Background: AP-1 (activator protein-1) is a transcription factor comprised of Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra-1, and Fra-2) family members.
Results: AP-1 comprised of JunD and c-Fos induces hematopoietic gene expression and is regulated by BMP-4.
Conclusion: AP-1JunD/c-Fos has a crucial role in hematopoiesis and is required for BMP-4-induced hematopoiesis.
Significance: This work provides new insights regarding the role of AP-1 in hematopoiesis.
Keywords: Cancer, Cancer Biology, Cell Biology, Cell Cycle, Hematopoiesis
Abstract
Activator protein-1 (AP-1) regulates a wide range of cellular processes including proliferation, differentiation, and apoptosis. As a transcription factor, AP-1 is commonly found as a heterodimer comprised of c-Jun and c-Fos proteins. However, other heterodimers may also be formed. The function of these dimers, specifically the heterodimeric AP-1 comprised of JunD and c-Fos (AP-1JunD/c-Fos), has not been elucidated. Here, we identified a function of AP-1JunD/c-Fos in Xenopus hematopoiesis. A gain-of-function study performed by overexpressing junD and c-fos and a loss-of-function study using morpholino junD demonstrate a critical role for AP-1JunD/c-Fos in hematopoiesis during Xenopus embryogenesis. Additionally, we confirmed that JunD of AP-1JunD/c-Fos is required for BMP-4-induced hematopoiesis. We also demonstrated that BMP-4 regulated JunD activity at the transcriptional regulation and post-translational modification levels. Collectively, our findings identify AP-1JunD/c-Fos as a novel hematopoietic transcription factor and the requirement of AP-1JunD/c-Fos in BMP-4-induced hematopoiesis during Xenopus hematopoiesis.
Introduction
Activator protein-1 (AP-1)3 is an evolutionarily conserved bZip family transcription factor composed of Jun family members (e.g. c-Jun, JunB, and JunD) and Fos family members (e.g. c-Fos, FosB, Fra-1, and Fra-2). Whereas the Fos proteins can only heterodimerize with members of the Jun family, the Jun proteins can both homodimerize and heterodimerize with Fos members to form transcriptionally active complexes (1). Each of the AP-1 components is differentially expressed and regulated to perform subtly different functions (2). Moreover, different combinations of AP-1 components have been implicated in a large variety of biological processes, including proliferation, differentiation, apoptosis, and development (2–5). However, despite increasing knowledge regarding the physiological functions of AP-1, a specific role for distinct AP-1 components in early embryogenesis has only been partially elucidated (5–8).
In the Xenopus embryo, two sites of hematopoiesis are present and include the ventral blood island (VBI), which is analogous to the yolk sac blood islands of higher vertebrates, and the dorsal lateral plate region that is analogous to the aorta, gonads and mesonephros region (AGM) of vertebrates (9). Specifically, the VBI is located on the ventral side of the embryo, and the hematopoietic stem cells of the VBI differentiate primarily into embryonic erythroid cells (primitive hematopoiesis) (9, 10).
BMP-4 (bone morphogenetic protein-4) and transforming growth factor-β (TGF-β) are known to be required during gastrulation for specifying the ventral character of the embryonic mesoderm and are therefore candidates for controlling the development of VBI-derived blood progenitors (11, 12). Ectopic expression of BMP-4 in animal cap explants induces expression of the hematopoietic-specific transcription factor, SCL (stem cell leukemia), GATA-1, GATA-2, and GATA-3, LMO2, Neptune, and globin blood marker (11, 13, 14).
Here, we characterize the novel function of heterodimeric AP-1 comprised of junD and c-fos (AP-1JunD/c-Fos) in hematopoiesis during Xenopus development. Furthermore, we provide evidence showing that AP-1JunD/c-Fos function is required for BMP-4-induced hematopoiesis. In addition, we demonstrate that BMP-4 controls AP-1JunD/c-Fos activity through transcriptional and post-translational regulation of junD during hematopoiesis.
EXPERIMENTAL PROCEDURES
Xenopus Embryo Manipulations and Animal Cap Assay
All procedures were followed as described previously (15).
Plasmid Constructs and in Vitro Transcription
XjunD (GenbankTM accession no. BC079782) and Xc-fos (GenbankTM accession no. BC079689) cDNAs were isolated from Xenopus and were inserted into the EcoRI/XbaI site of the pCS2 (+) vector and the FLAG- or HA-tagged pCS2 (+) vector by PCR. For examining XjunD-MO specificity, the XjunD cDNA was cloned into the pCS2-HA vector at the BamHI/EcoRV site. For rescue experiments, mouse junD cDNA was cloned into the pcDNA3.1 (+) vector. Capped mRNAs were synthesized from linearized vectors using with Ambion mMESSAGE Machine kit (Ambion, Austin, TX). The SCL-mAP1-luciferase reporter gene, which is mutated at the consensus AP-1 binding site, was generated by site-directed mutagenesis kit (Stratagene, LA Jolla, CA).
