Background: MeCP2 is required for synaptogenesis and proper development of neuronal circuits.
Results: MeCP2 phosphorylation on serine 421 is controlled by nuclear calcium signaling activating nuclear CaMKII.
Conclusion: This defines a novel pathway through which nuclear calcium regulates synaptic activity-driven genomic responses.
Significance: Nuclear calcium modulates the function of a key regulator of neuronal circuit development.
Keywords: Calcium Calmodulin-dependent Protein Kinase (CaMK); Calcium Signaling; Chromatin; Glutamate Receptors Ionotropic (AMPA, NMDA); Transcription Factors
Abstract
The function of MeCP2, a methylated DNA-interacting protein that may act as a global chromatin modifier, is controlled by its phosphorylation on serine 421. Here we show that in hippocampal neurons, nuclear calcium signaling controls synaptic activity-induced phosphorylation of MeCP2 on serine 421. Pharmacological inhibition of calcium/calmodulin-dependent protein (CaM)kinases blocked activity-induced MeCP2 serine 421 phosphorylation. CaM kinase II (CaMKII) but not CaMKIV, the major nuclear CaM kinase in hippocampal neurons, appeared to mediate this phosphorylation event. Biochemical subcellular fractionations and immunolocalization studies revealed that several isoforms of CaMKII (i.e. CaMKIIα, -β, -γ, and -δ) are expressed in the cytosol but are also detectable in the cell nucleus of hippocampal neurons, suggesting that nuclear CaMKII catalyzes MeCP2 serine 421 phosphorylation. Thus, in addition to the classical nuclear calcium-CaMKIV-CREB/CBP (cAMP-response element-binding protein/CREB-binding protein) pathway that regulates transcription of specific target genes, nuclear calcium may also modulate genome-wide the chromatin state in response to synaptic activity via nuclear CaMKII-MeCP2 signaling.
Introduction
Methyl-CpG-binding protein (MeCP)3 2 is a transcription factor that binds to methylated cytosine residues of CpG dinucleotides in DNA (1). It can function as a transcriptional repressor through the recruitment of the Sin3a/histone deacetylase corepressor complex to target promoters or by increasing the methylation of histone H3 on lysine 9 (2–7). However, several lines of evidence suggest that MeCP2 not only silences gene expression, but that it is also involved in the activation of transcription. First, in the hypothalamus, lack of MeCP2 leads to reduced expression of thousands of genes (3). Second, MeCP2 may form a complex with the transcriptional activator cAMP-response element-binding protein (CREB) and can cooperate with CREB in the transactivation of a transfected reporter gene that contains the promoter region of a MeCP2 target gene (3). Transcriptional activation by MeCP2 may also occur via “derepression,” which has been reported to play a role in the regulation of Bdnf expression in rat hippocampal and cortical neurons (4, 8). In these cells, KCl-induced membrane depolarization and subsequent calcium entry causes phosphorylation of MeCP2 and its release from Bdnf promoter IV, which renders Bdnf expression permissive for activation by other, either constitutively active or signal-regulated transcription factors (4, 8). Recent studies, however, suggested that MeCP2 may not regulate specific genes but instead acts in a histone-like fashion to modulate genome-wide the chromatin state in response to synaptic activity (9, 10).
Although MeCP2 is expressed in many tissues (5, 6), its function may be primarily in the development of synapses and the formation of circuits in the central nervous system. Mutations in the MeCP2 gene cause the majority of cases of Rett syndrome, an X-linked dominant neurodevelopmental disorder and leading cause of mental retardation and autistic behavior in girls and women (9–11). Patients with classic Rett syndrome appear to develop normally during the first 6–18 months of life, after which they begin to regress, gradually losing any acquired speech and replacing purposeful hand use with stereotypies (12–14). Mice that either lack or overexpress MeCP2 develop a phenotype that recapitulates many characteristic features of Rett syndrome, including normal early postnatal development followed by progressive motor and cognitive dysfunctions. In addition, similar to Rett syndrome patients, they show abnormalities in brain morphology and cyto-architecture, in particular a decrease in dendritic arborization and spine loss (8, 15).
The mechanism through which MeCP2 controls neurodevelopment is unknown. However, given the evidence that synaptogenesis and the proper wiring of the nervous system is a neuronal activity-driven process, it has been suggested that MeCP2 may relay neuronal activity patterns in early postnatal development to the transcriptional machinery (16). Failure of MeCP2 either to induce appropriate genome-wide chromatin changes or to activate or repress as yet unidentified target genes could lead to malfunction of circuit development, which may ultimately cause neuropsychiatric disorders. According to this concept, signal regulation is a key feature of MeCP2 and indeed critical for proper brain development. The best characterized, inducible post-translational modification of MeCP2 is its phosphorylation on serine 421 (8). This phosphorylation event is triggered by KCl-induced membrane depolarization or synaptic activity and requires calcium entry through NMDA receptors and/or voltage-gated calcium channels (8). Knock-in mice that lack either serine 421 of MeCP2 or serine 421 as well as serine 424, a second site of synaptic activity-induced phosphorylation, show alterations in synaptogenesis, synaptic plasticity, and spatial memory (9, 17), underscoring the importance of these phosphorylation sites in vivo. The calcium-dependent modulation of MeCP2 function suggests that its role in neural circuit development could be mediated by one or several components of a pool of about 1000 genes that are induced or repressed within a few hours following NMDA receptor stimulation and the activation of calcium signaling pathways (18, 19). Because calcium can act in different subcellular compartments (in particular cytosol versus nucleus) to differentially regulate transcription (20), it is important to determine the precise spatial requirement of the calcium signal needed to induce MeCP2 serine 421 phosphorylation. In this study, we focused on nuclear calcium, which has emerged as a key signal in several transcription-dependent forms of neuronal adaptations, including acquired neuroprotection and memory (19, 21–24). In hippocampal neurons, nuclear calcium transients are required for activity-dependent regulation of about 200 genes, many of which are targets of CREB and CBP, the prototypical transcription factor complex activated by nuclear calcium and the nuclear calcium/calmodulin-dependent protein (CaM) kinase IV (19). Here we identify MeCP2 as an alternative target of nuclear calcium signaling and provide evidence that unlike CREB/CBP regulation, a nuclear localized CaMKII mediates the effects of nuclear calcium on MeCP2.
