Background: The cascade of reactions and proteins conferring regulated exocytosis needs to be characterized.
Results: Synaptotagmin 1 is a primary vesicle-docking factor, and Munc18-1 accelerates subsequent v-/t-SNARE assembly/zippering.
Conclusion: Synaptotagmin 1, PI(4,5)P2, complexin II, and Munc18-1 function in a sequential and concerted manner to mediate vesicle docking, SNAREpin assembly, and fast Ca2+-triggered exocytosis.
Significance: Efficient Ca2+-regulated membrane fusion was reconstituted from a minimal set of components.
Keywords: Exocytosis, Inositol Phospholipid, Membrane Reconstitution, Snare, Synaptotagmin, Synaptic Vesicle
Abstract
Regulated exocytosis requires the general membrane fusion machinery–soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and Sec1/Munc18 (SM) proteins. Using reconstituted giant unilamellar vesicles containing preassembled t-SNARE proteins (syntaxin 1·SNAP-25), we determined how Munc18-1 controls the docking, priming, and fusion of small unilamellar vesicles containing the v-SNARE VAMP2 and the Ca2+ sensor synaptotagmin 1. In vitro assays allowed us to position Munc18-1 in the center of a sequential reaction cascade; vesicle docking by synaptotagmin 1 is a prerequisite for Munc18-1 to accelerate trans-SNARE complex (SNAREpin) assembly and membrane fusion. Complexin II stalls SNAREpin zippering at a late stage and, hence, contributes to synchronize membrane fusion in a Ca2+- and synaptotagmin 1-dependent manner. Thus, at the neuronal synapse, the priming factor Munc18-1 may accelerate the conversion of docked synaptic vesicles into a readily releasable pool by activating SNAREs for efficient membrane fusion.
Introduction
In the central nervous system synaptic vesicles are morphologically docked at the active zone and this docked pool of vesicles largely coincides with the readily releasable pool, which can fuse within less than a millisecond after sensing a local increase of the Ca2+ concentration (1, 2). Although numerous components and a series of reactions are involved in the initial vesicle tethering and priming steps, the number of proteins and lipids required for the final docking step and the subsequent fusion reaction are limited by the refined reaction mechanism and spatial constraints (3–5). Evidence has accumulated that a minimal set of six proteins might be functionally required at these last reaction steps. VAMP2, a v-SNARE2 on synaptic vesicles, forms a trans-SNARE complex, SNAREpin, with its cognate t-SNARE consisting of syntaxin 1 and SNAP-25 on the presynaptic plasma membrane, thereby bridging the two lipid bilayers (6). SNAREpin formation starts at the N-terminal membrane-distal end and progresses toward the C-terminal membrane-proximal end of the heptad-repeat-containing SNARE motifs, finally resulting in the formation of a four-helix bundle that extends through the membrane and, thus, also includes the membrane-spanning regions of VAMP2 and syntaxin 1 (7–10). Indeed, SNAREpin assembly is sufficient to drive membrane fusion in reconstituted assays (11–13). However, three late acting regulatory proteins, Munc18-1, synaptotagmin 1 (Syt1), and complexin (Cpx), control the formation of SNAREpins and the subsequent SNAREpin-zippering reaction (3–5, 14–17).
Synaptotagmins and complexins confer Ca2+ regulation to the general SNARE fusion machinery. Cpx plays a dual role in membrane fusion and arrests SNAREpins at a distinct assembly state (18). It binds via its central α-helix to the N-terminal membrane-distal region of the VAMP2 and syntaxin 1 SNARE motif in the SNAREpin, thereby likely stabilizing the initial v-/t-SNARE interactions (19–21). Simultaneously, its N-terminal accessory helix binds in a competitive mode with VAMP2, the C-terminal membrane-proximal part of a t-SNARE located in a second SNAREpin (21, 22). Thus, Cpx stabilizes partially assembled SNAREpins and blocks further SNAREpin zippering.
To release this block and to couple the reaction to a Ca2+ signal, Syt1, which is anchored via its N-terminal transmembrane domain in the vesicular membrane, binds with its two C2 domain anionic phospholipids in a Ca2+-dependent manner. These Ca2+-dependent lipid interactions locally perturb the membrane structure and together with Ca2+-dependent SNARE interactions likely release the Cpx clamp resulting in membrane fusion (23, 24). In addition to the classical Ca2+ binding loops in the C2 domains, the C2B domain also contains a polybasic amino acid cluster that confers Ca2+-independent interactions with the t-SNARE and PI(4,5)P2, which itself is known to be enriched at active zones and functions at several steps of the synaptic vesicle cycle (25). These Ca2+-independent interactions have been implicated in vesicle docking in vivo and in vitro (26–29).
Munc18-1, a SM protein, is essential for regulated exocytosis and has several functions in SNAREpin assembly (30–35). It stabilizes syntaxin 1, contributes to the transport of syntaxin 1 from the ER to the plasma membrane, and keeps syntaxin 1 in a closed conformation that blocks SNAP-25 binding and t-SNARE assembly (36–45). The release of this inhibition requires regulatory proteins such as Munc13 and the specific binding of Munc18-1 to VAMP2 or VAMP3 (46–50). The resulting syntaxin 1·SNAP-25 complex then provides a binding site for Syt1, conferring vesicle docking (27–29, 51). Thus, the dramatic decrease of vesicle docking observed in Munc18-1-deficient cells might be the result of impaired t-SNARE complex assembly (27, 32, 52–54). Indeed, the overexpression of SNAP-25 can rescue the docking phenotype in Munc18 knock-out cells (27). However, the cells overexpressing SNAP-25 are still exocytosis-deficient, indicating a post-docking role of Munc18-1 (27, 55). Interestingly, reconstituted liposome assays demonstrated that Munc18-1 stimulates specific vesicle docking and membrane fusion when preassembled t-SNAREs were reconstituted into liposomes (50, 54, 56). However, to observe the fusion stimulation in these reconstituted assays, Munc18-1 needed to be simultaneously preincubated with v- and t-SNARE liposomes at low temperature for an extended time period, indicating that another factor is required to prime the SNAREs for this Munc18-1 function. Thus, the mechanism of how Munc18-1 functions at this step still remains elusive.
In summary, each of the three regulatory proteins (Munc18-1, Syt1, Cpx) has been reported to mediate vesicle docking and to contribute indirectly or directly to SNAREpin formation, stability, and zippering. To assign Munc18-1 a role in this intricate protein network and to determine the sequence of events and synergistic functions, we have employed a reconstituted assay using purified components, small unilamellar vesicles (SUVs), and giant unilamellar vesicles (GUVs) mimicking synaptic vesicles and the flat presynaptic plasma membrane, respectively. First, we directly compared the vesicle docking efficiency provided by each individual regulatory component and combinations thereof establishing a binding hierarchy. Second, we determined which binding partners (PI(4,5)P2, t-SNARE, v-SNARE) have to interact with the regulatory components to confer vesicle docking. Third, the regulatory components were analyzed individually and in combination in a reconstituted lipid-mixing assay. Based on these analyses a defined sequence of events emerges. Briefly, Syt1 appears to be the predominant vesicle-docking factor. Its vesicle docking activity generates a reaction intermediate for Munc18-1 activity, which shifts SNAREpins and docked vesicles into a highly reactive state. CpxII together with Syt1 suppresses the Munc18-1 stimulation and renders the reaction Ca2+-sensitive. Thus, in the simplest model Munc18-1, Syt1, CpxII, and the neuronal SNAREs would be the basic machinery generating a readily releasable pool of docked vesicles, which immediately responds to a Ca2+ signal by membrane fusion.
EXPERIMENTAL PROCEDURES
Protein Purification
Recombinant mammalian His6-tagged proteins were expressed in Escherichia coli BL21(DE3) bacteria (Stratagene). Protein purifications for VAMP2, Syt1, syntaxin 1·SNAP-25, and CpxII were performed exactly as described previously via nickel-nitrilotriacetic acid affinity chromatography and subsequent ion exchange chromatography (13, 57).