Morpholino
The translation-blocking morpholino oligonucleotides (MOs) for Xenopus junD, MO1 (5′-CAGGTTAAGGTTCAGATCCTTTTTC-3′) and MO2 (5′-TGATAGAAGGGTATTTCCATC-ATCC-3′), were generated by GeneTools. MO1 and MO2 were designed to block translation of endogenous XjunD. For loss of function of XjunD, a mixture of two MOs was used and control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′), provided by GeneTools, was used as a toxicity control. Oligonucleotides were resuspended in sterile water and injected at doses of 20 ng per embryo.
qRT-PCR
Total RNA was prepared using the TRIzol reagent (Tel-Test, Inc., Friendswood, TX), and cDNA was synthesized using Superscript pre-amplification system (Invitrogen). The PCR primers and cycling conditions are described at the Xenopus Molecular Marker Resource (University of Texas).4 Additional primers are described in supplemental data. PCR reactions were carried out with SYBR Premix (Qiagen, Valencia, CA) and a thermal cycler real time system (Qiagen Rotor-Gene-Q, Valencia, CA).
Luciferase Assays
All procedures were followed as described previously (15).
Benzidine Staining of Blood
Animal caps were dissected from the injected embryos at stages 8 and 9 and cultured until stage 30. Animal caps were fixed for 5 min in 12% glacial acetic acid containing 0.4% benzidine (Sigma B-3503). The reaction was initiated by the addition of hydrogen peroxide to a final concentration of 0.3% and incubated at room temperature. The reaction was monitored for color development and photographed immediately.
Cell Lineage Tracing
GFP mRNA (200–300 pg) alone or together with mRNA of junD and c-fos was injected into dorsal animal blastomeres (D1) or ventral animal blastomeres (V1) of 8-cell stage embryos. Injected embryos were cultured to stages 27–30. The embryos were rinsed several times with 1× PBS and fixed for 1 h at room temperature in MEMFA (0.1 m MOPS, 2 mm EGTA, 1 mm MgSO4, 3.7% formaldehyde) and stored at 4 °C. Fixed embryos were observed with a fluorescent microscope (15).
Whole Mount in Situ Hybridization
Embryos were injected with the indicated mRNAs and then processed for whole-mount in situ hybridization by using standard methods (16) with α-globin probes.
Western Blot Analysis
All procedures were followed as described previously (15). For Western blotting, anti-HA-peroxidase conjugate (Roche Applied Science), anti-phospho-Jun (α-73) (Cell Signaling, Danvers, MA), and monoclonal anti-actin (Sigma) were used. Proteins were visualized using ECL Western blotting detection reagents (Amersham Biosciences).
RESULTS
AP-1JunD/c-Fos Has a Role in Hematopoiesis
To define the function of AP-1JunD/c-Fos in hematopoiesis, we analyzed the gene expression profile induced by AP-1JunD/c-Fos in embryonic stem cells of Xenopus animal caps explants. Co-expression of junD and c-fos induced hematopoietic transcription factors, GATA-1, SCL, LMO-2, and Neptune, which are involved in the initial blood program (Fig. 1A), and α-globin, the erythrocyte specific marker, in a dose-dependent manner (Fig. 1B). Otherwise, junD or c-fos mRNA alone could not induce globin (supplemental Fig. 1). Hematopoietic transcription factors such as GATA-1, SCL, LMO-2, and Neptune alone do not induce hematopoietic cells from the embryonic ectoderm. Interestingly, AP-1JunD/c-Fos alone is sufficient to induce blood cell formation from the ectoderm similar to BMP-4 signaling.
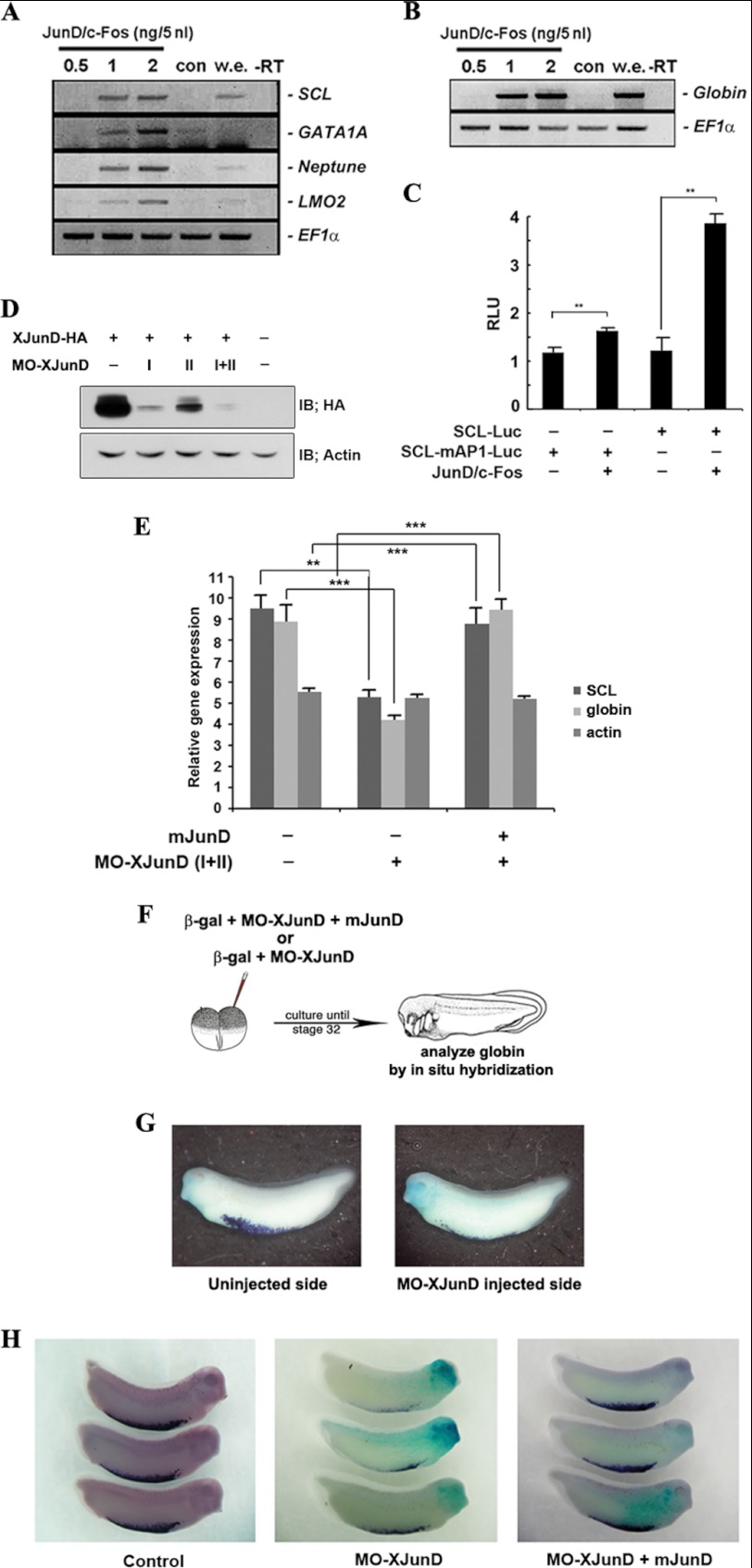
FIGURE 1.