EXPERIMENTAL PROCEDURES
Cell Culture, Virus Infection, and Stimulations
Hippocampal neurons from newborn C57Black6 mice were cultured in Neurobasal medium (Invitrogen) containing 1% rat serum, B27 (Invitrogen) and penicillin and streptomycin (Sigma-Aldrich, München, Germany). The procedure to isolate and culture hippocampal neurons has been described (25, 26). Stimulations were done after a culturing period of 10 days during which hippocampal neurons develop a rich network of processes, express functional NMDA-type and AMPA/kainate-type glutamate receptors, and form synaptic contacts (27). The following drugs were used: 10 μm KN62, 2 μm KN93 and bicuculline (Alexis, Läufelfingen, Germany); 10 μm SB203580 (Calbiochem, Darmstadt, Germany); 1 μm cyclosporine A (Sigma-Aldrich); 1 μm FK506 (Axxora, Lörrach, Germany). Bursts of action potential firing were induced by treatment of cultured hippocampal neurons with 50 μm bicuculline at day in vitro (DIV) 10.
Recombinant Adeno-associated Virus and Virus Infection
Recombinant adeno-associated virus (rAAV) vector with a CMV/CBA hybrid promoter for the expression of hrGFP (humanized Renilla reniformis green fluorescent protein), CaMBP4, or CaMKIV(1–313) have previously been described (18, 19). A rAAV vector containing the mouse CaMKII promoter (a gift from Ali Cetin and Peter Seeburg, Max Planck Institute for Medical Research Heidelberg, Germany), was used to generate rAAV-CaMKII(1–290)-Flag, rAAV-CaMKII(1–290)NLS-Flag, and rAAV-CaMKVI(1–313)NLS-Flag. rAAV vectors were generated by standard molecular biology techniques and verified by sequencing. Viral particles were produced and purified as described previously (18). Depending on the experimental conditions, hippocampal neurons were infected after DIV 3 at multiplicities of infection of 2 × 109 copies/ml and harvested at DIV 10.
Quantitative Reverse Transcription-PCR
Total RNA was isolated at DIV 10 from hippocampal primary neuron cultures with the RNeasy mini kit (Qiagen, Hilden, Germany) including an optional DNase I treatment at room temperature for 15 min according to the manufacturer's instructions (Qiagen). cDNAs were synthesized from 1.3 μg of total RNA using the High Capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative reverse transcription-PCR was done on an ABI7300 thermal cycler using universal quantitative reverse transcription-PCR master mix with TaqMan gene expression assays for the indicated genes (Applied Biosystems). The following TaqMan gene expression assays were used in this study: Gapdh (Mm99999915_m1), Gusb (Mm00446953_m1), and Bdnf (Mm00432069_m1). The expression of target genes was normalized against the expression of Gusb and Gapdh as endogenous control genes using the ΔΔCt method. Data represent mean value (±S.E.) from at least four independent experiments. The following primers were used to detect exon-specific Bdnf expression by quantitative reverse transcription-PCR using Power SYBR Green PCR master mix (Applied Biosystems): Bdnf promoter I, forward, 5′-AACAAGACACATTACCTTCCAGCAT-3′, reverse, 5′CTCTTCTCACCTGGTGGAACATT-3′; Bdnf promoter IV, forward: 5′-GCTGCCTTGATGTTTACTTTGA-3′, reverse, 5′-GCAACCGAAGTATGAAATAACC-3′. The following cycling conditions were used: 95 °C for 10 min followed by 45 cycles of 95 °C for 10 s, annealing temperature 55 °C for 10 s. Melting curve analysis was performed at the end of each reaction to confirm amplification of a single PCR product. All reactions were performed in duplicate. The expression of Bdnf was normalized to the expression of Hprt1 (forward, 5′-CAGTCCCAGCGTCGTGATTA-3′, reverse, 5′-AGCAAGTCTTTCAGTCCTGTC-3′) as endogenous control using the ΔΔCt method (28).
Subcellular Fractionation and Nuclear Protein Extraction
Nuclear protein extraction was performed using a QproteomeTM nuclear protein extraction kit (Qiagen) according to the manufacturer's instructions. Cytosolic and nuclear extracts were acetone-precipitated, and equal amounts were quantified by Western blot analysis.
Immunohistochemistry
Expression of endogenous and recombinant proteins was detected by indirect immunofluorescence staining using standard protocol. In brief, hippocampal neurons were fixed at DIV 10 in 4% paraformaldehyde, permeabilized in methanol, blocked in 10% normal goat serum in 0.1% Triton X-100 in PBS, and stained with the following primary (overnight at 4 °C) and secondary antibodies (1 h at room temperature): rabbit anti-FLAG polyclonal (1:1000, Sigma), goat anti-CaMKIIα (1:250, Santa Cruz Biotechnology, SC-5391), goat anti-CaMKIIβ (1:250, Santa Cruz Biotechnology, SC-1540), goat anti-CaMKIIγ (1:250; Santa Cruz Biotechnology, SC-1541), goat anti-CaMKIIδ (Santa Cruz Biotechnology, SC-5392), rabbit anti-MeCP2 (1:500, Millipore), rabbit anti-MeCP2pS421 (1:500, a gift from Michael E. Greenberg, Harvard Medical School, Boston, MA), goat anti-rabbit-Cy3 (1:500, Dianova), goat anti-rabbit Alexa Fluor 488 (1:500, Invitrogen), or donkey anti-goat Alexa Fluor 555 (1:500, Invitrogen). Counterstain of nuclei was done with Hoechst (1:5000, Serva). Samples were mounted in Mowiol. Stained samples were analyzed using a CCD camera (Spot Insight 2; Visitron Systems, Puchheim, Germany).
Immunoblot Analysis
Standard protocols for immunoblot analysis were used to detect the expression and/or phosphorylation of the indicated proteins. Antibodies against the following proteins and/or their phosphorylated forms were used: ATF3, CaMKIIα, CaMKIIβ, CaMKIIγ, CaMKIIδ, and SP1 (Santa Cruz Biotechnology); histone H3 (Abcam); dsRED (Clontech); hrGFP (Stratagene), FLAG, and tubulin (Sigma); MeCP2 and pCREB (Upstate Biotech Millipore); CREB and pERK1/2 (Cell Signaling); and MeCP2pS421. Immunoblot analysis of endogenously expressed tubulin was used to control for protein loading.