Munc18-1 Purification
Recombinant rat Munc18-1 encoded in the pEG(KG) vector (a kind gift of Dr. Richard Scheller) was transformed into BL21(DE3) bacteria (Stratagene). Bacterial cultures in 4 liters of LB medium containing 100 μg/ml ampicillin were grown at 37 °C to an optical density of 0.6 (at 600 nm). Protein expression was induced overnight at 16 °C by 1 mm isopropyl-β-d-thiogalactopyranoside. The following day bacteria were collected by centrifugation and washed once with breaking buffer (25 mm HEPES-KOH, pH 7.4, 150 mm KCl, 10% glycerol). After another round of centrifugation, bacteria were resuspended in a final volume of 100 ml of breaking buffer and snap-frozen for storage at −80 °C. After thawing the bacterial suspension, β-mercaptoethanol was added to a final concentration of 3 mm as well as a protease inhibitor mixture (final concentrations: leupeptin (1.5 μg/ml), antipain (2.5 μg/ml), turkey trypsin inhibitor (25 μg/ml), benzamidine (12.5 μg/ml), Pefabloc SC (6.25 μg/ml), aprotinin (1.25 μg/ml), chymostatin (5 μg/ml), and pepstatin (2.5 μg/ml)). For lysis, bacteria were passed through a Microfluidizer M110L (Microfluidics) at >10,000 p.s.i. A final concentration of 50 units/ml Benzonase (Merck) as well as 1 mm MgCl2 were added to the bacterial lysate followed by a 10-min incubation step. Subsequently, insoluble material was removed by ultracentrifugation for 1 h at 40,000 rpm at 4 °C in a 45Ti rotor (Beckman Coulter). 100 ml of the supernatant containing GST-tagged Munc18-1 were incubated with 2 ml of glutathione beads (GE Healthcare) for 1 h at 4 °C. Beads were washed 3× with 13 ml of breaking buffer followed by washing 3× with 13 ml of washing buffer (25 mm HEPES-KOH, pH 7.4, 500 mm KCl, 5% glycerol, 3 mm β-mercaptoethanol). Beads were resuspended in 2 ml of cleavage buffer (25 mm HEPES-KOH, pH 7.4, 150 mm KCl, 5% glycerol, 3 mm β-mercaptoethanol), and Munc18-1 was cleaved off the GST tag by the addition of thrombin at a final concentration of 50 units/ml for 1 h at room temperature. Munc18-1 eluate was collected with Biospin disposable chromatography columns (Bio-Rad). 50 μl of benzamidin-Sepharose 6B (GE Healthcare) (washed 3× with H2O and 3× with cleavage buffer) and 80 μl of Pefabloc SC (5 mm final concentration) were added for 1 h at room temperature. Beads were removed by centrifugation for 3 min at 200 × g and 4 °C. Protein aggregates were removed by another centrifugation step for 10 min at 55,000 rpm in a TLA55 rotor (Beckman Coulter), and the supernatant was desalted into fusion buffer (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 1 mm DTT) using a PD10 column (GE Healthcare). Finally, Munc18-1 aliquots were snap-frozen. The concentration of purified Munc18-1 was determined by SDS-PAGE and Coomassie Blue staining using BSA as standard protein and ImageJ software (NIH) for quantification.
Soluble t-SNARE Purification
Mouse SNAP-25 encoded in the pET-15b vector and rat syntaxin 1A amino acids 1–262 in the pET-24(a) vector were co-transformed into BL21(DE3) bacteria (Stratagene). Bacterial cultures in 22 liters of LB medium containing 100 μg/ml ampicillin and 100 μg/ml kanamycin were grown at 37 °C to an optical density of 0.6 (at 600 nm). Protein expression was induced by 1 mm isopropyl-β-d-thiogalactopyranoside for 3 h at 37 °C. Bacteria were collected by centrifugation and washed once with PBS. Cell pellets were resuspended in a final volume of 300 ml of breaking buffer (50 mm HEPES-KOH, pH 7.4, 100 mm KCl, 10% glycerol) and snap-frozen for storage at −80 °C. After thawing, β-mercaptoethanol was added to a final concentration of 3 mm as well as a protease inhibitor mixture described above. The further preparation of the bacterial lysate is outlined above in the section “Munc18-1 purification.” The resulting lysate was incubated with 6 ml of nickel-nitrilotriacetic acid beads (Qiagen) for 1.5 h at 4 °C on a rotating wheel. Beads were loaded into a column and washed with wash buffer (25 mm HEPES-KOH, pH 7.4, 400 mm KCl, 10% glycerol, 3 mm β-mercaptoethanol) followed by buffer A (25 mm HEPES-KOH, pH 7.4, 200 mm KCl, 10% glycerol, 3 mm β-mercaptoethanol, 50 mm imidazole). His-tagged SNAP-25·syntaxin 1A amino acid 1–262 complexes were eluted with buffer B (25 mm HEPES-KOH, pH 7.4, 200 mm KCl, 10% glycerol, 3 mm β-mercaptoethanol, 500 mm imidazole). Protein fractions were pooled and desalted into fusion buffer, and the protein concentration was determined as described above. Finally, SNAP-25·syntaxin 1A amino acid 1–262 complexes were snap-frozen.
Munc18-1 Binding Assay
For the binding assay, 100 ml of clarified bacterial lysate containing GST-Munc18-1 was incubated with 2 ml of glutathione beads (GE Healthcare). After 3× washing with 13 ml of breaking buffer and 13 ml of washing buffer, 40 μl of beads were incubated with 140 μg of VAMP2 or VAMP2 fragments generated by botulinum neurotoxin D (BoNT/D) cleavage (0.07 mg/ml BoNT/D, 1 h at 37 °C) in fusion buffer containing 1% n-octyl-beta-d-glucopyranoside in a final volume of 100 μl for 1.5 h at 4 °C (thus GST-Munc18-1 beads were incubated with a 25-fold molar excess of His6-tagged VAMP2). Beads were washed 3× with 1 ml of fusion buffer containing 1% n-octyl-beta-d-glucopyranoside. Finally, 20 μl of Laemmli buffer were added, and beads were boiled at 98 °C for 3 min. As a control, purified GST was bound to GSH beads, and 40 μl of beads were incubated with 140 μg of His-tagged VAMP2 or BoNT/D-fragmented His-tagged VAMP2. Beads were washed 3× with 1 ml of fusion buffer containing 1% n-octyl-beta-d-glucopyranoside. Finally, 100 μl of Laemmli buffer was added, and beads were boiled at 98 °C for 3 min. SDS-PAGE was performed with 10 μl of each binding reaction.
Proteins were made visible by using the silver-staining method. First, gels were fixed in 30% methanol and 10% acetic acid for 1 h followed by 2 washes in 10% ethanol for 15 min each. After treatment with 0.02% Na2S2O3 for 1 min, gels were rinsed with H2O and incubated with staining solution (2 mg/ml AgNO3, 0.037% formaldehyde) for 15 min followed by rinsing with H2O. Subsequently, developing solution (60 mg/ml Na2CO3, 0.0185% formaldehyde, 0.0002% Na2S2O3) was applied until protein bands appeared. Finally, gels were fixed in 7% acetic acid.
Protein Reconstitution into Liposomes
SUVs and GUVs were prepared exactly as described previously (57). All lipids were from Avanti Polar Lipids with the exception of 3H-labeled 1,2-dipalmitoyl phosphatidylcholine (3H-DPPC), which was from Amersham Biosciences. The VAMP2/Syt1 lipid mix was 30 mol% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 15 mol% 1,2-dioleoyl-sn-glycero-3-phosphoserine, 22.6 mol% 1-hexadecanoyl-2-octadecenoyl-sn-glycero-3-phosphoethanolamine, 5 mol% liver PI (l-α-phosphatidylinositol), 25 mol% cholesterol (from ovine wool), 1.6 mol% rhodamine-DPPE (N-(lissamine rhodamine B sulfonyl) 1,2-dipalmitoyl phosphatidylethanolamine), 0.8 mol% NBD-DPPE (N-(7-nitro-2,1,3-benzoxadiaziole-4-yl)-1,2-dipalmitoyl phosphatidylethanolamine), and trace amounts of 3H-DPPC; 3 μmol of total lipid. The syntaxin1·SNAP-25 lipid mix for docking assays was 34.5 mol% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 15 mol% 1,2-dioleoyl-sn-glycero-3-phosphoserine, 20 mol% 1-hexadecanoyl-2-octadecenoyl-sn-glycero-3-phosphoethanolamine, 3 mol% liver PI (l-α-phosphatidylinositol), 2 mol% brain PI(4,5)P2 (l-α-phosphatidylinositol-4,5-bisphosphate), 25 mol% cholesterol (from ovine wool), and 0.5 mol% rhodamine-DPPE; 5 μmol of total lipid. The syntaxin1·SNAP-25 lipid mix for fusion assays contained no rhodamine-DPPE but 35 mol% of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and trace amounts of 3H-DPPC. Rhodamine-DPPE and 3H-DPPC were used for GUV lipid recovery calculations.