AP-1JunD/c-Fos is required for hematopoiesis. A and B, co-expression of junD and c-fos induces hematopoietic makers (SCL, GATA1A, Neptune, and LMO2) and globin. Animal caps, explanted from embryos injected with the indicated concentration of mRNAs encoding junD or/and c-fos, were incubated until stages 18–20 (A) or 24–28 (B) and used for qRT-PCR analysis. C, co-expression of junD and c-fos enhances the promoter activities of the SCL but not the mutant SCL-mAP1. Embryos injected with the SCL- or SCL-mAP1-luciferase reporter gene alone or together with 2 ng of junD and c-fos were incubated until stages 18–20. Luciferase activity was measured. Values are shown as means ± S.D. from at least three independent experiments. RLU, relative luciferase activity. D, MO junD (20 ng) specifically knocks down the translation of the overexpressed C-terminal HA-tagged XJunD protein at stage 18. Actin served as a specificity control. E, mouse junD rescues SCL and globin, which are repressed by MO junD expression without changing the dorsal mesoderm marker, actin. qRT-PCR analysis of whole embryos expressing MO junD (20 ng) alone or in combination with 1 ng of mouse junD at stages 20–24. F, illustration of the scheme of the experiment. One blastomere of two-cell embryos was injected with mRNA encoding β-galactosidase together with MO junD (20 ng) or mouse junD (1 ng) (mjunD) as illustrated. G and H, embryos were stained for β-galactosidase (β-gal) activity at stage 30 (blue stain) followed by in situ hybridization analysis of globin expression (purple stain). G, the expression of globin is repressed in the MO junD-injected side. H, mouse junD mRNA (mjunD) rescues globin expression that is repressed by expression of MO junD. EF1α, loading control; w.e., whole embryo was used as a positive control for PCR; −RT, control reaction without reverse transcriptase; con, animal cap samples obtained from non-injected embryos. **, p value < 0.01; ***, p value < 0.001. IB, immunoblot.
Additionally, we examined whether AP-1JunD/c-Fos could regulate the transcription of SCL using an SCL promoter-luciferase reporter gene. AP-1JunD/c-Fos enhanced the promoter activity of this gene (Fig. 1C) but not the activity of SCL-mAP-1-luciferase, which is mutated at the consensus AP-1 binding site, suggesting the involvement of AP-1JunD/c-Fos in the transcriptional regulation of the SCL gene.
To further determine whether AP-1JunD/c-Fos is indeed necessary for hematopoiesis in Xenopus laevis development, we performed a loss-of-function study using morpholino antisense directed against junD (MO-junD). We generated two types of antisense morpholino oligonucleotides (MO1 and MO2) capable of depleting the XJunD protein. The MO-junDs were effective in specifically reducing the level of the 3′-terminal end HA-tagged XJunD protein (XJunD-HA) (Fig. 1D), whereas the control MO had no effect on the translation of XjunD mRNA (supplemental Fig. 2A). qRT-PCR analysis showed that the junD-depleted embryos repressed the expression of SCL and globin compared with control embryos without affecting the dorsal mesoderm marker, actin. Additionally, co-injection of mRNA of mouse junD (mjunD) rescued these markers that had been repressed by MO-junDs (Fig. 1E). The control MO-injected embryos had no effect on the expression of these markers (supplemental Fig. 2B). Furthermore, XjunD- or control MO were injected into one blastomere of two-cell embryos, together with the lineage tracer β-galactosidase (200 pg; Fig. 1F). Embryos were cultured until stage 32 and stained for β-galactosidase activity to confirm the accuracy of injections (blue staining, Fig. 1, G and H), followed by in situ hybridization to detect expression of globin, a marker of differentiated red blood cells (RBCs) (purple staining, Fig. 1, G and H). Embryos injected with MO-junD appeared normal but showed a severe repression of globin expression in the MO-junD-injected side relative to those of non-injected side or control MO-injected side (Fig. 1G). Expression of globin was effectively rescued by co-injection of mouse junD (Fig. 1H). Taken together, these results indicate that AP-1JunD/c-Fos is required for normal primitive erythropoiesis of X. laevis.