Calcium Imaging
Hippocampal neurons were loaded with 3.4 μm Fluo-3 AM (Invitrogen, Karlsruhe, Germany) in the dark for 20 min at room temperature in buffered, CO2-independent, salt-glucose-glycine solution containing (in mm) 140.1 NaCl, 5.3 KCl, 1.0 MgCl2, 2.0 CaCl2, 10.0 Hepes (pH 7.4), 1.0 glycine, 30.0 glucose, and 0.5 sodium pyruvate. Hippocampal neurons were incubated for another 20 min in the darkness in salt-glucose-glycine. Fluo-3 signals of neurons mounted in a perfusion chamber (Life Imaging Services, Reinach, Switzerland) were measured at room temperature in salt-glucose-glycine using an inverted Leica SP2 confocal microscope with an HCX PL APO CS 40.0 × 1.25 NA oil UV objective (Leica, Wetzlar, Germany). Images were taken every 1.635 s. To calibrate the fluorescence signal (F), Fluo-3 was saturated by adding 50 μm ionomycin (Fmax) (Sigma-Aldrich Chemie GmbH, München, Germany) and then quenched with MnCl2 (Fmin). [Ca2+] was expressed as a function of the Fluo-3 fluorescence Kd × ((F − Fmin)/(Fmax − F)) (29).
Data Analysis
All data plotted in histograms represent the means ± S.E. One-way analysis of variance with Tukey's post hoc test was used for all statistical analysis.
RESULTS
Blockade of Nuclear Calcium Signaling
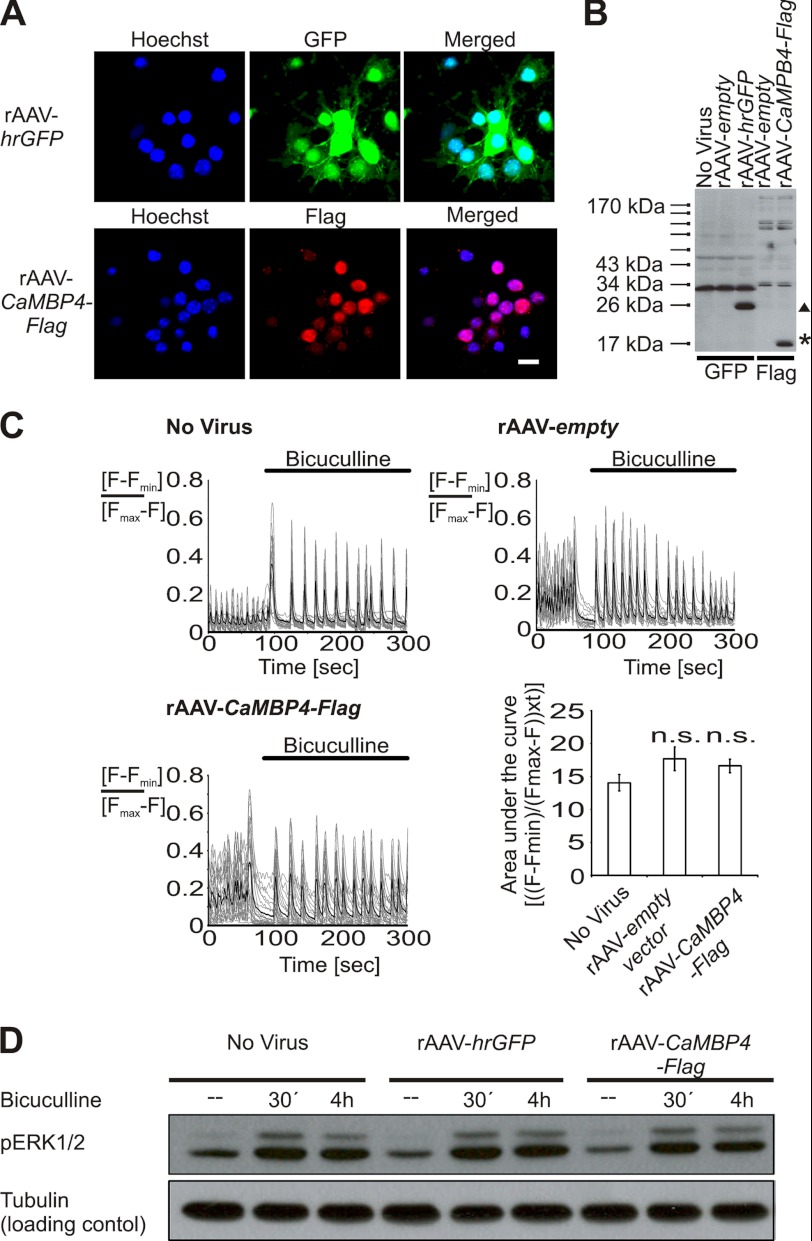
To investigate the role of nuclear calcium signaling in the regulation of MeCP2 phosphorylation on serine 421, we infected primary mouse hippocampal neurons with an rAAV containing an expression cassette for CaMBP4 (rAAV-CaMBP4-Flag). CaMBP4 is a nuclear protein that contains four repeats of the M13 calmodulin-binding peptide from the skeletal muscle myosin light chain kinase; it binds to and inactivates the nuclear calcium/CaM complex (30) and has been used previously to identify nuclear calcium-regulated genes (18, 19, 23). Expression of CaMBP4, as well as that of hrGFP after infection with rAAV-CaMBP4 and rAAV-hrGFP, was readily detectable immunocytochemically in 80–95% of the viable cells (Fig. 1A). The correct size proteins were also detected in immunoblots (Fig. 1B). Neither expression of CaMBP4 after infection with rAAV-CaMBP4-Flag nor infection with the rAAV vector alone (rAAV-empty) interfered with the ability of the neurons to generate robust intracellular calcium transients following the induction of bursts of action potential (AP) firing (Fig. 1C). To induce AP bursting, we exposed the cultures to the GABAA receptor antagonist bicuculline; this led to the removal of tonic inhibition from the network, giving rise to periodically occurring AP bursts, each of which is associated with an increase in the cytoplasmic and nuclear calcium concentration (31, 32) (Fig. 1C). Infection with rAAV-CaMBP4-Flag also did not compromise AP bursting-induced early signaling events such as the activation of ERK1/2 (25, 33), which was assessed in immunoblots using antibodies that recognize the phosphorylated, i.e. activated, forms of ERK1/2 (Fig. 1D).
FIGURE 1.
Blockade of nuclear calcium signaling. A, immunocytochemical analysis of rAAV-mediated expression of hrGFP and CaMBP4 in hippocampal neurons. Hoechst staining was used to identify nuclei. The scale bar is 20 μm. B, immunoblot analysis of rAAV-mediated expression of hrGFP (∼26 kDa; indicated by ▴) and CaMBP4-Flag (∼17 kDa; indicated by *) in hippocampal neurons. C, Fluo-3 calcium imaging (line graphs) and the corresponding quantitative analysis (bar graph) of the area under the curve of AP bursting-induced calcium transients in uninfected hippocampal neurons and in hippocampal neurons infected with the indicated rAAVs. AP bursting in hippocampal neurons was induced at the indicated time points with bicuculline (50 μm). Representative traces are shown. Measurements of individual cells are depicted in thin gray lines, and their means are shown in bold black lines. The area under the curve represents the integral of Fluo-3 signal above base line at the time of bicuculline application for a period of 200 s (n = 3). n.s., not significant. D, immunoblot analysis of ERK1/2 phosphorylation (pERK1/2) in uninfected hippocampal neurons and in hippocampal neurons infected with rAAV-hrGFP or rAAV-CaMBP4-Flag. Neurons were left unstimulated or were stimulated for 30 min or 4 h with bicuculline (50 μm). Tubulin was used as loading control. A representative of three independent experiments is shown.
