Mast Cells in the Evolution to Congestive Heart Failure. J Exp Med. 2002;195:375–81. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–7. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 4.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol. 2002;283:H518–H525. doi: 10.1152/ajpheart.00218.2000. [DOI] [PubMed] [Google Scholar]
- 5.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Cardiac Fail. 2005;11:548–56. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.McLarty JL, Melendez GC, Brower GL, Janicki JS, Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–70. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008;45:56–61. doi: 10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiota N, Rysa J, Kovanen PT, Ruskoaha H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1823–5. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Panizo A, Mindan FJ, Galindo MF, Cenarruzabeitia E, Hernandez M, Diez J. Are mast cells involved in hypertensive heart disease? J Hypertens. 1995;13:1201–8. doi: 10.1097/00004872-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Engels W, Reiters PH, Daemen MJ, Smits JF, van der Vusse GJ. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J Pathol. 1995;177:423–9. doi: 10.1002/path.1711770414. [DOI] [PubMed] [Google Scholar]
- 11.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–98. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JA, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–9. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 13.Batlle M, Roig E, Perez-Villa F, Lario S, Cejudo-Martin P, Garcia-Pras E, et al. Increased expression of the renin-angiotensin system and mast cell density but not of angiotensin-converting enzyme ii in late stages of human heart failure. J Heart Lung Transplant. 2006;25:1117–25. doi: 10.1016/j.healun.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Forman MF, Brower GL, Janicki JS. Rat cardiac mast cell maturation and differentiation following acute ventricular volume overload. Inflamm Res. 2006;55:408–15. doi: 10.1007/s00011-006-6016-z. [DOI] [PubMed] [Google Scholar]
- 15.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci U S A. 1991;88:6382–6. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai M, Shih LS, Newlands GF, Takeishi T, Langley KE, Zsebo KM, et al. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med. 1991;174:125–31. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli SJ, Iemura A, Garlick DS, Gamba-Vitalo C, Zsebo KM, Andrews RG. Reversible expansion of primate mast cell populations in vivo by stem cell factor. J Clin Invest. 1993;91:148–52. doi: 10.1172/JCI116164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol Heart Circ Physiol. 1996;271:H2071–H2078. doi: 10.1152/ajpheart.1996.271.5.H2071. [DOI] [PubMed] [Google Scholar]
- 19.Tomimori Y, Muto T, Fukami H, Saito K, Horikawa C, Tsuruoka N, et al. Mast cell chymase regulates dermal mast cell number in mice. Bioch and Biophy Res Commu. 2002;290:1478–1482. doi: 10.1006/bbrc.2002.6365. [DOI] [PubMed] [Google Scholar]
- 20.D’llario L, Francolini I, Martinelli A, Piozzi A. Insight into the heparin-toluidine blue (C. I basic blue 17) interaction. Dyes and Pigments. 2009;80:343–348. [Google Scholar]
- 21.Galli SJ, Dvorak AM, Marcum JA, Ishizaka T, Nabel G, Simonian HD, et al. Mast cell clones: A model for the analysis of cellular maturation. J Cell Biol. 1982;95:435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernex M, Sternby NH. Mast cells and coronary heart disease. Relationship between number of mast cells in the myocardium, severity of coronary atherosclerosis and myocardial infarction in an autopsy series of 672 cases. Acta Pathol Microbiol Scand. 1964;62:525–38. doi: 10.1111/apm.1964.62.4.525. [DOI] [PubMed] [Google Scholar]
- 23.Estensen RD. Eosinophilic myocarditis: a role for mast cells? Arch Pathol Lab Med. 1984;108:358–9. [PubMed] [Google Scholar]
- 24.Li QY, Raza-Ahmad A, MacAulay MA, Lalonde LD, Rowden G, Trethewey E, et al. The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts. Transplantation. 1992;53:1047–51. doi: 10.1097/00007890-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Patella V, de Crescenzo G, Lamparter-Schummert B, De Rosa G, Adt M, Marone G. Increased cardiac mast cell density and mediator release in patients with dilated cardiomyopathy. Inflamm Res. 1997;46:S31–S32. [PubMed] [Google Scholar]
- 26.Patella V, Marino I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–8. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 27.Jaggi AS, Singh M, Sharma A, Singh D, Singh N. Cardioprotective effects of mast cell modulators in ischemia-reperfusion-induced injury in rats. Methods Find Exp Clin Pharmacol. 2007;29:593–600. doi: 10.1358/mf.2007.29.9.1161005. [DOI] [PubMed] [Google Scholar]
- 28.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–22. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 30.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005;102:11408–13. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102:18105–10. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamur MC, Moreno AN, Mello LF, Souza Junior DA, Campos MR, Pastor MV, et al. Mast cell repopulation of the peritoneal cavity: contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol. 2010;11:32. doi: 10.1186/1471-2172-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da Silva CA, Reber L, Frossard N. Stem cell factor expression, mast cells and inflammation in asthma. Fundam Clin Pharmacol. 2006;20:21–39. doi: 10.1111/j.1472-8206.2005.00390.x. [DOI] [PubMed] [Google Scholar]
- 34.Murray DB, Levick SP, Brower GL, Janicki JS. Inhibition of matrix metalloproteinase activity prevents increases in myocardial tumor necrosis factor-alpha. J Mol Cell Cardiol. 2010;49:245–50. doi: 10.1016/j.yjmcc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longley BJ, Tyrrell L, Ma Y, Williams DA, Halaban R, Langley K, et al. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci U S A. 1997;94:9017–21. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]