SUVs were formed in the presence of VAMP2 (protein to lipid ratio 1/200) and Syt1 (1/800) using the lipid mix defined above and the previously described technique of dilution and dialysis followed by a Nycodenz gradient centrifugation (13). t-SNARE-GUVs (protein to lipid ratio 1/1000) were formed by electro-swelling as described previously (57).
SUV/GUV Binding Assay
All SUV/GUV binding studies were carried out in an ice bath to suppress fusion (13, 57). Potential aggregates of 3H-DPPC-labeled SUVs were removed by centrifugation at 16,000 × g for 1 min. Where indicated, GUVs (42 nmol lipid) were preincubated for 5 min with 6 μm CpxII and/or for 10 min with 0.9 μm Munc18-1 on ice in 180 μl of fusion buffer (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 0.1 mm EGTA, 1 mm MgCl2, 1 mm DTT). Subsequently, 20 μl of SUVs (7.5 nmol lipid) were added to GUVs in a final volume of 200 μl of buffer. After 5 min or 1 h of co-incubation, GUVs and the associated SUVs were isolated by centrifugation for 5 min at 5000 × g, 4 °C. 190 μl of the supernatant were discarded, and the counts per minute in the resuspended pellet were determined. Where indicated, BoNT/D and botulinum neurotoxin C (BoNT/C) cleavage of SNARE proteins before SUV/GUV mixing was performed for 1 h at 37 °C with toxin concentrations of 0.07 and 0.15 mg/ml, respectively. To determine unspecific SUV-GUV interactions and to control for the SUVs remaining in the dead volume of the pellet, SUVs and GUVs were pretreated with 0.05 mg/ml proteinase K (Sigma) for 30 min at 37 °C. Absolute background measurements (usually 5% of the input) were subtracted from all samples, and the percentage of pulled-down SUVs was calculated based on input measurements. Average values of three independent experiments were calculated with corresponding 95% confidence intervals as error bars (Microsoft Excel 2008).
SUV/GUV Fusion Assay
The fusion assay was performed as described previously (57). GUVs were preincubated with 6 μm CpxII (5 min) and/or 0.9 μm Munc18-1 (10 min) at room temperature. SUVs and GUVs were mixed at room temperature and immediately transferred into a prewarmed 96-well plate (37 °C). Samples, in which VAMP2-SUVs and t-SNARE-GUVs were preincubated in the presence of Munc18-1 for 1 h on ice, were subsequently transferred to a 96-well plate at room temperature to delay the onset of fusion. Samples were measured at 37 °C in a Synergy 4 plate reader (BioTek Instruments GmbH) at intervals of 10 s. After 5 min, Ca2+ was added to a final concentration of 100 μm. The NBD fluorescence obtained from control incubations containing SUVs pretreated with BoNT/D was subtracted from individual measurement sets. The fusion-dependent fluorescence was normalized to the maximum fluorescent signal obtained in the presence of 0.4% dodecylmaltoside (Fluka). Three independent fusion experiments were performed for each condition.
RESULTS
Syt1-SUVs Efficiently Bind PI(4,5)P2- and t-SNARE-containing GUVs
To determine the function of Munc18-1 in the protein/lipid network (v-SNARE, t-SNARE, Syt1, CpxII, Munc18-1, and PI(4,5)P2) conferring vesicle docking/fusion, it is necessary to systematically analyze the role of each protein in a comparative manner and to determine at which step it functions. To this end, we have established a vesicle-docking assay using SUVs, which contain reconstituted VAMP2 and/or membrane anchored Syt1 as well as GUVs containing syntaxin 1·SNAP-25 and/or PI(4,5)P2. VAMP2 and Syt1 were reconstituted at a protein to lipid ratio of 1/200 and 1/800, respectively, corresponding to the physiological concentrations found in synaptic vesicles (58). These SUVs were radiolabeled by the incorporation of 3H-DPPE to allow the quantification of the SUV/GUV interaction. GUVs, containing preassembled syntaxin 1·SNAP-25 complexes at a protein to lipid ratio of 1/1000 and/or 2 mol% PI(4,5)P2, were prepared by electro-swelling and labeled by the fluorescent lipid rhodamine-DPPE to determine lipid recovery. When GUVs (filled with 250 mm sucrose) are resuspended in an iso-osmolar reaction buffer of lower density, GUVs will sediment at low centrifugal force, thus allowing their separation from free SUVs, which remain in the supernatant. The protein pattern of reconstituted liposomes and the purity of all regulatory components used in this study are shown in supplemental Fig. S1.
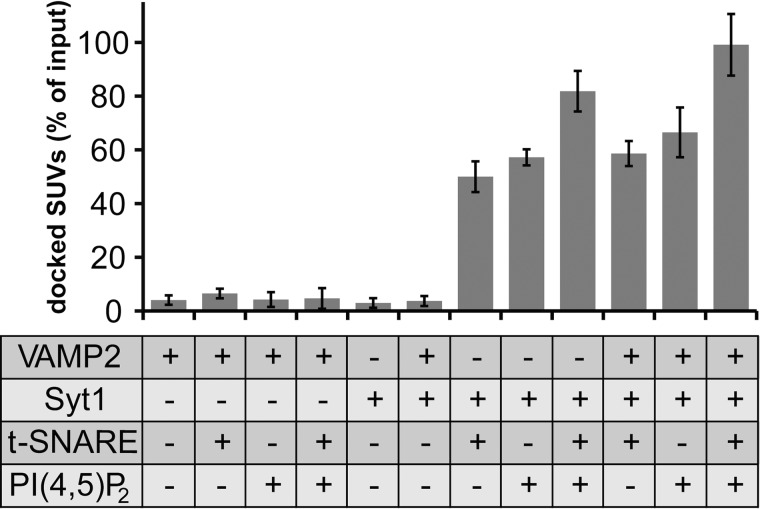
For the interaction studies, SUVs (7.5 nmol lipid) and GUVs (42 nmol lipid) were incubated for 5 min in the absence or presence of the preferred regulatory component(s) in an ice-bath. Free and docked SUVs were separated by centrifugation at 5000 × g for 5 min, and the percentage of SUVs docked to GUVs was determined by measuring the radioactivity recovered in the pellet. The inactivation of VAMP2 and t-SNARE by BoNT/D and BoNT/C cleavage, respectively, shows that unspecific protein-independent interaction of SUVs with GUVs is minimal (≤5% for all controls) (Fig. 1). Proteinase K-treated samples were used to determine absolute background values that were subtracted from all samples. Remarkably, VAMP2-SUVs and t-SNARE-GUVs display very weak binding (6.5 ± 1.8%) within the 5-min incubation period on ice, indicating that SNAREs located in their native membrane environment inefficiently form SNAREpins or that SNAREpins are unstable. The presence of PI(4,5)P2, which is known to bind to the t-SNARE, did not increase v-/t-SNARE-mediated docking (4.7 ± 3.8%). In contrast, vesicle docking was significantly enhanced when membrane-anchored Syt1 was incorporated into SUVs and either syntaxin 1·SNAP-25 or PI(4,5)P2 was present in the GUVs. This Syt1-mediated docking was largely VAMP2-independent. Syt1/PI(4,5)P2-dependent docking (57 ± 4.0%) was slightly more efficient than Syt1/t-SNARE-dependent docking (50 ± 5.7%), which might be due to the 20-fold higher surface concentration of PI(4,5)P2 compared with the t-SNARE. Because the interaction of PI(4,5)P2 with positively charged amino acids close to the transmembrane domain of syntaxin 1 generates syntaxin 1 and PI(4,5)P2 clusters, we also tested if such PI(4,5)P2 clusters might have an effect on Syt1-mediated vesicle docking (59). To this end, GUVs containing 2% PI(4,5)P2 or GUVs containing 2% PI(4,5)P2 and the membrane remnants of BoNT/C-cleaved syntaxin 1 (amino acids 254–288) were generated. We did not observe any significant difference in Syt1-mediated vesicle docking between these two GUV populations, suggesting that PI(4,5)P2 clusters are not required for Syt1/PI(4,5)P2-mediated vesicle docking under the conditions employed (supplemental Fig. S2). Most efficient docking was observed when all components were present and nearly 100% of SUVs added to the reaction were recovered in the GUV pellet upon the 5-min co-incubation period. Thus, in contrast to the weak SUV binding mediated by v-/t-SNARE interactions, Syt1 efficiently links SUVs with GUVs when its binding partners PI(4,5)P2 and/or t-SNARE are present on the GUV surface.