Role of Nuclear Calcium in Synaptic Activity-induced Phosphorylation of MeCP2
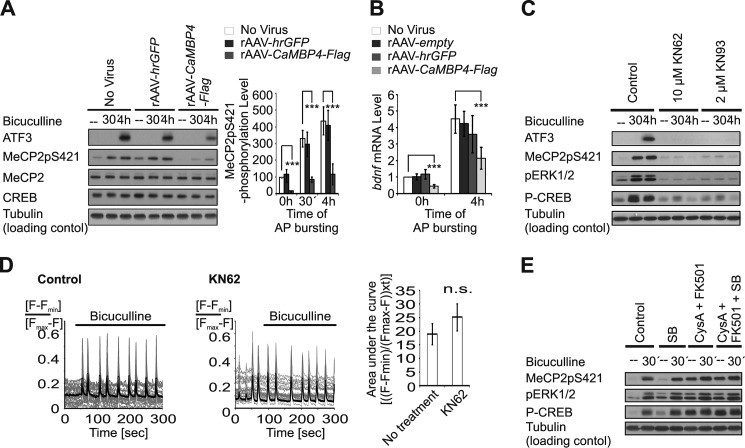
We next assessed synaptic activity-induced MeCP2 phosphorylation on serine 421 using phospho-MeCP2-specific antibodies and immunoblot analysis. We detected a robust increase in MeCP2 phosphorylation 30 min and 4 h after the induction of AP bursting in mouse hippocampal neurons using bicuculline. This phosphorylation event was blocked in neurons infected with rAAV-CaMBP4-Flag but not in neurons infected with rAAV-hrGFP. In parallel, we investigated the expression of ATF3, an activity-regulated transcriptional response known to be controlled by nuclear calcium (22), and that of Bdnf from promoter IV. The AP bursting-induced expression of both genes was sensitive to inhibition of nuclear calcium signals using CaMBP4 (Fig. 2, A and B). We detected a similar dependence on nuclear calcium signaling for synaptic activity-driven Bdnf transcription from promoter I (data not shown).
FIGURE 2.
Role of nuclear calcium in synaptic activity-induced phosphorylation of MeCP2. A, immunoblot analysis of AP bursting-induced phosphorylation of MeCP2 on serine 421 in hippocampal neurons infected with the indicated rAAVs. Expression of ATF3, MeCP2, and CREB and MeCP2 phosphorylation on serine 421 (MeCP2pS421) were analyzed in lysates of hippocampal neurons before and after induction of AP bursting with 50 μm bicuculline. Tubulin served as control. (left panel). Quantitative analysis of MeCP2pS421 level is shown in the right panel (n = 3). ***, p < 0.001. B, quantitative reverse transcription-PCR analysis of Bdnf expression from promoter IV in uninfected hippocampal neurons and in neurons infected with the indicated rAAVs. Neurons were stimulated for 4 h with 50 μm bicuculline or were left unstimulated (n = 7). C, immunoblot analysis of the effects of KN62 (10 μm) or KN93 (2 μm) on AP bursting-induced expression of ATF3 and phosphorylation of ERK1/2 (pERK1/2), CREB on serine 133 (P-CREB), and MeCP2 on serine 421 (MeCP2pS421). Hippocampal neurons pretreated for 1 h with the indicated drugs were stimulated for the indicated periods of time with bicuculline (50 μm) or were left unstimulated. D, Fluo-3 calcium imaging (line graphs) and the corresponding quantitative analysis of the area under the curve (bar graph) showing the effect of KN62 (10 μm) on AP bursting-induced calcium transients. KN62 and bicuculline were applied as in C. Representative traces are shown. Measurements of individual cells are depicted in thin gray lines, and their means are shown in bold black lines (n = 3). n.s., not significant. E, immunoblot analysis of AP bursting-induced phosphorylation of ERK1/2 (pERK1/2), CREB on serine 133 (P-CREB), and MeCP2 on serine 421 (MeCP2pS421) in hippocampal neurons with or without treatment with the indicated drugs. Pharmacological blockers were added to the cultures 1 h prior to stimulation with bicuculline (50 μm). A representative of three independent experiments is shown.
The observation that MeCP2 phosphorylation on serine 421 requires nuclear calcium signaling suggests an involvement of nuclear calcium/calmodulin-dependent protein kinases. Indeed, consistent with previously reported pharmacological experiments (8), we found that two inhibitors of CaM kinases, KN62 and KN93, blocked AP bursting-induced MeCP2 phosphorylation on serine 421 (Fig. 2C). To rule out “signal distortion” by KN62 and KN93 (i.e. disruption of AP bursting-induced calcium transients due to the known inhibitory effects of these drugs on voltage-gated calcium channels) (34, 35), we carried out calcium imaging experiments. These experiments demonstrated that in the hippocampal culture system, KN62 used at a concentration of 10 μm did not compromise AP bursting-induced calcium transients (Fig. 2D). As expected, KN62 and KN93 blocked the increase in ATF3 expression following AP bursting as well as the increase in ERK1/2 phosphorylation, which in mouse neurons is activated by synaptic activity via a CaM kinase-dependent process (Fig. 2C) (36). We found no pharmacological evidence for a critical involvement of other calcium signal-regulated pathways such as the p38 MAP kinase pathway or the calcineurin pathway in the induction of MeCP2 phosphorylation on serine 421. Blockade of p38 MAP kinase or calcineurin using SB203580 or cyclosporine A plus FK506, respectively, had no effect on MeCP2 phosphorylation on serine 421 (Fig. 2E). However, cyclosporine A plus FK506 did increase MeCP2 phosphorylation on serine 421 under basal conditions and led to a superinduction of this phosphorylation event after AP bursting (Fig. 2E). These findings suggest that a nuclear CaM kinase is responsible for the synaptic activity-induced increase in MeCP2 phosphorylation on serine 421 and that calcineurin plays a role in the dephosphorylation of MeCP2 on this site.
