Background: Immunoglobulin E (IgE) has two cellular receptors, FcϵRI and CD23, that mediate distinct functional effects.
Results: We have identified the CD23 binding site on IgE and show that FcϵRI and CD23 allosterically compete for binding.
Conclusion: A mechanism of communication exists within the IgE molecule to prevent simultaneous engagement with the two receptors.
Significance: Competition between IgE receptors explains ligand cross-regulation.
Keywords: Allergy, Allosteric Regulation, Antibodies, Immunology, NMR, Protein-Protein Interactions, CD23, Immunoglobulin E
Abstract
IgE, the antibody that mediates allergic responses, acts as part of a self-regulating protein network. Its unique effector functions are controlled through interactions of its Fc region with two cellular receptors, FcϵRI on mast cells and basophils and CD23 on B cells. IgE cross-linked by allergen triggers mast cell activation via FcϵRI, whereas IgE-CD23 interactions control IgE expression levels. We have determined the CD23 binding site on IgE, using a combination of NMR chemical shift mapping and site-directed mutagenesis. We show that the CD23 and FcϵRI interaction sites are at opposite ends of the Cϵ3 domain of IgE, but that receptor binding is mutually inhibitory, mediated by an allosteric mechanism. This prevents CD23-mediated cross-linking of IgE bound to FcϵRI on mast cells and resulting antigen-independent anaphylaxis. The mutually inhibitory nature of receptor binding provides a degree of autonomy for the individual activities mediated by IgE-FcϵRI and IgE-CD23 interactions.
Introduction
Immunoglobulin E (IgE) is the antibody isotype responsible for mediating allergic reactions. It functions through interactions with its two receptors, FcϵRI4 and CD23 (also known as FcϵRII). The binding of IgE to FcϵRI is essential for type I hypersensitivity, whereas the interaction between CD23 and IgE is crucial for IgE-mediated facilitated allergen binding, processing, and presentation (1). Through interactions with membrane IgE, soluble CD23 fragments can up- or down-regulate synthesis of IgE, depending on the oligomerization state of CD23 (2). IgE expression can also be controlled by a negative feedback mechanism through an interaction of IgE·allergen complexes with membrane-bound CD23 on IgE+ B cells (3). Because CD23 both positively and negatively regulates IgE expression, a critical role for CD23 in IgE homeostasis has been proposed.
High-resolution structures have been determined for Fc fragments of IgE (4, 5), the extracellular region of FcϵRIα (6), the C-type lectin domain of CD23 (7, 8), and complexes of IgE-Fc·FcϵRIα (4, 9). The structures of the complex explain the 1:1 stoichiometry observed for the IgE-Fc-FcϵRIα interaction; one FcϵRIα molecule engages two IgE Cϵ3 domains simultaneously near the Cϵ2-Cϵ3 domain interface. In contrast, the stoichiometry of binding CD23 to IgE is 2:1 (10), with a biphasic affinity, trimeric CD23 apparently binding with an affinity an order of magnitude higher than monomeric CD23 (11).
The structure of IgE is noteworthy for a marked bend between the second and third constant domains of the Fc region. It has been suggested that this bend imparts conformational constraints on the Fab arms, which might favor cross-linking of mast cell-bound IgE by allergens with specific disposition of epitopes (12). The IgE Fc region shows an intriguing mixture of structural rigidity and conformational flexibility, with the aforementioned rigid bend between the Cϵ2 and Cϵ3 domains (5) and an unusual degree of intrinsic structural lability within the Cϵ3 domain (13). Conformational flexibility around the Cϵ3-Cϵ4 interface has been noted previously (14); motions around an axis at this interface control whether both Cϵ3 domains are in a correct orientation to bind simultaneously to the FcϵRIα receptor. If only one Cϵ3 domain binds to FcϵRIα, then the affinity is about 10,000-fold weaker than when both Cϵ3 domains are engaged (15).