FIGURE 1.
Syt1, but not the v-SNARE VAMP2, mediates efficient vesicle docking by specific interactions with PI(4,5)P2- and/or t-SNARE (syntaxin 1·SNAP-25)-GUVs in the absence of Ca2+. VAMP2- or VAMP2/Syt1-SUVs (7.5 nmol of lipid, 38 pmol of VAMP2, 9.4 pmol of Syt) labeled with 3H-DPPC were mixed with GUVs (42 nmol of lipid, 42 pmol of syntaxin 1·SNAP-25) lacking or containing PI(4,5)P2 (0.84 nmol) in a final volume of 200 μl and incubated on ice for 5 min. Where indicated (−), VAMP2 and syntaxin 1·SNAP-25 were specifically cleaved by treatment with BoNT/D and BoNT/C, respectively. SUVs bound to GUVs were isolated by centrifugation, and the radioactivity associated with the pellet was measured as described under “Experimental Procedures.” Percentages were calculated based on input radioactivity. Error bars are 95% confidence intervals (n = 3).
The Polybasic Motif in the C2B Domain of Syt1 Provides an Overlapping Binding Site for the t-SNARE and PI(4,5)P2 and Mediates Membrane Docking
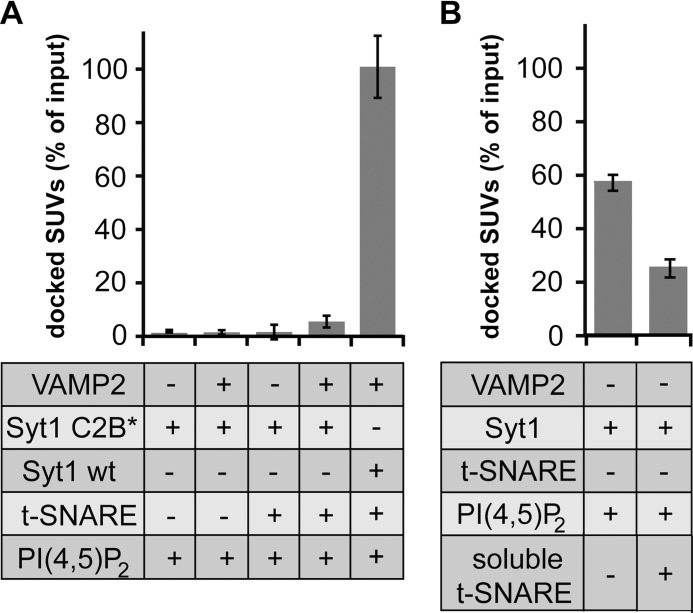
Next, we determined which binding site within Syt1 confers the interaction with PI(4,5)P2 and the t-SNARE. Fig. 2A reveals that a triple mutation (K326Q,K327Q,K331Q) in the polybasic cluster of the C2B domain of Syt1 abolished both PI(4,5)P2- and t-SNARE-dependent docking. This result is consistent with previous observations where the same mutants inhibit Syt1/t-SNARE interactions and fusion in vitro (60). Thus, the polybasic cluster of Syt1 might also significantly contribute to synaptic vesicle docking at the presynaptic plasma membrane in vivo. Our result also raises the question if PI(4,5)P2 and the t-SNARE can simultaneously bind to Syt1. Hence, we preincubated Syt1-SUVs (9.4 pmol of Syt1) with 60 pmol of soluble t-SNARE complexes. The soluble t-SNARE was produced by the co-expression of syntaxin 1 (amino acids 1–262) and His6-SNAP-25 (amino acids 1–206). Affinity purification, via the His6 tag ensured the efficient formation of 1:1 syntaxin 1·SNAP-25 complexes. Fig. 2B shows that the preincubation step with the soluble t-SNARE reduced vesicle docking more than 2-fold, indicating a competitive binding to a common or overlapping binding site, containing Lys-326, -327, and -331 as critical amino acids.
FIGURE 2.
The polybasic motif in the C2B domain of Syt1 is required for vesicle docking via redundant PI(4,5)P2 and t-SNARE interactions. A, mutations in the polybasic motif of Syt1C2B impair both PI(4,5)P2- and t-SNARE-dependent vesicle docking. v-SNARE-SUVs containing the Syt1C2B* triple mutation K326Q,K327Q,K331Q or Syt1 wt were incubated with t-SNARE-GUVs as described in the legend to Fig. 1. Where indicated (−), VAMP2 and syntaxin 1·SNAP-25 were cleaved by the specific BoNTs (last bar reproduced from Fig. 1). B, t-SNARE and PI(4,5)P2 compete for binding to Syt1. Syt1-SUVs were preincubated with a 6-fold molar excess of soluble t-SNARE and subsequently incubated with PI(4,5)P2-GUVs on ice for 5 min and analyzed as described under “Experimental Procedures.” Error bars are 95% confidence intervals (n = 3).
Munc18-1 Enhances VAMP2-SUV Docking to t-SNARE-GUVs after Prolonged Incubation Periods
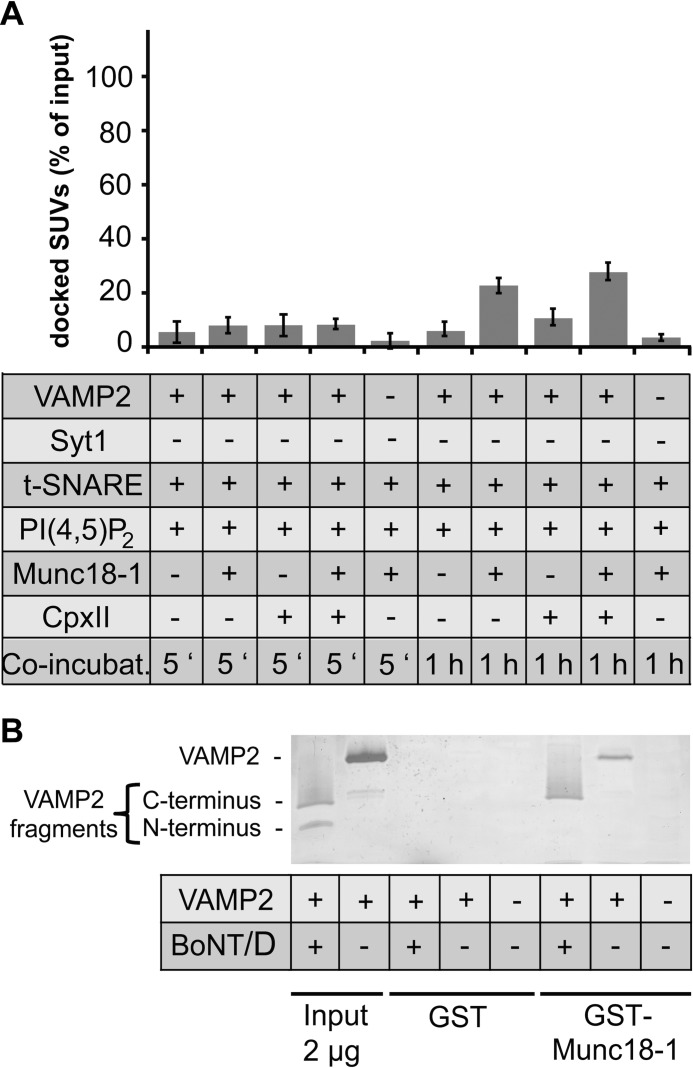
Because Munc18-1 has been reported to confer vesicle docking, we tested the role of Munc18-1 in the SUV/GUV docking assay. t-SNARE-GUVs were preincubated for 10 min with 0.9 μm Munc18-1, which are saturating amounts as shown by the maximal signals obtained in a reconstituted Ca2+-regulated lipid mixing assay (supplemental Fig. S3). Because of the preincubation step, Munc18-1 was already preloaded onto the t-SNARE-GUVs and could directly function as a docking factor via its VAMP2 interaction. A 5-min co-incubation in the presence of Munc18-1 did not significantly increase the SUV/GUV docking efficiency (7.2 ± 1.1%) (Fig. 3A). However, after 1 h of co-incubation, Munc18-1 stimulated vesicle docking 3-fold (22 ± 3.0%). Parallel lipid mixing experiments revealed that the prolonged co-incubation on ice does not result in membrane fusion, demonstrating that the vesicles are still at the docking stage (supplemental Fig. S4). In summary, Munc18-1 is capable of increasing vesicle docking, but only after prolonged incubation times, confirming the role of Syt1 as primary docking factor.