Role of Nuclear CaMKII in MeCP2 Phosphorylation
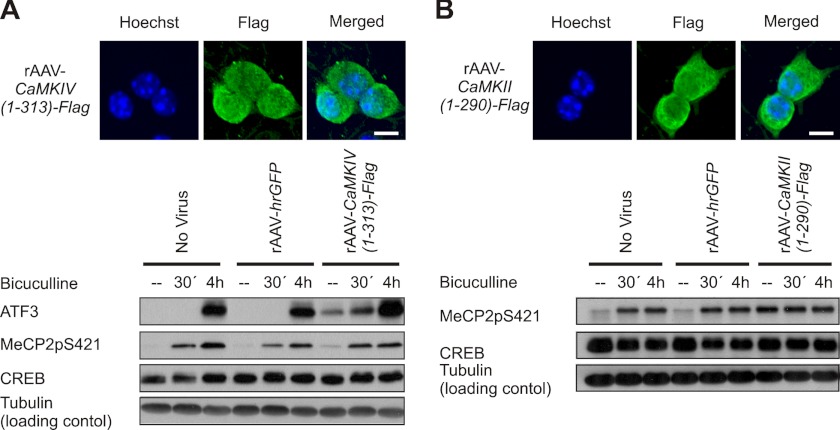
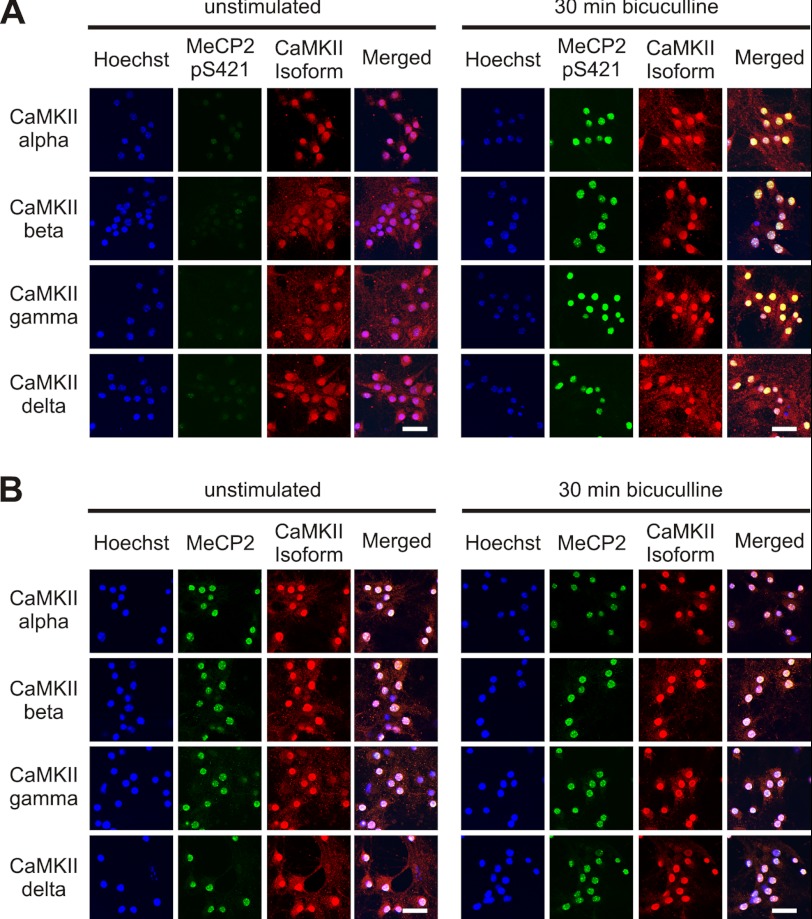
One of the most prominent nuclear CaM kinases is CaMKIV, which mediates as part of the nuclear calcium-CaMKIV-CREB/CBP pathway the regulation of many genes in the nervous system (20, 31, 37). However, it has been reported that CaMKIV is not involved in the phosphorylation of MeCP2 phosphorylation on serine 421 (8). We can confirm that the expression of a constitutively active form of CaMKIV using rAAV-CaMKIV(1–313)-Flag does not lead to an increase of MeCP2 phosphorylation on serine 421 in hippocampal neurons, although it does, as has been reported previously (22), increase ATF3 expression (Fig. 3A). We therefore considered the possibility that another CaM kinase mediates the effects of synaptic activity on MeCP2 phosphorylation on serine 421. CaMKII has been suggested to play a role in this phosphorylation event (8), and indeed, we found that expression of a constitutively active form of CaMKII using rAAV-CaMKII(1–290)-Flag can lead to an increase of MeCP2 phosphorylation on serine 421 in hippocampal neurons (Fig. 3B). However, CaMKII is generally considered a cytosolic enzyme, although in some cell types, including cerebellar granule cells, astrocytes, and heart muscle, a nuclear localization of certain CaMKII isoforms has been reported (38–41). To investigate the localization of CaMKII in hippocampal neurons, we carried out immunostainings as well as subcellular fractionations followed by immunoblotting using antibodies to the CaMKIIα, -β, -γ, and -δ isoforms. We found that in hippocampal neurons, all isoforms of CaMKII analyzed were readily detected in the cytosol but that a significant fraction was localized also to the cell nucleus (Figs. 4 and 5). MeCP2 and the serine 421 phosphorylated form of MeCP2 were analyzed in parallel and were found virtually exclusively localized to the cell nucleus (Figs. 4 and 5). Neither MeCP2, the serine 421 phosphorylated form of MeCP2, nor any of the CaMKII isoforms analyzed underwent a detectable nucleocytoplasmic redistribution within the first 30 min of induction of action potential bursting using bicuculline treatment (Figs. 4 and 5).
FIGURE 3.
Role of CaMKII in MeCP2 phosphorylation. A, immunofluorescence analysis (upper panel) and immunoblot analysis (lower panel) of rAAV-mediated expression of recombinant CaMKIV(1–313)-Flag in hippocampal neurons and assessment of its effect on AP bursting-induced expression of CREB and ATF3 and MeCP2 phosphorylation on serine 421 (MeCP2pS421). CaMKIV(1–313)-Flag was detected with anti-FLAG antibody. Hoechst staining was used to identify nuclei. The scale bar is 10 μm (upper panel). Tubulin was used as loading control in the immunoblot analysis (n = 3) (lower panel). B, immunofluorescence analysis (upper panel) and immunoblot analysis (lower panel) of rAAV-mediated expression of recombinant CaMKII(1–290)-Flag expression in hippocampal neurons and assessment of its effect on AP bursting-induced expression of CREB and MeCP2 phosphorylation on serine 421 (MeCP2pS421). Hoechst staining was used to identify nuclei. The scale bar is 10 μm. Representative immunoblots from n = 3 experiments are shown.