In this study, we define the CD23 binding site on the Cϵ3 domain of IgE using NMR spectroscopy and site-directed mutagenesis. We show that the CD23 and FcϵRI binding sites occur on opposite ends of the Cϵ3 domain of IgE. We demonstrate that allosteric inhibition prohibits simultaneous binding of these two receptors and that this mechanism prevents engagement and cross-linking of IgE bound to mast cells by soluble CD23.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Recombinant human IgE-Fc (composed of domains Cϵ2-Cϵ4) (5), the αγ-fusion protein (the FcϵRIα extracellular region fused to an IgG4 Fc) (10), soluble FcϵRIα (13), derCD23 (7), and the Cϵ3 domain (13) were each produced and purified as described previously. mAb 7.12 was produced from a B cell hybridoma (16).
NMR Spectroscopy
NMR spectroscopy was performed on protein samples in a buffer containing 25 mm Tris, 125 mm NaCl, 4 mm CaCl2, pH 6.8, at protein concentrations between 120 and 900 μm. Data were collected at 25 °C on Bruker spectrometers equipped with CryoProbes operating at 500 and 700 MHz. For chemical shift perturbation experiments, unlabeled derCD23 ligands were concentrated to 2 mm and then titrated in small aliquots to samples of 200 μm 15N-labeled Cϵ3 until saturation was seen. The NMR chemical shifts of the urea denatured and native state Cϵ3 domain are available from the BioMagResBank database under accession numbers 18482 and 18483.
Surface Plasmon Resonance
All experiments were performed on a Biacore T100 instrument (GE Healthcare), essentially as described previously (7, 9). All measurements were done independently at least twice, using standard double reference subtraction methods for data analysis (17). Specific binding surfaces were prepared using standard amine coupling methods for derCD23 and the αγ-fusion protein, whereas IgE-Fc was biotinylated and captured on a streptavidin surface. Ligands in HEPES-buffered saline (10 mm HEPES, pH 7.4, 150 mm NaCl, 4 mm CaCl2, 0.005% surfactant P20) were injected at 25 μl/min with a 1-min association phase followed by a 15-min dissociation phase. For the sandwich binding experiments, ∼90 resonance units of IgE-Fc was captured on an αγ-fusion protein surface during a 1-min injection of a 10 nm IgE-Fc sample; after a 3-min stabilization period, 0–100 μm derCD23 was injected for 1 min followed by a 15-min dissociation phase.
FRET Assay
Inhibition assays were performed by competing 1 μm terbium chelate-labeled derCD23 and 0–20 μm Alexa Fluor 647-labeled IgE-Fc with a range of concentrations of unlabeled αγ-fusion protein. Protein mixtures were prepared in LanthaScreen buffer (Invitrogen) in triplicate, in 384-well plates (Greiner Bio-One), and equilibrated overnight at room temperature. FRET measurements were made on an Artemis plate reader (Berthold Technologies). TR-FRET ratios were calculated for each well as the emission of acceptor at 665 nm divided by the emission of donor at 620 nm and then multiplied by 10,000.
Mast Cell Degranulation Assay
The human mast cell line LAD-2 (National Institutes of Health) was primed by the addition of 2.5 nm IgE (National Institute for Biological Standards and Control) or a buffer-only control for 1 h before the addition of cross-linking reagents. Polyclonal rabbit anti-human IgE (Dako) was added at 20 nm, and then soluble CD23 constructs were added at 0.1, 1 and 10 μm and incubated for 1 h at 37 °C. Supernatants were harvested and tested for β-hexosaminidase release, as described previously (18). The level of degranulation measured for Triton X-treated cells was defined as 100% release, and all samples were compared with that.
B Cell Activation Assays
Human tonsillar B cells were activated with IL-4 (200 IU/ml) (R&D Systems), anti-CD40 antibody (1 μg/ml) (G28.5; ATCC), and either 1 μm derCD23 or 1 μm triCD23, as described previously (19).