AP-1JunD/c-Fos Converts Dorsal-fated Tissue into Ventral-fated tissue, Specifically VBI
To elucidate the in vivo function of AP-1JunD/c-Fos during Xenopus embryogenesis, we performed a cell lineage tracing experiment. Embryos at the eight-cell stage were injected with GFP mRNA alone or together with AP-1 mRNA (junD and c-fos) into animal blastomeres at the dorsal portion of the embryos (D1) (Fig. 2A). Two of the dorsal animal blastomeres at the eight-cell stage embryo (D1) differentiated into dorsal and anterior neural tissues. Thus, when GFP mRNA alone was injected into the D1 region, fluorescent cells were detected in dorsal and anterior structures (Fig. 2B). However, when GFP and a low dose of AP-1JunD/c-Fos mRNAs (500 pg) were co-injected into the D1 regions, fluorescent cells were detected in the VBI region but also remained in the dorsal region (Fig. 2C). The VBI is indicated by in situ hybridization of globin (Fig. 2, B and C, lower panels). When AP-1JunD/c-Fos mRNAs were injected into animal blastomeres of the ventral part of the embryos (V1) at the eight-cell stage as a control experiment (Fig. 2D), GFP was expressed at the ventroposterior epidermis (Fig. 2E) (17, 18). A similar pattern was seen with control embryos injected with GFP mRNA into the ventral part of animal blastomeres (Fig. 2F). These results indicate that junD and c-fos not only induce hematopoiesis but also have the ability to change a part of dorsal fated tissue into VBI tissue in whole embryos.
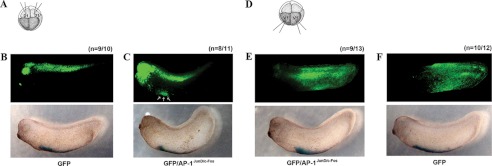
FIGURE 2.
AP-1JunD/c-Fos converts dorsal-fated tissue into ventral-fated tissue (ventral blood island). A and D, illustration of the scheme of the experiment. GFP mRNA (200 pg, B) only (B and F) or GFP together with AP-1 (junD and c-fos, 500 pg) mRNA (C and E) were injected into dorsal animal blastomeres (D1) or ventral animal blastomeres (V1) of eight-cell stage embryos and then cultured until stages 28–30. Embryos were fixed, and GFP expression was observed by green fluorescent microscopy. B and C, dorsally expressed GFP (B, upper panel) is partially transferred into the ventral blood island region (C, three arrows in upper panel). E and F, embryos injected with either GFP alone (F, upper panel) or together with AP-1JunD/c-Fos (E, upper panel) show a similar expression pattern of GFP, which is expressed at the ventroposterior epidermis. The ventral blood island is indicated by in situ hybridization of globin (B–F, lower panel). The number (n) of phenotypes for each group is presented.
AP-1JunD/c-Fos Is Required for the BMP-4-induced Hematopoiesis
BMP-4 is a TGF-β family member that has been shown to be an essential factor in the differentiation of primitive blood cells in the X. laevis embryo.
To further verify the role of AP-1JunD/c-Fos in hematopoiesis, we investigated whether the role of AP-1JunD/c-Fos in blood formation is associated with BMP-4. First, we determined whether AP-1JunD/c-Fos and BMP-4 have synergistic effects in hematopoiesis. For analyzing the synergistic effect between AP-1 and BMP-4, we used a low dose of mRNA. A low dose of AP-1JunD/c-Fos or BMP-4 alone had no apparent effect on the expression of hematopoietic markers (Fig. 3A). As indicated earlier, for hematopoietic markers and globin expression, a high dose of BMP-4 mRNA is required in animal cap explants. A combination of AP-1JunD/c-Fos and BMP-4 resulted in a marked increase in the level of hematopoietic markers, including globin (Fig. 3A). The expression of hematopoietic markers and globin induced by the combination of AP-1JunD/c-Fos and BMP-4 is higher than the additive value of each alone. To further confirm this finding, we performed benzidine staining, a specific histochemical stain for differentiated red blood cells. Animal caps derived from embryos injected with β-galactosidase as a control (Fig. 3B, fourth panel) were not stained by benzidine. The low dose of BMP-4-injected animal caps also did not exhibit detectable benzidine staining (Fig. 3B, third panel). However, consistent with the qRT-PCR data, a combination of AP-1JunD/c-Fos and BMP-4 enhanced benzidine staining (Fig. 3B, second panel) compared with each alone (Fig. 3B, first panel). These results indicate a synergistic effect of AP-1JunD/c-Fos and BMP-4 on blood formation.