FIGURE 4.
Localization of CaMKII in hippocampal neurons. A, localization of MeCP2, MeCP2pS421, and the different CaMKII isoforms in the hippocampal neurons. A and B, immunocytochemical analysis of MeCP2pS421 (A), MeCP2 (B), and the α, β, γ, and δ CaMKII isoforms (A and B) in hippocampal neurons before and 30 min after induction of AP bursting using bicuculline (50 μm). Hoechst staining was used to identify nuclei. The scale bar is 20 μm.
FIGURE 5.
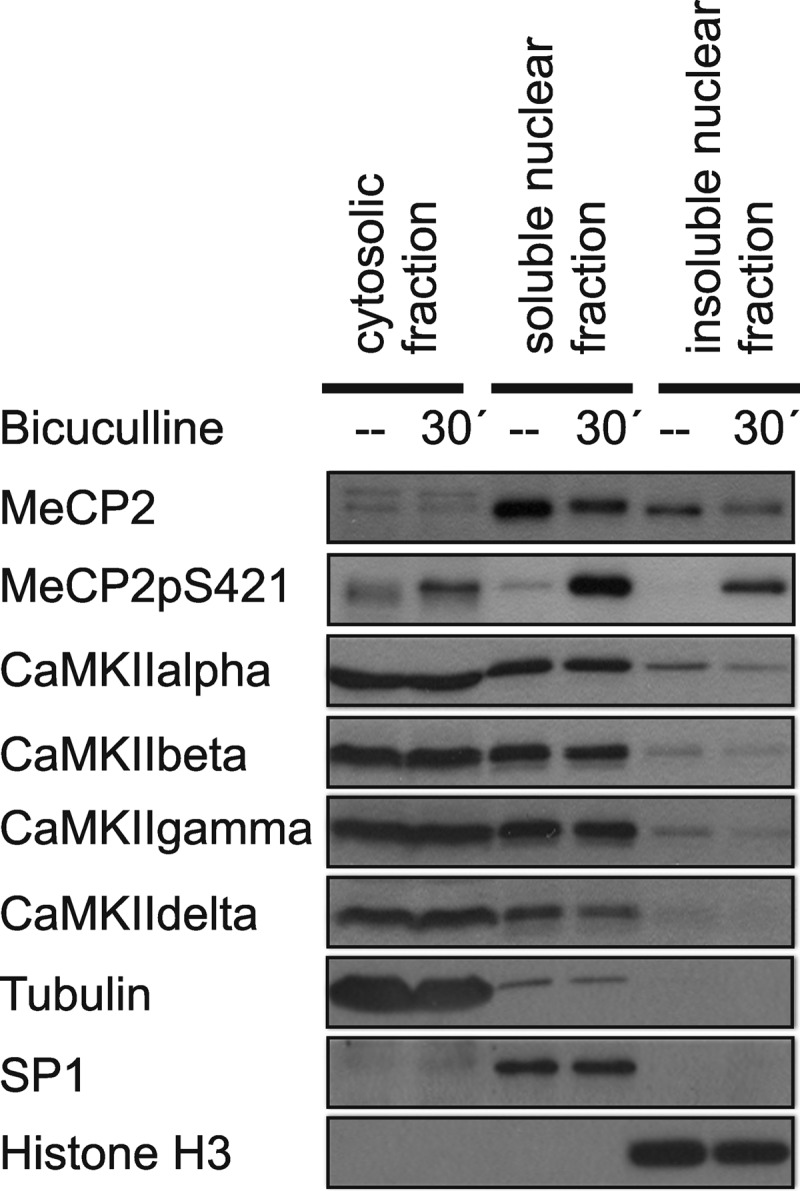
Analysis of the subcellular localization of CaMKII isoforms. Immunoblot analysis of MeCP2, MeCP2pS421, and the α, β, γ, and δ isoforms of CaMKII in the cytosolic and in the soluble and insoluble nuclear fractions obtained from unstimulated hippocampal neurons and neurons 30 min after induction of AP bursting with 50 μm bicuculline. Tubulin, SP1, and histone H3 were used as controls for the different fractions of the cell lysates. A representative of three independent experiments is shown.
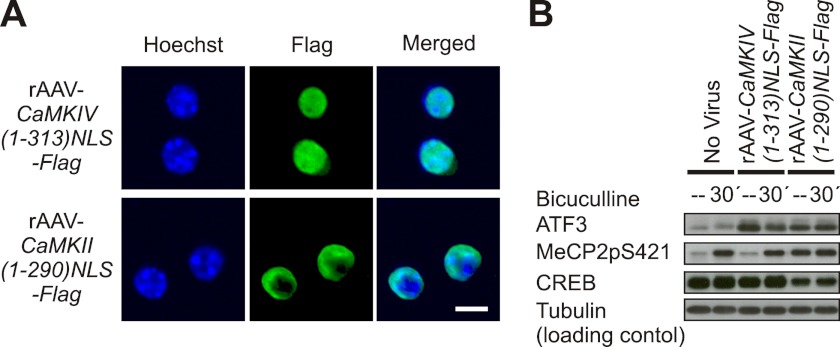
To finally determine whether nuclear CaMKII is sufficient for causing MeCP2 phosphorylation on serine 421, we infected hippocampal neurons with rAAV-CaMKII(1–290)NLS-Flag to express a nuclear targeted, constitutively active form of CaMKII. We found that indeed, a constitutively active form of CaMKII localized to the cell nucleus led to an increase of MeCP2 phosphorylation on serine 421 (Fig. 6). Under the conditions used, it also caused an increase in ATF3 expression and in expression of Bdnf from promoter I but not from promoter IV (Fig. 6; data not shown). In contrast, the expression of a nuclear targeted, constitutively active form of CaMKIV using rAAV-CaMKIV(1–313)NLS-Flag, which similar to infection with rAAV-CaMKII(1–290)NLS-Flag led to an increase in ATF3 expression, failed to cause phosphorylation of MeCP2 on serine 421 (Fig. 6).
FIGURE 6.
A constitutively active form of nuclear CaMKII is sufficient to cause phosphorylation of MeCP2 on serine 421. A, immunofluorescence analysis of rAAV-mediated expression of CaMKIV(1–313)NLS-Flag and CaMKII(1–290)NLS-Flag. Hoechst was used to identify nuclei. The scale bar is 10 μm. B, immunoblot analysis of MeCP2 phosphorylation on serine 421 (MeCP2pS421) and the expression of CREB and ATF3 in hippocampal neurons infected with the indicated rAAVs before and 30 min after induction of AP bursting using 50 μm bicuculline. Tubulin served as loading control. A representative immunoblot from n = 3 experiments is shown.