RESULTS
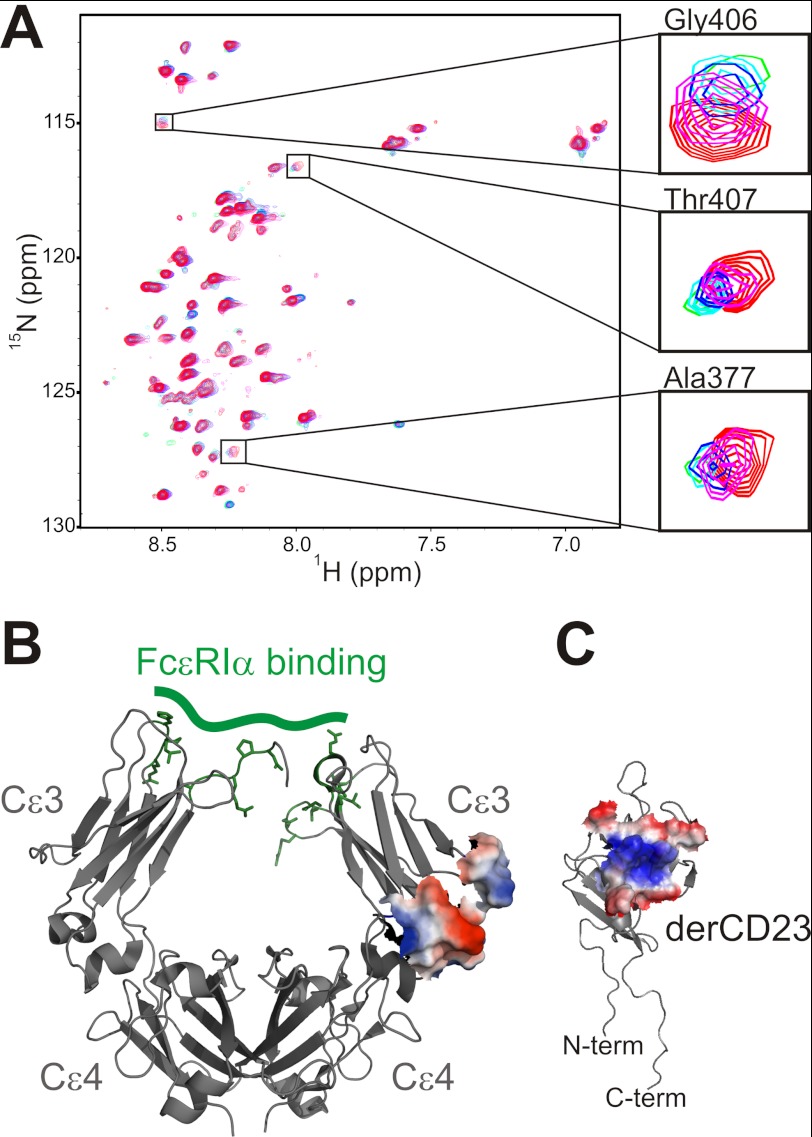
In an earlier study, we identified the IgE binding site on CD23 using NMR chemical shift perturbation studies (7). Here we performed the reciprocal NMR binding experiment, mapping the interaction site of CD23 onto the Cϵ3 domain from IgE. Using an approach described by Schulman et al. (20), we assigned the backbone resonances of the molten globule Cϵ3 domain by first performing resonance assignments of Cϵ3 denatured in 6 m urea and then, through gradual titration of buffer conditions, tracking those resonances to the native state Cϵ3 domain. Next, we titrated unlabeled monomeric CD23 protein (derCD23) against an 15N-labeled Cϵ3 sample and used the assigned NMR spectra to identify residues that were affected by the addition of ligand. Similar to what was observed on derCD23 in the reciprocal titration (7), a small number of Cϵ3 residues showed peak shifting and line broadening during the derCD23 titration (Fig. 1A), consistent with an interaction showing intermediate and fast/intermediate exchange kinetics. When mapped onto the surface of the protein, the identified residues from three discontinuous sequences (amino acids 405–407, 409–411, and 413 from the E-F helix, amino acids 377–380 from the C-D loop, and residue 436 from the C-terminal region) form a contiguous surface representing the binding site on Cϵ3 for CD23 (Fig. 1B). A plot of change in peak intensity versus residue number can be seen in supplemental Fig. S1.
FIGURE 1.