FIGURE 3.
Munc18-1 stimulates docking of v-SNARE-SUVs to t-SNARE-GUVs, which requires both SNARE-SNARE and Munc18-1-SNARE interactions and a prolonged incubation on ice. A, Munc18-1 shows more pronounced vesicle docking than CpxII. The weak docking activity of CpxII is statistically not considered to be significant (p < 0.2, independent t-test). t-SNARE-GUVs were preincubated with 0.9 μm Munc18-1 for 10 min and/or 6 μm CpxII for 5 min on ice. v-SNARE-SUVs were added, and the incubation was continued for the indicated time periods on ice. Where indicated (VAMP2 −), VAMP2 was cleaved by BoNT/D (the first bar was reproduced from Fig. 1). Error bars are 95% confidence intervals (n = 3). B, Munc18-1 binds the C-terminal but not the N-terminal fragment of BoNT/D-cleaved VAMP2. GST-Munc18-1 was immobilized on glutathione beads followed by a 1-h incubation with full-length VAMP2 or BoNT/D-cleaved VAMP2. VAMP2 was present in a 25-fold molar excess over Munc18-1. Bound proteins were separated by SDS-PAGE and visualized by silver staining; a representative experiment is shown.
Partially assembled SNAREpins also provide high affinity binding sites for complexins, which in turn can stabilize SNAREpins and vesicle docking. To determine if CpxII enhances vesicle docking, 6 μm CpxII were added to t-SNARE-GUVs 5 min before mixing with VAMP2-SUVs before co-incubations of 5 min or 1 h on ice. 6 μm CpxII are saturating amounts as shown by the maximal signal obtained in a reconstituted Ca2+-regulated lipid mixing assay (57). CpxII reproducibly stimulated vesicle docking but only after the 1-h incubation time (Fig. 3A). However, the statistical analysis showed that this stimulation was not significant (p < 0.2, independent t-test). Nevertheless, a combination of Munc18-1 and CpxII also showed a small additive effect after 1 h co-incubation (Fig. 3A). Thus, CpxII is a much weaker stimulator than Munc18-1 and Syt1. In the absence of Syt1, docking stimulation by each Munc18-1 and CpxII was strictly VAMP2-dependent. Finally, combinations of CpxII with Syt1 in the absence or presence of Munc18-1 were tested. The addition of CpxII and Munc18-1 did not significantly enhance the prominent docking effect of Syt1 under any condition chosen (supplemental Figs. S5 and S6).
Munc18-1-SNARE and N-terminal SNARE-SNARE Interactions Synergize to Stimulate Vesicle Docking
Next, we attempted to further refine the molecular mechanism by which Munc18-1 enhances vesicle docking and SNAREpin formation. It is known that Munc18-1 shows a specific but very weak interaction with VAMP2 (49). Such weak interactions between single molecules are difficult to detect by classical binding experiments but can be visualized after protein cross-linking or by NMR (49). However, in the membrane environment and in the presence of several Munc18-1 and VAMP2 copies, the simultaneous occurrence of such low affinity interactions could result in efficient vesicle docking by increased avidity. To further characterize the mechanism, we made use of the fact that the binding site for Munc18-1 has been mapped by cross-linking experiments to amino acids 87–91 and by NMR studies to amino acids 75–95 (49). This region is located in the C-terminal half of VAMP2 and remains membrane-anchored after BotNT/D cleavage. BoNT/D is a site-specific protease that cleaves VAMP2 before Lys-60, removing the N-terminal part of the VAMP2 SNARE motif but leaving behind the binding site for Munc18-1 on the truncated VAMP2 remnant (61). Thus, if the single interaction of Munc18-1 with the membrane-proximal binding site on VAMP2 is sufficient for efficient vesicle docking, BoNT/D cleavage should not affect docking. However, Fig. 3A shows that BoNT/D cleavage efficiently abolished Munc18-1-mediated vesicle docking. Thus, the dual binding of Munc18-1 to the C terminus of VAMP2 and the N-terminal portions of the v-/t-SNARE motifs synergize to confer SNAREpin formation/assembly. To exclude the remote possibility that the N terminus of VAMP2 harbors a yet unidentified binding site for Munc18-1, GST-Munc18-1 was immobilized on glutathione beads and incubated with full-length VAMP2 or VAMP2 cleaved by BoNT/D, generating the N- and C-terminal fragments. To detect the low affinity Munc18-1/VAMP2 interactions, bead-associated proteins were visualized by silver-staining. Fig. 3B shows that both full-length VAMP2 and the C-terminal fragment show similar binding activities to GST-Munc18-1. An interaction of the N-terminal VAMP2 fragment with Munc18-1 was not detectable. Binding was specific because neither full-length VAMP2 nor its fragments bind GST. Thus, both SNARE-SNARE interactions and Munc18-1-SNARE interactions are required to enhance vesicle docking and to stabilize SNAREpins.
Vesicle Docking by Syt1 Is a Prerequisite for Munc18-1 to Accelerate Lipid Mixing
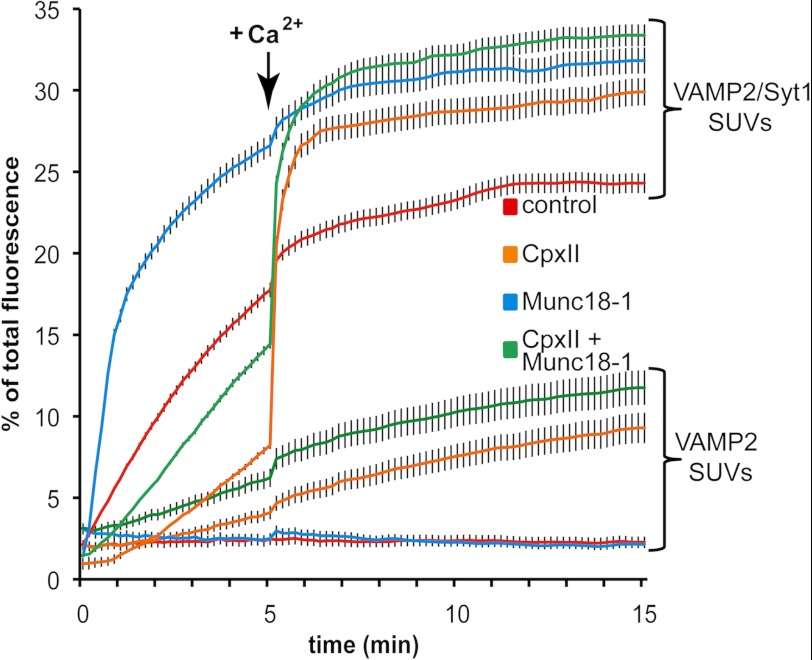
Finally, we determined how the various regulatory components and their effects on vesicle docking influence membrane fusion, measured by lipid mixing. For this purpose similar conditions as in the docking assay were employed, but the VAMP2-SUVs now contain in addition a quenched pair of lipid-coupled fluorophores (0.8 mol% NBD-DPPE and 1.6 mol% rhodamine-DPPE). Fusion of labeled SUVs (2.5 nmol of lipid) with unlabeled t-SNARE-GUVs (14 nmol lipid) results in a dramatic dilution of the fluorophores, and consequently the NBD fluorescence increases due to dequenching. Thus, membrane fusion is monitored by this well established lipid mixing assay (13, 62) (Fig. 4).