DISCUSSION
This study revealed that synaptic activity-induced phosphorylation of MeCP2 on serine 421 requires nuclear calcium signaling. We established that significant fractions of all endogenously expressed isoforms of CaMKII tested are localized to the cell nucleus of hippocampal neurons. These results together with the observation that the expression of a constitutively active, nuclear form of CaMKII is sufficient for causing MeCP2 phosphorylation on serine 421, whereas the expression of constitutively active forms of CaMKIV are not, indicates that nuclear calcium stimulating nuclear CaMKII regulates MeCP2 function. Thus, in addition to the classical nuclear calcium-CaMKIV-CREB/CBP pathway, nuclear calcium appears to also function as a global regulator of chromatin structure by acting via nuclear CaMKII and MeCP2. Precisely how the nuclear calcium-regulated phosphorylation of MeCP2 on serine 421 affects MeCP2 function is unclear, although it has been suggested that it may cause a relief of a genome-wide repressive chromatin state maintained by MeCP2 (10). This type of chromatin remodeling may be required for full transcriptional responses mediated by nuclear calcium-CaMKIV-CREB/CBP as well as by other, nuclear calcium-independent signaling pathways such as the ERK-MAP kinase cascade. Thus, the ability of nuclear calcium signaling to impact on bulk chromatin may facilitate concurrent signal-regulated transcription of specific target genes. In addition, together with other epigenetic modifiers of chromatin structure such as de novo DNA methyltransferases, histone acetyl transferases, and histone deacetylases, whose expression or function is also regulated by synaptic activity and nuclear calcium signaling (42–46), nuclear CaMKII regulation of MeCP2 may lead to long-lasting genomic adaptations that could affect the efficacy of subsequent stimuli to induce transcriptional responses.
In light of the critical role of MeCP2 in synaptogenesis and neural circuit development (6, 7, 9, 16, 17, 47, 48), our findings indicate that synaptic activity-driven processes that shape synaptic connectivity via MeCP2 require calcium transients to invade the cell nucleus. Nuclear calcium, which is known to control spine density as well as length and arborization of dendrite (23), may therefore also be a key signal that instructs, through the induction of genomic responses, the formation of appropriately connected networks needed for the development of normal cognitive abilities.
Acknowledgments
We are very grateful to Michael E. Greenberg for providing the antibody against the serine 421 phosphorylated form of MeCP2. We thank I. Bünzli-Ehret for help with the preparation of hippocampal cultures.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), the Alexander von Humboldt Foundation (Wolfgang Paul Prize to H. B.), an European Research Council Advanced Grant (to H. B.), the Sonderforschungsbereich (SFB) 488 and SFB 636 of the DFG, and the Graduate Academy (Excellence Initiative) of Heidelberg University.
- MeCP
- methyl-CpG-binding protein
- CREB
- cAMP-response element-binding protein
- CBP
- CREB-binding protein
- CaM
- calcium/calmodulin-dependent protein
- CaMK
- CaM kinase
- rAAV
- recombinant adeno-associated virus
- hrGFP
- humanized R. reniformis green fluorescent protein
- NLS
- nuclear localization signal
- DIV
- day in vitro
- AP
- action potential.
REFERENCES
- 1. Hendrich B., Bird A. (1998) Identification and characterization of a family of mammalian methyl-CpG-binding proteins. Mol. Cell Biol. 18, 6538–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuks F., Hurd P. J., Wolf D., Nan X., Bird A. P., Kouzarides T. (2003) The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 278, 4035–4040 [DOI] [PubMed] [Google Scholar]
- 3. Chahrour M., Jung S. Y., Shaw C., Zhou X., Wong S. T., Qin J., Zoghbi H. Y. (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320, 1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen W. G., Chang Q., Lin Y., Meissner A., West A. E., Griffith E. C., Jaenisch R., Greenberg M. E. (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 [DOI] [PubMed] [Google Scholar]
- 5. Shahbazian M. D., Antalffy B., Armstrong D. L., Zoghbi H. Y. (2002) Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 11, 115–124 [DOI] [PubMed] [Google Scholar]
- 6. Alvarez-Saavedra M., Carrasco L., Sura-Trueba S., Demarchi Aiello V., Walz K., Neto J. X., Young J. I. (2010) Elevated expression of MeCP2 in cardiac and skeletal tissues is detrimental for normal development. Hum. Mol. Genet. 19, 2177–2190 [DOI] [PubMed] [Google Scholar]
- 7. Guy J., Cheval H., Selfridge J., Bird A. (2011) The role of MeCP2 in the brain. Annu. Rev. Cell Dev. Biol. 27, 631–652 [DOI] [PubMed] [Google Scholar]
- 8. Zhou Z., Hong E. J., Cohen S., Zhao W. N., Ho H. Y., Schmidt L., Chen W. G., Lin Y., Savner E., Griffith E. C., Hu L., Steen J. A., Weitz C. J., Greenberg M. E. (2006) Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52, 255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen S., Gabel H. W., Hemberg M., Hutchinson A. N., Sadacca L. A., Ebert D. H., Harmin D. A., Greenberg R. S., Verdine V. K., Zhou Z., Wetsel W. C., West A. E., Greenberg M. E. (2011) Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron 72, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skene P. J., Illingworth R. S., Webb S., Kerr A. R., James K. D., Turner D. J., Andrews R., Bird A. P. (2010) Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell 37, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzales M. L., LaSalle J. M. (2010) The role of MeCP2 in brain development and neurodevelopmental disorders. Curr. Psychiatry Rep. 12, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagberg B., Aicardi J., Dias K., Ramos O. (1983) A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett syndrome: report of 35 cases. Ann. Neurol 14, 471–479 [DOI] [PubMed] [Google Scholar]
- 13. Bienvenu T., Chelly J. (2006) Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat. Rev. Genet. 7, 415–426 [DOI] [PubMed] [Google Scholar]
- 14. Chahrour M., Zoghbi H. Y. (2007) The story of Rett syndrome: from clinic to neurobiology. Neuron 56, 422–437 [DOI] [PubMed] [Google Scholar]
- 15. Chen R. Z., Akbarian S., Tudor M., Jaenisch R. (2001) Deficiency of methyl-CpG-binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 27, 327–331 [DOI] [PubMed] [Google Scholar]