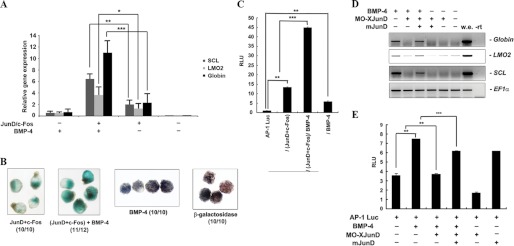
FIGURE 3.
AP-1JunD/c-Fos is required for hematopoiesis induced by BMP-4. A and B, animal caps, explanted from embryos injected with the indicated mRNAs were incubated until stage 20–24 and used for qRT-PCR analysis (A) or benzidine staining (B). A, AP-1JunD/c-Fos and BMP-4 synergistically induce hematopoietic markers and globin. B, blood formation stained by benzidine is synergistically enhanced by AP-1JunD/c-Fos and BMP-4. C, the activity of the (AP-1)4-luciferase reporter gene is synergistically activated by co-injection of AP-1JunD/c-Fos and BMP-4. An (AP-1)4-luciferase assay using animal cap explants derived from embryos injected with the (AP-1)4-luciferase reporter gene alone or in combination with the indicated mRNA was performed. Luciferase activity was measured at stage 18. Values are averages from at least three independent experiments. RLU, relative luciferase activity. D, animal caps, explanted from embryos injected with the indicated mRNAs or MO junD (20 ng), were incubated until stage 20–24 and used for qRT-PCR analysis. MO JunD selectively blocks BMP-4-induced expression of globin, LMO2, and SCL. Injection of mouse junD mRNA rescues the BMP-4 induction of globin, LMO2, and SCL as well as the activity of the (AP-1)4-luciferase reporter gene in MO junD-injected animal caps (E). EF1α, loading control; w.e., whole embryo was used as a positive control for PCR; −rt, control reaction without reverse transcriptase. **, p value < 0.01; ***, p value < 0.001.
On the basis of these results, we examined whether BMP signaling acts upstream of AP-1JunD/c-Fos during blood formation. We first determined whether BMP-4 could regulate AP-1 activity in an animal cap assay. The activity of the (AP-1)4-luciferase reporter gene, which contains four AP-1 binding sequences (TGAC/GTCA), was enhanced by AP-1 (junD and c-fos) or BMP-4 alone. These results suggest that BMP-4 regulates AP-1JunD/c-Fos activity.
Additionally, co-injection of AP-1 and BMP-4 caused a synergistic effect on the activation of AP-1 (Fig. 3C). This result is consistent with the synergistic effect of AP-1JunD/c-Fos and BMP-4 on blood formation of Xenopus embryos.
To confirm whether AP-1JunD/c-Fos is indeed necessary for BMP-4-induced blood formation, we investigated whether depletion of junD could inhibit the expression of BMP-4-induced hematopoietic markers. MO-junD (Fig. 3D) but not control MO (supplemental Fig. 3) effectively inhibited the BMP-4-induced expression of hematopoietic transcription markers (SCL and LMO2), including globin. Consistent with this result, the activity of the AP-1 reporter gene that was enhanced by BMP-4 was suppressed by depletion of junD (Fig. 3E, lanes 1–3). Additionally, the expression of hematopoietic markers and the activity of the AP-1 reporter gene, which were suppressed by MO-junD in the presence of BMP-4, were effectively rescued by co-overexpression of wild-type mjunD mRNA (Fig. 4, D and E). Taken together, these data support the idea that AP-1JunD/c-Fos is required for BMP-4-mediated hematopoiesis during X. laevis development.
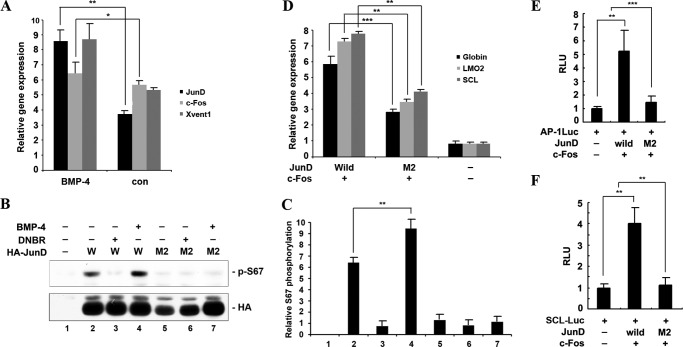
FIGURE 4.
Transcriptional and post-translational modification of AP-1JunD/c-Fos by BMP-4 is required for hematopoiesis. A, BMP-4 activates the transcription of junD, but not c-fos. Xvent1 (ventral marker) was used as a positive control for BMP-4. Animal caps derived from embryos injected with the indicated mRNA were excised and cultured until stage 13 and used for qRT-PCR analysis. con, animal cap samples obtained from non-injected embryos. B, BMP-4 enhances phosphorylation of XJunD at serine 67; in contrast, dominant-negative BMP receptor inhibits the phosphorylation of XJunD. Embryos injected with wild-type HA-junD (W) or mutant HA-junD (M2) alone or in combination with 1 ng of BMP-4 or dominant-negative BMP receptor mRNA were used for Western blotting. Phosphorylation of JunD was analyzed by Western blot using anti-phospho-Jun (α-73). Western blotting with anti-HA shows that equal amounts of expressed JunD were loaded. C, band density was measured using the NIH ImageJ program. D–F, animal caps, explanted from embryos injected with the indicated mRNAs or in combination with the (AP-1)4-luciferase reporter gene or SCL-luciferase reporter gene, were used for qRT-PCR analysis (D) and the luciferase assay (E and F). The concentration of each mRNA injected into embryos was 1 ng. D, AP-1M2JunD/c-Fos shows a lower induction of hematopoietic markers and globin, compared with AP-1JunD/c-Fos. E and F, the AP-1- and SCL-luciferase activities are consistent with D. Data are shown as means ± S.D. of values from at least three independent experiments. RLU, relative luciferase activity. **, p value < 0.01; ***, p value < 0.001.