NMR mapping of the CD23 and IgE interaction surfaces. A, increasing amounts of unlabeled derCD23 were added to a 200 μm sample of 15N-labeled Cϵ3; five spectra of the titration are overlaid (red, zero derCD23; magenta, 50 μm; blue, 100 μm; cyan, 150 μm; green, 200 μm). Insets show magnified views of the indicated regions. B, the NMR-derived derCD23 interaction site on Cϵ3 was mapped onto the structure of IgE-Fc (1F6A (6)) and shown as surface representation. For comparison, the residues of IgE that interact with FcϵRI are indicated in green. C, the IgE interaction surface on CD23 was defined previously (Ref. 7 and shown here as a surface representation). The interacting surfaces of IgE and CD23 are colored according to electrostatic potential and coded such that regions with a potential <−4 kBT are red, whereas those >4 kBT are blue (kB, Boltzmann constant; T, absolute temperature).
This region is at the end of the Cϵ3 domain, near to the interface with Cϵ4, in contrast to the interaction site for FcϵRI, which is at the other end of Cϵ3 near the interface with Cϵ2 (4, 9) (Fig. 1B). Among other immunoglobulin-receptor interactions, sites analogous to the Cϵ3-Cϵ4 interface are utilized in the interactions of FcαRI with IgA (21), CHIR-AB1 with IgY (22), and FcRn, protein A, and protein G with IgG (23–25). A comparison of the CD23 binding surface on IgE with the analogous IgA and IgG binding surfaces shows areas of overlap but a nonconserved interaction motif, in contrast to the striking conservation of interaction surfaces for IgE·FcϵRIα and IgG·FcγR complexes (4).
The identification of this CD23 interaction site on IgE provides a structural explanation for the experimentally observed 2:1 (CD23:IgE) stoichiometry (10) as the dimeric IgE-Fc can bind to two separate CD23 lectin head domains. The two CD23 interactions were shown to have slightly different binding affinities and thermodynamic characteristics (10), as was also observed for the FcαRI-IgA interaction (21). The two binding affinities imply an asymmetry of the two CD23 binding sites, which may possibly be allosterically induced. The capacity of CD23 to induce a conformational change in IgE is discussed further below.
Following the NMR mapping of interaction epitopes for both proteins, we used site-directed mutagenesis to validate the interaction site in the context of the full IgE-Fc construct and to define the energetic contributions of individual residues. Ten mutants from derCD23 and 11 mutants from IgE-Fc (domains Cϵ2–4) were produced, purified, and characterized; their binding affinities were measured using an SPR assay (7). Table 1 summarizes the results of the site-directed mutagenesis studies. Mutations on both proteins that affect binding are entirely consistent with the NMR-defined interaction sites. Charged residues have the largest energetic effect on binding. CD23 mutations D227A, E257A, R224A, and R188A all show a change in binding free energy (ΔΔG) of about +6 kJ mol−1 (Table 1). Uncharged residues also contributed to the binding energy; a prominently exposed tyrosine residue (Tyr-189) in the center of the IgE binding site of CD23 made a substantial contribution to binding energy. The CD23 binding surface on IgE was also predominantly electrostatic, with residues Asp-409, Glu-412, Arg-376, and Lys-380 showing the largest effects on CD23 binding energetics. Because the NMR data indicated a site on Cϵ3 very near to the Cϵ4 interface (Fig. 1B) and because binding sites from several other immunoglobulin-receptor interactions involve sites analogous to the Cϵ3-Cϵ4 interface (21–23), we also made a pair of mutations in the F-G loop of the Cϵ4 domain, close to the CD23 binding site in Cϵ3. However, neither Q535A nor Q538A appeared to affect CD23 binding, leading us to believe that the CD23 binding surface on IgE is largely restricted to residues from Cϵ3.
TABLE 1.