- 16. West A. E., Greenberg M. E. (2011) Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb. Perspect. Biol. 3, a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H., Zhong X., Chau K. F., Williams E. C., Chang Q. (2011) Loss of activity-induced phosphorylation of MeCP2 enhances synaptogenesis, LTP, and spatial memory. Nat. Neurosci. 14, 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang S. J., Steijaert M. N., Lau D., Schütz G., Delucinge-Vivier C., Descombes P., Bading H. (2007) Decoding NMDA receptor signaling: identification of genomic programs specifying neuronal survival and death. Neuron 53, 549–562 [DOI] [PubMed] [Google Scholar]
- 19. Zhang S. J., Zou M., Lu L., Lau D., Ditzel D. A., Delucinge-Vivier C., Aso Y., Descombes P., Bading H. (2009) Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 5, e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardingham G. E., Chawla S., Johnson C. M., Bading H. (1997) Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385, 260–265 [DOI] [PubMed] [Google Scholar]
- 21. Limbäck-Stokin K., Korzus E., Nagaoka-Yasuda R., Mayford M. (2004) Nuclear calcium/calmodulin regulates memory consolidation. J. Neurosci. 24, 10858–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang S. J., Buchthal B., Lau D., Hayer S., Dick O., Schwaninger M., Veltkamp R., Zou M., Weiss U., Bading H. (2011) A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J. Neurosci. 31, 4978–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mauceri D., Freitag H. E., Oliveira A. M., Bengtson C. P., Bading H. (2011) Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron 71, 117–130 [DOI] [PubMed] [Google Scholar]
- 24. Hardingham G. E., Bading H. (2010) Synaptic versus extrasynaptic NMDA receptor signaling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bading H., Greenberg M. E. (1991) Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science 253, 912–914 [DOI] [PubMed] [Google Scholar]
- 26. Bading H., Ginty D. D., Greenberg M. E. (1993) Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260, 181–186 [DOI] [PubMed] [Google Scholar]
- 27. Bading H., Segal M. M., Sucher N. J., Dudek H., Lipton S. A., Greenberg M. E. (1995) N-Methyl-d-aspartate receptors are critical for mediating the effects of glutamate on intracellular calcium concentration and immediate early gene expression in cultured hippocampal neurons. Neuroscience 64, 653–664 [DOI] [PubMed] [Google Scholar]
- 28. Kairisalo M., Korhonen L., Sepp M., Pruunsild P., Kukkonen J. P., Kivinen J., Timmusk T., Blomgren K., Lindholm D. (2009) NF-κB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 30, 958–966 [DOI] [PubMed] [Google Scholar]
- 29. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 30. Wang J., Campos B., Jamieson G. A., Jr., Kaetzel M. A., Dedman J. R. (1995) Functional elimination of calmodulin within the nucleus by targeted expression of an inhibitor peptide. J. Biol. Chem. 270, 30245–30248 [DOI] [PubMed] [Google Scholar]
- 31. Hardingham G. E., Arnold F. J., Bading H. (2001) Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 4, 261–267 [DOI] [PubMed] [Google Scholar]
- 32. Arnold F. J., Hofmann F., Bengtson C. P., Wittmann M., Vanhoutte P., Bading H. (2005) Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity. J. Physiol. 564, 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardingham G. E., Arnold F. J., Bading H. (2001) A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat. Neurosci. 4, 565–566 [DOI] [PubMed] [Google Scholar]
- 34. Sihra T. S., Pearson H. A. (1995) Ca/calmodulin-dependent kinase II inhibitor KN62 attenuates glutamate release by inhibiting voltage-dependent Ca2+ channels. Neuropharmacology 34, 731–741 [DOI] [PubMed] [Google Scholar]
- 35. Gao L., Blair L. A., Marshall J. (2006) CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem. Biophys. Res. Commun. 345, 1606–1610 [DOI] [PubMed] [Google Scholar]
- 36. Vanhoutte P., Barnier J. V., Guibert B., Pagès C., Besson M. J., Hipskind R. A., Caboche J. (1999) Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol. Cell Biol. 19, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chow F. A., Anderson K. A., Noeldner P. K., Means A. R. (2005) The autonomous activity of calcium/calmodulin-dependent protein kinase IV is required for its role in transcription. J. Biol. Chem. 280, 20530–20538 [DOI] [PubMed] [Google Scholar]
- 38. Mishra S., Gray C. B., Miyamoto S., Bers D. M., Brown J. H. (2011) Location matters: clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ. Res. 109, 1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang T., Kohlhaas M., Backs J., Mishra S., Phillips W., Dybkova N., Chang S., Ling H., Bers D. M., Maier L. S., Olson E. N., Brown J. H. (2007) CaMKIIδ isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J. Biol. Chem. 282, 35078–35087 [DOI] [PubMed] [Google Scholar]
- 40. Takeuchi Y., Yamamoto H., Matsumoto K., Kimura T., Katsuragi S., Miyakawa T., Miyamoto E. (1999) Nuclear localization of the δ subunit of Ca2+/calmodulin-dependent protein kinase II in rat cerebellar granule cells. J. Neurochem. 72, 815–825 [DOI] [PubMed] [Google Scholar]
- 41. Takeuchi Y., Yamamoto H., Fukunaga K., Miyakawa T., Miyamoto E. (2000) Identification of the isoforms of Ca2+/calmodulin-dependent protein kinase II in rat astrocytes and their subcellular localization. J. Neurochem. 74, 2557–2567 [DOI] [PubMed] [Google Scholar]
- 42. Chawla S., Hardingham G. E., Quinn D. R., Bading H. (1998) CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 281, 1505–1509 [DOI] [PubMed] [Google Scholar]
- 43. Chawla S., Vanhoutte P., Arnold F. J., Huang C. L., Bading H. (2003) Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 85, 151–159 [DOI] [PubMed] [Google Scholar]
- 44. Miller C. A., Sweatt J. D. (2007) Covalent modification of DNA regulates memory formation. Neuron 53, 857–869 [DOI] [PubMed] [Google Scholar]
- 45. Guo J. U., Ma D. K., Mo H., Ball M. P., Jang M. H., Bonaguidi M. A., Balazer J. A., Eaves H. L., Xie B., Ford E., Zhang K., Ming G. L., Gao Y., Song H. (2011) Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat. Neurosci. 14, 1345–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira A. M., Hemstedt T. J., Bading H. (2012) Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat. Neurosci. 15, 1111–1113 [DOI] [PubMed] [Google Scholar]
- 47. Smrt R. D., Eaves-Egenes J., Barkho B. Z., Santistevan N. J., Zhao C., Aimone J. B., Gage F. H., Zhao X. (2007) Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 27, 77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deng J. V., Rodriguiz R. M., Hutchinson A. N., Kim I. H., Wetsel W. C., West A. E. (2010) MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat. Neurosci. 13, 1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]