FIGURE 4.
In the presence of Syt1, Munc18-1 stimulates lipid mixing without a SUV/GUV preincubation step. VAMP2- or VAMP2/Syt1-SUVs (2.5 nmol of lipid, 12.5 pmol of VAMP2, 3.1 pmol of Syt1) labeled with rhodamine-DPPE and NBD-DPPE were mixed with unlabeled syntaxin 1·SNAP-25-GUVs (14 nmol of lipid, 14 pmol of t-SNARE) in the absence or presence of 90 pmol Munc18-1 and/or 600 pmol CpxII in a final volume of 100 μl, and the increase in NBD fluorescence was monitored. After 5 min at 37 °C, Ca2+ was added to a final concentration of 100 μm, and the measurement continued for another 10 min. The results were normalized to the maximum NBD fluorescence signal after detergent lysis of the liposomes. Error bars are S.E. (n = 3).
Lipid mixing was measured for 5 min in the absence of Ca2+ and, subsequently Ca2+ was added (100 μm final concentration) to monitor the kinetics and extent of Ca2+-synchronized membrane fusion for an additional 10 min (Fig. 4). As already shown in our previous study, VAMP2-SUVs and t-SNARE-GUVs by themselves do not show any significant membrane fusion under the conditions employed (low lipid concentrations and low protein to lipid ratio) (57). The addition of CpxII shows a weak stimulatory effect consistent with its weak stimulation of docking with progressing incubation times. Membrane-anchored Syt1 (in the absence of both CpxII and Ca2+) profoundly stimulates membrane fusion, consistent with its prominent stimulation of vesicle docking. The addition of Ca2+ results in a significant but limited burst of fast membrane fusion. The addition of CpxII suppresses the Ca2+-independent stimulation by Syt1 but results in a pronounced fast Ca2+-dependent burst of membrane fusion. Furthermore, the final fusion signal significantly exceeds the signal obtained in the presence of Syt1 alone, suggesting cooperative functions of Syt1 and CpxII. The addition of Munc18-1 to fusion reactions containing only SNARE liposomes did not enhance membrane fusion during the 15-min measurement period. This result is expected because VAMP2-SUVs and t-SNARE-GUVs need to be co-incubated together with Munc18-1 on ice for 1 h (non-fusogenic condition) to observe vesicle docking and the previously reported stimulation of membrane fusion (50, 56). Indeed, a prolonged co-incubation results in a pronounced stimulation of the initial fusion rate (supplemental Fig. S4). Remarkably, in the presence of Syt1, Munc18-1 profoundly stimulates lipid mixing even in the absence of any co-incubation together with SUVs and GUVs. Thus, vesicle docking mediated by Syt1 has generated an intermediate that allows Munc18-1 to efficiently promote SNAREpin assembly and fusion. The addition of CpxII partially inhibits the Ca2+-independent stimulation and again results in a rapid Ca2+-synchronized fusion burst. Thus, after the Syt1 vesicle docking reaction, Munc18-1 efficiently stimulates SNARE complex assembly and lipid mixing, whereas CpxII functions as a fusion clamp.
DISCUSSION
In our approach to assign Munc18-1 to a defined late-acting reaction step in regulated membrane fusion, we have resolved how Syt1, Munc18-1, and CpxII sequentially and synergistically function to control SNAREpin formation/assembly and generate a pool of vesicles that responds to a Ca2+ signal with efficient and fast synchronized lipid mixing. 1) A direct comparison of the vesicle-docking capabilities of the three regulatory components reveals that only Syt1 provides fast and efficient vesicle docking, which is consistent with recent in vitro and in vivo studies of Syt1 function (27–29, 57). The strong enhancement of vesicle docking by Syt1 results in a significant stimulation of membrane fusion. Correspondingly, the weak docking activity of CpxII coincides with a weak stimulation of membrane fusion. As expected, in the presence of Syt1, CpxII blocks membrane fusion, further confirming that these two proteins function synergistically as a fusion clamp (57). 2) The Syt1-mediated docking occurs VAMP2-independently but requires either PI(4,5)P2 or syntaxin 1·SNAP-25 on the opposite membrane. Under the employed conditions, vesicle docking does not require PI(4,5)P2 clusters. 3) PI(4,5)P2 and t-SNAREs compete for binding to Syt1 implying a sequential binding modus. 4) Syt1-mediated vesicle docking results in a reaction intermediate that becomes an efficient substrate for Munc18-1 action. The observation that Munc18-1 shows a more prominent liposome docking phenotype than CpxII, but does not stimulate membrane fusion (in the absence of the low temperature co-incubation), already suggests a requirement for an earlier acting factor, Syt1. In the presence of Syt1, Munc18-1 not only dramatically increases the initial fusion rate but also the final extent of membrane fusion, consistent with its SNAREpin assembly function. Thus, the majority of the vesicles docked by Syt1 are efficiently shifted into a reactive pool, which would be consistent with studies in living cells that demonstrated that Munc18-1 can regulate the size of the readily releasable pool of vesicles (63, 64). Interestingly, recent in vitro reconstitution experiments showed that Syt1 docks vesicles, but a considerable time passes before these vesicles can fuse (28). Our data now indicate that Munc18-1 actually accelerates this vesicle priming step. 5) The presence of CpxII inhibits this stimulation by Munc18-1 to a significant degree and aids in synchronizing the reaction pathway toward the Ca2+-dependent reaction. However, CpxII exerts only a partial block, and a distinct fraction of the vesicles still fuses in a Ca2+-independent manner with progressing time. It remains to be shown if this Ca2+-independent membrane fusion in the reconstituted in vitro assay, which only uses a limited number of purified components, reflects some shortcoming of the assay or potentially reproduces spontaneous vesicle fusion in vivo. Interestingly, Munc18-1 stimulates both spontaneous and evoked release in neurons, consistent with our in vitro data (64).