Phosphorylation and Transcriptional Regulation of JunD by BMP-4 Is Important for the Function of AP-1JunD/c-Fos in Hematopoiesis
As demonstrated above, the biological ability of AP-1JunD/c-Fos is required for BMP-4-induced hematopoiesis. To study whether BMP-4 regulates AP-1JunD/c-Fos in the embryo, we investigated transcriptional regulation of AP-1JunD/c-Fos by BMP-4. Interestingly, BMP-4 increased the transcription of junD, but not c-fos (Fig. 4A). Xvent1 was used for positive control of BMP-4 (19, 20). Additionally, we investigated whether BMP-4 could phosphorylate JunD in embryos. Serine phosphorylation is a mechanism for regulating AP-1-dependent gene transcription (21, 22). A phospho-Jun antibody (α-73) was used for recognizing junD only if it is phosphorylated at serine 100 (23, 24). To examine phosphorylation of junD by BMP-4, we generated HA-tagged wild-type junD and mutant (M2) junD in which Ser-66, a sequence conserved with Ser-100 of mouse junD, was replaced with alanine. Phosphorylation of Ser-66 of JunD was detected using a phospho-Jun antibody (α-73). BMP-4 stimulation enhanced JunD phosphorylation at Ser-66, whereas dominant-negative BMP receptor efficiently inhibited phosphorylation of JunD (Ser-66) (Fig. 4B). The M2 mutant was not affected by BMP-4 or dominant-negative BMP receptor (Fig. 4B). The band density was measured using the NIH ImageJ program (Fig. 4C). This result indicates that BMP-4 is sufficient to regulate Ser-66 phosphorylation of JunD.
To study the biological role of Ser-66 phosphorylation in hematopoiesis of the X. laevis embryo, we compared the activity of mutant M2 junD and wild-type JunD in hematopoiesis of Xenopus embryos. Compared with wild-type junD and c-fos (AP-1JunD/c-Fos), the mutant junD (M2) and c-fos (AP-1M2JunD/c-Fos) induced hematopoietic markers at a lower level as determined by an animal cap assay (Fig. 4D). Consistently, both AP-1- and SCL-luciferase activities were less activated by AP-1M2JunD/c-Fos compared with AP-1JunD/c-Fos (Fig. 4, E and F). Taken together, the results suggest that transcriptional regulation and phosphorylation by BMP-4 is important for the biological role of AP-1JunD/c-Fos in hematopoiesis of Xenopus development.
DISCUSSION
In the current study, we provide evidence for a novel function of the transcription factor AP-1JunD/c-Fos in hematopoiesis during Xenopus development. Furthermore, we suggest that transcriptional and post-translational regulation of junD, which is induced by BMP-4, is important for the biological function of AP-1JunD/c-Fos in hematopoiesis (supplemental Fig. 4).
The transcription factor AP-1 is composed of different possible dimer combinations formed between the Jun and Fos family of “bZip” transcription factors. These different AP-1 dimers exhibit similar DNA binding specificities (TGAC/GTCA) but have differences in their transactivation efficiencies (5–8), indicating that distinct AP-1 dimers differentially regulate AP-1 target genes. Thus, we hypothesized that the composition of AP-1 would determine the expression of specific target genes and allow prediction of the nature of the dimeric combinations, which are physiologically relevant under various conditions.
Previously, we have shown that AP-1 is involved in mesoderm induction as a major downstream mediator of FGF signaling (25, 26). Moreover, we demonstrated that heterodimeric AP-1, comprised of c-Jun and c-Fos, mediated activin-induced Spemann organizer gene expression (15). We also reported the involvement of AP-1 in BMP-4 signaling and in BMP-4 expression (26, 27). Although the role of other AP-1 components has been reported in X. laevis development, the physiological function of heterodimeric AP-1 comprised of JunD and c-Fos has not yet been elucidated in X. laevis development. In the present study, we elucidated the function of heterodimeric AP-1 comprised of JunD and c-Fos in hematopoiesis of X. laevis development.
To determine the specificity of junD in BMP-4-induced hematopoesis, we examined whether c-jun had an effect in BMP-4-induced hematopoiesis. Depletion of c-jun using MO c-jun had no effect on BMP-4-induced hematopoiesis; in contrast, MO c-jun effectively inhibited activin-induced gene expression (15), suggesting a specific biological function of AP-1JunD/c-Fos downstream of BMP-4 (supplemental Fig. 5). Additionally, depletion of junD using MO junD had no effect on activin-induced dorsal mesoderm and endoderm formation (supplemental Fig. 6). Taken together, these results indicated that c-Jun and JunD have distinct roles downstream of activin and BMP-4, suggesting that specific AP-1 composition regulated by diverse signaling determines the expression of specific target genes.