Effects of mutations on the IgE-CD23 interaction
| IgE-Fc mutation | KD | ΔΔG | derCD23 mutation | KD | ΔΔG |
|---|---|---|---|---|---|
| μm | kJ mol−1 | μm | kJ mol−1 | ||
| Wild type | 2.3 | Wild type | 2.3 | ||
| D409A | 26.3 | +6.0 | D227A | 30.9 | +6.4 |
| E412A | 24.2 | +5.8 | E257A | 26.7 | +6.1 |
| R376A | 19.7 | +5.3 | R224A | 26.2 | +6.0 |
| K380A | 13.3 | +4.3 | R188A | 25.0 | +5.9 |
| K435A | 5.0 | +1.9 | Y189A | 15.6 | +4.7 |
| K352A | 3.8 | +1.2 | K276A | 10.9 | +3.9 |
| R351A | 2.6 | +0.3 | L226A | 6.6 | +2.6 |
| D347A | 2.5 | +0.2 | D236A | 5.8 | +2.3 |
| P439A | 2.5 | +0.2 | D192A | 4.3 | +1.6 |
| Q535A | 2.5 | +0.2 | E265A | 2.5 | +0.2 |
| Q538A | 2.4 | +0.1 |
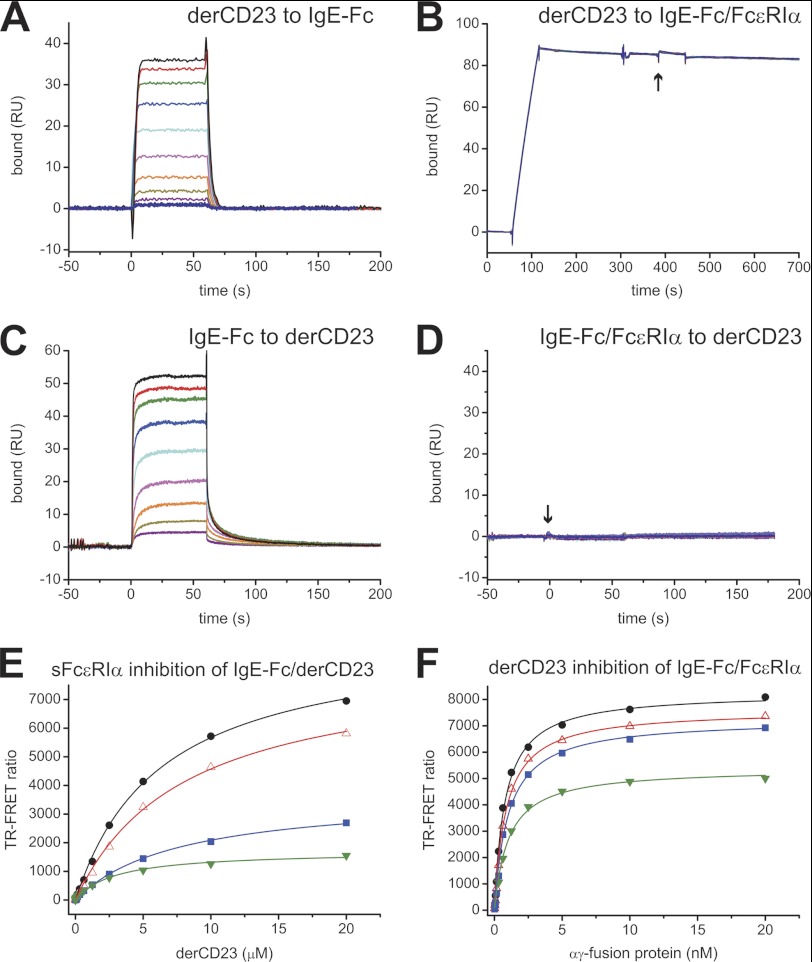
Earlier studies indicated that soluble CD23 can compete with FcϵRI binding, and this was attributed to steric competition for an overlapping binding site within the Cϵ3 domain (26, 27). However, our data show that the CD23 and FcϵRI binding sites are spatially distinct and suggest that the mechanism of mutual inhibition must be allosteric in nature. We performed a set of competitive binding assays to confirm this experimentally. Firstly, using an SPR assay, we showed that derCD23 can bind to IgE-Fc immobilized to an SPR chip but cannot bind to IgE-Fc captured by immobilized FcϵRIα (Fig. 2, A and B); a positive control, a Fab fragment of the anti-IgE antibody 7.12 (16), directed against the Cϵ2 domain, did bind to FcϵRIα-captured IgE-Fc (data not shown). Secondly, we showed that IgE-Fc can bind to immobilized derCD23, but an IgE-Fc·sFcϵRIα complex cannot bind to derCD23 (Fig. 2, C and D). These data indicated that CD23 and FcϵRI interactions with IgE are mutually inhibitory. Finally, we also tested the ability of the receptors to compete for binding to IgE in a solution TR-FRET experiment (28). This assay can be performed under equilibrium binding conditions, allowing a different set of mechanistic properties to be tested than in the kinetic SPR experiments. Under equilibrium conditions, different inhibition patterns are observed for competitive and allosteric inhibitors. A competitive inhibitor affects the apparent binding affinity, with inhibitor I reducing the apparent affinity by a ratio of (1+[I]/KI), whereas an allosteric inhibitor affects the apparent Bmax of the interaction without changing the apparent KD (29). Soluble FcϵRIα inhibited the IgE-Fc-derCD23 interaction (Fig. 2E) and derCD23 inhibited the IgE-Fc-FcϵRIα interaction (Fig. 2F), and both inhibitors resulted in a decrease of apparent interaction Bmax values without affecting the apparent KD of the interactions. These experiments confirmed mutual inhibition by the two IgE receptors and offer experimental evidence that an allosteric mechanism is involved.