Overall, our results together with previous work suggest the following cascade of reactions. SNAREs at their physiological membrane concentrations do not show efficient vesicle docking and membrane fusion. Syt1 acts as an initial vesicle-docking factor. Thus, the presence of Syt1 is a prerequisite for efficient SNAREpin formation. This order of events ensures that the Ca2+ sensor will inevitably be incorporated into the fusion machinery, rendering the machinery Ca2+-responsive. Initially, Syt1 binds with low affinity to PI(4,5)P2 on the presynaptic plasma membrane requiring its polybasic motif. This interaction may already occur in the immediate vicinity of the t-SNAREs because syntaxin 1 interacts with PI(4,5)P2 and forms PI(4,5)P2-dependent clusters (59, 65). Because the t-SNARE and PI(4,5)P2 compete for the binding to the polybasic motif and Syt1 has a higher affinity for the t-SNARE than for PI(4,5)P2 (∼KD = 250 μm), the initial low affinity Syt1-PI(4,5)P2 interaction will be replaced by the higher affinity Syt1-t-SNARE interaction (66, 67). This Syt1-t-SNARE interaction will bring v-SNAREs and t-SNAREs on the opposite membranes in close proximity, and SNAREpin assembly can start. Henceforth, SNAREpin assembly is controlled by CpxII and Munc18-1. Munc18-1 binds partially assembled t-SNARE complexes, the C-terminal part of the VAMP2 SNARE motif, and the subsequent linker sequence (36, 49, 56, 68). Because Munc18-1 specifically interacts with VAMP2 and VAMP3, only vesicles containing these v-SNAREs will be efficiently primed by accelerating SNAREpin assembly (50, 56). In addition, it is of note that Munc18-1 is also a high affinity partner of syntaxin 1 and keeps syntaxin 1 in a closed conformation. To release this inhibition and to allow subsequent SNARE complex assembly, a Munc13-t-SNARE and a Munc18-1-VAMP2 interaction are required (48, 50). However, these earlier regulatory steps are bypassed in our assay, because preassembled t-SNARE complexes were used to focus on late steps of SNAREpin assembly. While Munc18-1 targets the C-terminal part of VAMP2, CpxII is directed toward the assembled N-terminal part of the partially assembled SNAREpin. Indeed, it has been shown that both complexin and Munc18-1 can simultaneously bind SNARE complexes (64). An already available structure of a complexin-SNAREpin mimetic demonstrates that complexin binds via its central helix in an anti-parallel manner to the N-terminal end of partially assembled SNAREpins (21). The N-terminal accessory helix of complexin interacts in trans with a second SNAREpin and blocks SNAREpin assembly by competing with VAMP2 for binding to the membrane proximal part of the t-SNARE complex (21). These bridging functions of complexin also result in SNAREpin oligomerization. Thus, the SNAREpins are now in an arrested state containing the complexin clamp, Syt1, the Ca2+ sensor, and presumably Munc18-1. How this reaction intermediate is organized in structural terms remains to be shown. Ca2+ binding to Syt1 then mediates local perturbations in the lipid bilayer and the release of the complexin clamp likely via Ca2+-dependent SNAREpin interactions results in membrane fusion (23, 24). Post fusion, complexin does not any longer bridge SNARE complexes, SNARE complex oligomers are resolved, and the accessory/inhibitory helix is solvent-exposed (19). Having resolved basic vesicle docking and subsequent priming steps in a reconstituted assay and having assigned the corresponding machinery to distinct reactions, future work still needs to address the biophysics of the fusion reaction.
Supplementary Material
Acknowledgments
Expression vectors encoding the light chains of neurotoxins were kind gifts of Dr. Thomas Binz and the late Dr. Heiner Niemann. The expression vector encoding GST-Munc18-1/nSec1 was a gift of Dr. Richard Scheller.
This work was supported by a grant of German Research Foundation (SFB/TRR 83; to D. P. and T. H. S.).

This article contains supplemental Figs. S1–S6.
- v-SNARE
- vesicle SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)
- t-SNARE
- target SNARE
- Munc18-1
- mammalian unc18-1
- Syt1
- synaptotagmin 1
- CpxII
- complexin II
- PI(4,5)P2
- phosphatidylinositol (PI) 4,5-bisphosphate
- GUV
- giant unilamellar vesicle
- SUV
- small unilamellar vesicle
- DPPC
- 1,2-dipalmitoyl phosphatidylcholine
- rhodamine-DPPE
- N-(lissamine rhodamine B sulfonyl) 1,2-dipalmitoyl phosphatidylethanolamine
- NBD-DPPE
- N-(7-nitro-2,1,3-benzoxadiaziole-4-yl)-1,2-dipalmitoyl phosphatidylethanolamine
- BoNT/D
- botulinum neurotoxin D
- BoNT/C
- botulinum neurotoxin C.
REFERENCES
- 1. Bruns D., Jahn R. (1995) Real-time measurement of transmitter release from single synaptic vesicles. Nature 377, 62–65 [DOI] [PubMed] [Google Scholar]
- 2. Schikorski T., Stevens C. F. (2001) Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 4, 391–395 [DOI] [PubMed] [Google Scholar]
- 3. Jahn R., Scheller R. H. (2006) SNAREs. Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 [DOI] [PubMed] [Google Scholar]
- 4. Malsam J., Kreye S., Söllner T. H. (2008) Membrane fusion. SNAREs and regulation. Cell. Mol. Life Sci. 65, 2814–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Südhof T. C., Rothman J. E. (2009) Membrane fusion. Grappling with SNARE and SM proteins. Science 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 [DOI] [PubMed] [Google Scholar]
- 7. Melia T. J., Weber T., McNew J. A., Fisher L. E., Johnston R. J., Parlati F., Mahal L. K., Sollner T. H., Rothman J. E. (2002) Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell Biol. 158, 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pobbati A. V., Stein A., Fasshauer D. (2006) N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 313, 673–676 [DOI] [PubMed] [Google Scholar]
- 9. Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 [DOI] [PubMed] [Google Scholar]
- 10. Stein A., Weber G., Wahl M. C., Jahn R. (2009) Helical extension of the neuronal SNARE complex into the membrane. Nature 460, 525–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu C., Ahmed M., Melia T. J., Söllner T. H., Mayer T., Rothman J. E. (2003) Fusion of cells by flipped SNAREs. Science 300, 1745–1749 [DOI] [PubMed] [Google Scholar]
- 12. McNew J. A., Parlati F., Fukuda R., Johnston R. J., Paz K., Paumet F., Söllner T. H., Rothman J. E. (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153–159 [DOI] [PubMed] [Google Scholar]
- 13. Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) SNAREpins. Minimal machinery for membrane fusion. Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 14. Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. (1990) Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345, 260–263 [DOI] [PubMed] [Google Scholar]
- 15. Hata Y., Slaughter C. A., Südhof T. C. (1993) Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366, 347–351 [DOI] [PubMed] [Google Scholar]
- 16. Brose N., Petrenko A. G., Südhof T. C., Jahn R. (1992) Synaptotagmin, A calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 [DOI] [PubMed] [Google Scholar]
- 17. McMahon H. T., Missler M., Li C., Südhof T. C. (1995) Complexins. Cytosolic proteins that regulate SNAP receptor function. Cell 83, 111–119 [DOI] [PubMed] [Google Scholar]
- 18. Reim K., Mansour M., Varoqueaux F., McMahon H. T., Südhof T. C., Brose N., Rosenmund C. (2001) Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104, 71–81 [DOI] [PubMed] [Google Scholar]
- 19. Chen X., Tomchick D. R., Kovrigin E., Araç D., Machius M., Südhof T. C., Rizo J. (2002) Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409 [DOI] [PubMed] [Google Scholar]
- 20. Malsam J., Seiler F., Schollmeier Y., Rusu P., Krause J. M., Söllner T. H. (2009) The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl. Acad. Sci. U.S.A. 106, 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kümmel D., Krishnakumar S. S., Radoff D. T., Li F., Giraudo C. G., Pincet F., Rothman J. E., Reinisch K. M. (2011) Complexin cross-links prefusion SNAREs into a zigzag array. Nat. Struct. Mol. Biol. 18, 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li F., Pincet F., Perez E., Giraudo C. G., Tareste D., Rothman J. E. (2011) Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state. Nat. Struct. Mol. Biol. 18, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang J., Maximov A., Shin O. H., Dai H., Rizo J., Südhof T. C. (2006) A complexin/synaptotagmin1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 [DOI] [PubMed] [Google Scholar]
- 24. Martens S., Kozlov M. M., McMahon H. T. (2007) How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 [DOI] [PubMed] [Google Scholar]
- 25. Di Paolo G., Moskowitz H. S., Gipson K., Wenk M. R., Voronov S., Obayashi M., Flavell R., Fitzsimonds R. M., Ryan T. A., De Camilli P. (2004) Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431, 415–422 [DOI] [PubMed] [Google Scholar]