Transcription factors are tightly regulated at the transcriptional, post-transcriptional, and post-translational levels. In the current studies, we discovered that AP-1JunD/c-Fos is regulated downstream of BMP-4 signaling at the transcriptional and post-translational levels, and the regulation is important for AP-1JunD/c-Fos-induced hematopoiesis. Serine phosphorylation is a representative mechanism for regulating AP-1-dependent gene transcription (21–22). Although numerous studies on the phosphorylation of c-Jun have been reported, JunD phosphorylation and its possible regulatory function have not been well characterized. Previously, JunD has been shown to be phosphorylated in vivo and in vitro (23–24, 28–31). The N terminus of junD contains three sites for MAP kinase phosphorylation (serines 90 and 100 and threonine 117), which are essentially identical to the well characterized regulatory phosphorylation sites of c-Jun (serines 63 and 73 and threonine 91). These sites share a conserved sequence with serines 56 and 66 and threonine 83 of Xenopus JunD. Generally, phosphorylation of serine 100 of JunD was studied to examine JunD activity because the phospho-Jun antibody (α-73) recognizes phosphorylated serine 100 of JunD. Thus, in the present study, we confirmed that phosphorylation of serine 66 of Xenopus JunD, which is identical to serine 100 of mouse JunD, was also detected with the phospho-Jun antibody (α-73). Results indicated that BMP-4 signaling could regulate phosphorylation of serine 66 of Xenopus JunD. However, the kinase activated by BMP-4 that is responsible for phosphorylating JunD at serine 66 remains to be identified in future studies (supplemental Fig. 4).
Additionally, we characterized the biological role of serine 66 of XJunD in hematopoiesis. Overexpression of the phosphorylation mutant form of AP-1M2JunD/c-Fos partially retained activity for its biological role in hematopoiesis, suggesting that other phosphorylation sites might also contribute to the complete biological function of AP-1JunD/c-Fos in hematopoiesis.
In the present study, we suggested a new function for AP-1 in hematopoiesis during Xenopus development providing additional evidence of a specific role for individual AP-1 members in distinct cellular process. Furthermore, we suggest that AP-1JunD/c-Fos functions downstream of BMP-4 signaling and BMP-4 alters AP-1JunD/c-Fos function at the post-translational level by phosphorylation of JunD at serine 66.
Supplementary Material
Acknowledgment
We thank Ms. Tonya Poorman for help submitting our manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants CA077646, CA111536, CA120388, R37 CA081064, and ES016548. This work was also supported by The Hormel Foundation, the Basic Science Research Program (2009-0077052) through the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology, and Hallym University Research Fund, 2011 (HRF-201109-058).

This article contains supplemental “Experimental Procedures” and Figs. 1–6.
E. M. De Robertis, personal communication.
- AP-1
- activator protein-1
- VBI
- ventral blood island
- MO
- morpholino oligonucleotide
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Angel P., Karin M. (1991) The role of Jun, Fos, and the AP-1 complex in cell proliferation and transformation. Biochim. Biophys. Acta 1072, 129–157 [DOI] [PubMed] [Google Scholar]
- 2. Jochum W., Passegué E., Wagner E. F. (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20, 2401–2412 [DOI] [PubMed] [Google Scholar]
- 3. Ameyar M., Wisniewska M., Weitzman J. B. (2003) A role for AP-1 in apoptosis: The case for and against. Biochimie 85, 747–752 [DOI] [PubMed] [Google Scholar]
- 4. Shaulian E., Karin M. (2002) AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4, E131–136 [DOI] [PubMed] [Google Scholar]
- 5. Shaulian E., Karin M. (2001) AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 [DOI] [PubMed] [Google Scholar]
- 6. Chinenov Y., Kerppola T. K. (2001) Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20, 2438–2452 [DOI] [PubMed] [Google Scholar]
- 7. Tulchinsky E. (2000) Fos family members: Regulation, structure, and role in oncogenic transformation. Histol. Histopathol. 15, 921–928 [DOI] [PubMed] [Google Scholar]
- 8. Vogt P. K. (2001) Jun, the oncoprotein. Oncogene 20, 2365–2377 [DOI] [PubMed] [Google Scholar]
- 9. Mangia F., Procicchiami G., Manelli H. (1970) On the development of the blood island in Xenopus laevis embryos: light and electron microscope study. Acta Embryol. Exp. (Palermo) 2, 163–184 [PubMed] [Google Scholar]
- 10. Kau C. L., Turpen J. B. (1983) Dual contribution of embryonic ventral blood island and dorsal lateral plate mesoderm during ontogeny of hemopoietic cells in Xenopus laevis. J. Immunol. 131, 2262–2266 [PubMed] [Google Scholar]
- 11. Maéno M. (2003) Regulatory signals and tissue interactions in the early hematopoietic cell differentiation in Xenopus laevis embryo. Zoolog. Sci. 20, 939–946 [DOI] [PubMed] [Google Scholar]