FIGURE 2.
Competition binding experiments between derCD23 and sFcϵRIα for IgE-Fc. A and B, the binding of derCD23 was tested against IgE-Fc immobilized on a sensor surface (A) and IgE-Fc captured on an FcϵRIα-immobilized surface (B); the start of the derCD23 injection is indicated with an arrow. DerCD23 bound to immobilized IgE-Fc with a KD of 2.3 μm; no measureable binding was observed for derCD23 to IgE-Fc complexed to FcϵRIα. RU, resonance units. C and D, the binding of IgE-Fc to immobilized derCD23 (C) was compared with the binding of a complex of IgE-Fc·sFcϵRIα (D) to the same surface; the start of the injection of the complex is indicated with an arrow. IgE-Fc bound to derCD23 with a KD of 2.4 μm, but the IgE-Fc·sFcϵRIα complex did not bind to derCD23. All SPR binding experiments were performed using identical 2-fold serial dilutions of ligands, from 40 μm to 78 nm. E, binding between terbium-labeled derCD23 and Alexa Fluor 647-labeled IgE-Fc was measured in a solution TR-FRET assay in the presence of increasing concentrations of unlabeled αγ-fusion protein as inhibitor: 0 nm (black), 0.5 nm (red), 2.5 nm (blue), and 5 nm (green). F, binding between terbium-labeled αγ-fusion protein and Alexa Fluor 647-labeled IgE-Fc was measured with increasing concentrations of unlabeled derCD23 as inhibitor: 0 μm (black), 25 μm (red), 50 μm (blue), and 185 μm (green).
Given the location of the CD23 binding site, the most obvious mechanism for allostery is a conformational change around the interface between the Cϵ3 and Cϵ4 domains. Crystal structures of IgE-Fc and IgE-Fc·FcϵRIα complexes indicate that the Cϵ3 domains can exist in “open” and “closed” states, with only an open state being capable of binding FcϵRI (4, 9, 14). A detailed study of the open and closed states concluded that it is the motions around the Cϵ3 A-B helix, sitting at the Cϵ3-Cϵ4 interface, that control the orientation of the two Cϵ3 domains (14). Indeed, Wurzburg et al. (14) suggested that the Cϵ3-Cϵ4 domain interface might serve as a drug target for allosteric inhibitors of FcϵRI binding. It appears that nature has already utilized this approach to modulate FcϵRI binding of IgE by CD23.