- 26. Mahal L. K., Sequeira S. M., Gureasko J. M., Söllner T. H. (2002) Calcium-independent stimulation of membrane fusion and SNAREpin formation by synaptotagmin I. J. Cell Biol. 158, 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Wit H., Walter A. M., Milosevic I., Gulyás-Kovács A., Riedel D., Sørensen J. B., Verhage M. (2009) Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell 138, 935–946 [DOI] [PubMed] [Google Scholar]
- 28. Wang Z., Liu H., Gu Y., Chapman E. R. (2011) Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. J. Cell Biol. 195, 1159–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim J. Y., Choi B. K., Choi M. G., Kim S. A., Lai Y., Shin Y. K., Lee N. K. (2012) Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 31, 2144–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison S. D., Broadie K., van de Goor J., Rubin G. M. (1994) Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron 13, 555–566 [DOI] [PubMed] [Google Scholar]
- 31. Verhage M., Maia A. S., Plomp J. J., Brussaard A. B., Heeroma J. H., Vermeer H., Toonen R. F., Hammer R. E., van den Berg T. K., Missler M., Geuze H. J., Südhof T. C. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869 [DOI] [PubMed] [Google Scholar]
- 32. Weimer R. M., Richmond J. E., Davis W. S., Hadwiger G., Nonet M. L., Jorgensen E. M. (2003) Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 6, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toonen R. F., Verhage M. (2007) Munc18-1 in secretion. Lonely Munc joins SNARE team and takes control. Trends Neurosci. 30, 564–572 [DOI] [PubMed] [Google Scholar]
- 34. Carr C. M., Rizo J. (2010) At the junction of SNARE and SM protein function. Curr. Opin. Cell Biol. 22, 488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han G. A., Malintan N. T., Collins B. M., Meunier F. A., Sugita S. (2010) J. Neurochem. 115, 1–10 [DOI] [PubMed] [Google Scholar]
- 36. Rickman C., Medine C. N., Bergmann A., Duncan R. R. (2007) Functionally and spatially distinct modes of munc18-syntaxin 1 interaction. J. Biol. Chem. 282, 12097–12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rowe J., Calegari F., Taverna E., Longhi R., Rosa P. (2001) Syntaxin 1A is delivered to the apical and basolateral domains of epithelial cells. The role of munc-18 proteins. J. Cell Sci. 114, 3323–3332 [DOI] [PubMed] [Google Scholar]
- 38. Medine C. N., Rickman C., Chamberlain L. H., Duncan R. R. (2007) Munc18-1 prevents the formation of ectopic SNARE complexes in living cells. J. Cell Sci. 120, 4407–4415 [DOI] [PubMed] [Google Scholar]
- 39. McEwen J. M., Kaplan J. M. (2008) UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol. Biol. Cell 19, 3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han L., Jiang T., Han G. A., Malintan N. T., Xie L., Wang L., Tse F. W., Gaisano H. Y., Collins B. M., Meunier F. A., Sugita S. (2009) Rescue of Munc18-1 and -2 double knockdown reveals the essential functions of interaction between Munc18 and closed syntaxin in PC12 cells. Mol. Biol. Cell 20, 4962–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rowe J., Corradi N., Malosio M. L., Taverna E., Halban P., Meldolesi J., Rosa P. (1999) Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells. Prevention by rbSEC1. J. Cell Sci. 112, 1865–1877 [DOI] [PubMed] [Google Scholar]
- 42. Fernandez I., Ubach J., Dulubova I., Zhang X., Südhof T. C., Rizo J. (1998) Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell 94, 841–849 [DOI] [PubMed] [Google Scholar]
- 43. Misura K. M., Scheller R. H., Weis W. I. (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404, 355–362 [DOI] [PubMed] [Google Scholar]
- 44. Burkhardt P., Hattendorf D. A., Weis W. I., Fasshauer D. (2008) Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 27, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. MacDonald C., Munson M., Bryant N. J. (2010) Autoinhibition of SNARE complex assembly by a conformational switch represents a conserved feature of syntaxins. Biochem. Soc. Trans. 38, 209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maruyama I. N., Brenner S. (1991) A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 88, 5729–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brose N., Hofmann K., Hata Y., Südhof T. C. (1995) Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 270, 25273–25280 [DOI] [PubMed] [Google Scholar]
- 48. Ma C., Li W., Xu Y., Rizo J. (2011) Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat. Struct. Mol. Biol. 18, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu Y., Su L., Rizo J. (2010) Binding of Munc18-1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry 49, 1568–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schollmeier Y., Krause J. M., Kreye S., Malsam J., Söllner T. H. (2011) Resolving the function of distinct Munc18-1/SNARE protein interaction modes in a reconstituted membrane fusion assay. J. Biol. Chem. 286, 30582–30590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schiavo G., Stenbeck G., Rothman J. E., Söllner T. H. (1997) Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc. Natl. Acad. Sci. U.S.A. 94, 997–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Voets T., Toonen R. F., Brian E. C., de Wit H., Moser T., Rettig J., Südhof T. C., Neher E., Verhage M. (2001) Munc18-1 promotes large dense-core vesicle docking. Neuron 31, 581–591 [DOI] [PubMed] [Google Scholar]
- 53. Toonen R. F., Kochubey O., de Wit H., Gulyas-Kovacs A., Konijnenburg B., Sørensen J. B., Klingauf J., Verhage M. (2006) Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 25, 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tareste D., Shen J., Melia T. J., Rothman J. E. (2008) SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc. Natl. Acad. Sci. U.S.A. 105, 2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gulyás-Kovács A., de Wit H., Milosevic I., Kochubey O., Toonen R., Klingauf J., Verhage M., Sørensen J. B. (2007) Munc18-1. Sequential interactions with the fusion machinery stimulate vesicle docking and priming. J. Neurosci. 27, 8676–8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen J., Tareste D. C., Paumet F., Rothman J. E., Melia T. J. (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 [DOI] [PubMed] [Google Scholar]
- 57. Malsam J., Parisotto D., Bharat T. A., Scheutzow A., Krause J. M., Briggs J. A., Söllner T. H. (2012) Complexin arrests a pool of docked vesicles for fast Ca(2+)-dependent release. EMBO J. 31, 3270–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takamori S., Holt M., Stenius K., Lemke E. A., Grønborg M., Riedel D., Urlaub H., Schenck S., Brügger B., Ringler P., Müller S. A., Rammner B., Gräter F., Hub J. S., De Groot B. L., Mieskes G., Moriyama Y., Klingauf J., Grubmüller H., Heuser J., Wieland F., Jahn R. (2006) Molecular anatomy of a trafficking organelle. Cell 127, 831–846 [DOI] [PubMed] [Google Scholar]
- 59. van den Bogaart G., Meyenberg K., Risselada H. J., Amin H., Willig K. I., Hubrich B. E., Dier M., Hell S. W., Grubmüller H., Diederichsen U., Jahn R. (2011) Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rickman C., Archer D. A., Meunier F. A., Craxton M., Fukuda M., Burgoyne R. D., Davletov B. (2004) Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579 [DOI] [PubMed] [Google Scholar]
- 61. Yamasaki S., Baumeister A., Binz T., Blasi J., Link E., Cornille F., Roques B., Fykse E. M., Südhof T. C., Jahn R. (1994) Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J. Biol. Chem. 269, 12764–12772 [PubMed] [Google Scholar]
- 62. Struck D. K., Hoekstra D., Pagano R. E. (1981) Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20, 4093–4099 [DOI] [PubMed] [Google Scholar]
- 63. Toonen R. F., Wierda K., Sons M. S., de Wit H., Cornelisse L. N., Brussaard A., Plomp J. J., Verhage M. (2006) Munc18-1 expression levels control synapse recovery by regulating readily releasable pool size. Proc. Natl. Acad. Sci. U.S.A. 103, 18332–18337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deák F., Xu Y., Chang W. P., Dulubova I., Khvotchev M., Liu X., Südhof T. C., Rizo J. (2009) Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J. Cell Biol. 184, 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. James D. J., Kowalchyk J., Daily N., Petrie M., Martin T. F. (2009) CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc. Natl. Acad. Sci. U.S.A. 106, 17308–17313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rickman C., Davletov B. (2003) Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J. Biol. Chem. 278, 5501–5504 [DOI] [PubMed] [Google Scholar]
- 67. van den Bogaart G., Meyenberg K., Diederichsen U., Jahn R. (2012) Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J. Biol. Chem. 287, 16447–16453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khvotchev M., Dulubova I., Sun J., Dai H., Rizo J., Südhof T. C. (2007) Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J. Neurosci. 27, 12147–12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.