- 12. Maeno M., Mead P. E., Kelley C., Xu R. H., Kung H. F., Suzuki A., Ueno N., Zon L. I. (1996) The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood 88, 1965–1972 [PubMed] [Google Scholar]
- 13. Huber T. L., Zon L. I. (1998) Transcriptional regulation of blood formation during Xenopus development. Semin. Immunol. 10, 103–109 [DOI] [PubMed] [Google Scholar]
- 14. Perry C., Soreq H. (2002) Transcriptional regulation of erythropoiesis. Fine tuning of combinatorial multidomain elements. Eur. J. Biochem. 269, 3607–3618 [DOI] [PubMed] [Google Scholar]
- 15. Lee S. Y., Yoon J., Lee H. S., Hwang Y. S., Cha S. W., Jeong C. H., Kim J. I., Park J. B., Lee J. Y., Kim S., Park M. J., Dong Z., Kim J. (2011) The function of heterodimeric AP-1 comprised of c-Jun and c-Fos in activin mediated Spemann organizer gene expression. PloS one 6, e21796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore K. B., Mood K., Daar I. O., Moody S. A. (2004) Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev. Cell 6, 55–67 [DOI] [PubMed] [Google Scholar]
- 17. Itoh K., Tang T. L., Neel B. G., Sokol S. Y. (1995) Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development 121, 3979–3988 [DOI] [PubMed] [Google Scholar]
- 18. Huang S., Johnson K. E., Wang H. Z. (1998) Blastomeres show differential fate changes in 8-cell Xenopus laevis embryos that are rotated 90 degrees before first cleavage. Dev. Growth Differ. 40, 189–198 [DOI] [PubMed] [Google Scholar]
- 19. Friedle H., Rastegar S., Paul H., Kaufmann E., Knöchel W. (1998) Xvent-1 mediates BMP-4-induced suppression of the dorsal-lip-specific early response gene XFD-1' in Xenopus embryos. EMBO J. 17, 2298–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gawantka V., Delius H., Hirschfeld K., Blumenstock C., Niehrs C. (1995) Antagonizing the Spemann organizer: Role of the homeobox gene Xvent-1. EMBO J. 14, 6268–6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weston C. R., Davis R. J. (2002) The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12, 14–21 [DOI] [PubMed] [Google Scholar]
- 22. Yazgan O., Pfarr C. M. (2002) Regulation of two JunD isoforms by Jun N-terminal kinases. J. Biol. Chem. 277, 29710–29718 [DOI] [PubMed] [Google Scholar]
- 23. Gallo A., Cuozzo C., Esposito I., Maggiolini M., Bonofiglio D., Vivacqua A., Garramone M., Weiss C., Bohmann D., Musti A. M. (2002) Menin uncouples Elk-1, JunD, and c-Jun phosphorylation from MAP kinase activation. Oncogene 21, 6434–6445 [DOI] [PubMed] [Google Scholar]
- 24. Vinciguerra M., Vivacqua A., Fasanella G., Gallo A., Cuozzo C., Morano A., Maggiolini M., Musti A. M. (2004) Differential phosphorylation of c-Jun and JunD in response to the epidermal growth factor is determined by the structure of MAPK targeting sequences. J. Biol. Chem. 279, 9634–9641 [DOI] [PubMed] [Google Scholar]
- 25. Dong Z., Xu R. H., Kim J., Zhan S. N., Ma W. Y., Colburn N. H., Kung H. (1996) AP-1/jun is required for early Xenopus development and mediates mesoderm induction by fibroblast growth factor but not by activin. J. Biol. Chem. 271, 9942–9946 [DOI] [PubMed] [Google Scholar]
- 26. Kim J., Lin J. J., Xu R. H., Kung H. F. (1998) Mesoderm induction by heterodimeric AP-1 (c-Jun and c-Fos) and its involvement in mesoderm formation through the embryonic fibroblast growth factor/Xbra autocatalytic loop during the early development of Xenopus embryos. J. Biol. Chem. 273, 1542–1550 [DOI] [PubMed] [Google Scholar]
- 27. Xu R. H., Dong Z., Maeno M., Kim J., Suzuki A., Ueno N., Sredni D., Colburn N. H., Kung H. F. (1996) Involvement of Ras/Raf/AP-1 in BMP-4 signaling during Xenopus embryonic development. Proc. Natl. Acad. Sci. U.S.A. 93, 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stocco C. O., Lau L. F., Gibori G. (2002) A calcium/calmodulin-dependent activation of ERK1/2 mediates JunD phosphorylation and induction of nur77 and 20α-hsd genes by prostaglandin F2α in ovarian cells. J. Biol. Chem. 277, 3293–3302 [DOI] [PubMed] [Google Scholar]
- 29. Rosenberger S. F., Finch J. S., Gupta A., Bowden G. T. (1999) Extracellular signal-regulated kinase 1/2-mediated phosphorylation of JunD and FosB is required for okadaic acid-induced activator protein 1 activation. J. Biol. Chem. 274, 1124–1130 [DOI] [PubMed] [Google Scholar]
- 30. Smart D. E., Green K., Oakley F., Weitzman J. B., Yaniv M., Reynolds G., Mann J., Millward-Sadler H., Mann D. A. (2006) JunD is a profibrogenic transcription factor regulated by Jun N-terminal kinase-independent phosphorylation. Hepatology 44, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 31. Wang H., Xie Z., Scott R. E. (1996) JunD phosphorylation and expression of AP-1 DNA binding activity modulated by serum growth factors in quiescent murine 3T3T cells. Oncogene 13, 2639–2647 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.