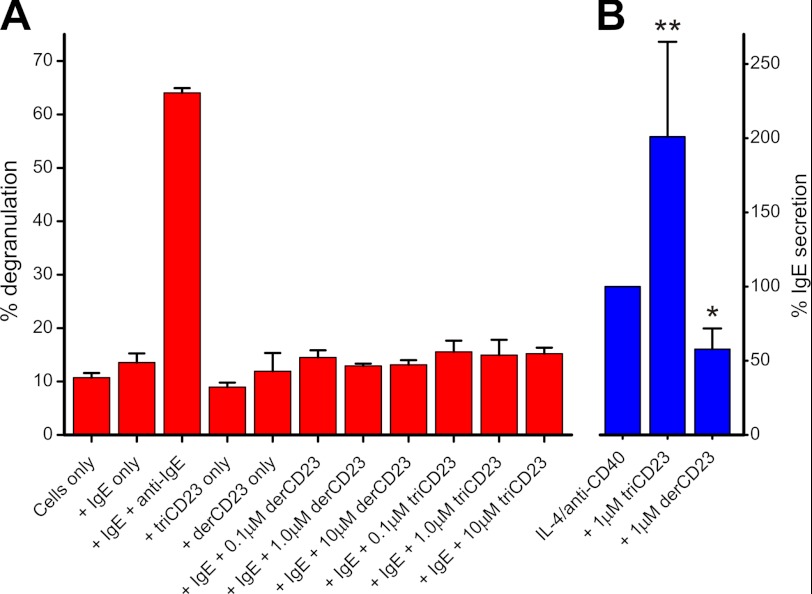
Soluble trimeric CD23 has been shown to bind to and cross-link membrane IgE on B cells, resulting in B cell activation (19). However, it is essential that trimeric CD23 not cross-link IgE bound to FcϵRI on the surface of mast cells. If this were to occur, then high levels of CD23 would result in mast cell activation in the absence of allergens. Our data from binding experiments (Fig. 2B) predicted that soluble CD23 cannot directly cross-link IgE bound to FcϵRI on mast cells. We tested this prediction in a mast cell degranulation assay using the FcϵRI+ LAD-2 human mast cell line. In this assay, cells were first primed by adding IgE followed by the addition of potential cross-linking reagents and measurement of release of the mast cell granule-associated enzyme β-hexosaminidase. An anti-IgE antibody resulted in FcϵRI-mediated activation of the mast cell and robust β-hexosaminidase release, but the addition of either the monomeric derCD23 or a trimeric CD23 construct (triCD23) failed to induce mast cell degranulation (Fig. 3A). In contrast, trimeric CD23 effectively cross-linked IgE on B cells, resulting in activation of these cells and increased production of soluble IgE (19) (Fig. 3B).
FIGURE 3.
Soluble CD23 does not cross-link IgE bound to FcϵRI on mast cells. The ability of soluble CD23 to engage IgE on B cells and mast cells was tested. A, after preincubation of IgE, the addition of anti-IgE antibody resulted in activation of the FcϵRIα+ LAD-2 mast cell line, as measured by release of β-hexosaminidase. Neither monomeric derCD23 nor trimeric triCD23 was able to cross-link IgE and activate mast cells in this assay. B, in contrast, triCD23 effectively cross-links membrane IgE on the surface of IgE+ human tonsillar B cells, resulting in activation of these cells and increased secretion of IgE. B cell cultures were activated with IL-4 and anti-CD40, and soluble CD23 was added at 1 μm; supernatants were harvested 12 days after activation, tested for IgE levels, and compared with levels for cells treated with IL-4/anti-CD40 alone (* = p < 0.05; ** = p < 0.01). The regulatory activities of soluble CD23 on IgE+ B cells are described in detail in Ref. 19.
DISCUSSION
Immunoglobulins have evolved two separate sites for binding to receptors. One site is near the hinge region in IgG and at the Cϵ2-Cϵ3 interface in IgE, whereas the other is at the interface of the C-terminal domain and the penultimate domain: the Cϵ3-Cϵ4 interface in IgE. A mechanism of communication has evolved within the IgE molecule between these two distant sites to prevent simultaneous engagement of CD23 and FcϵRI. This may be a unique property of IgE; it is known, for example, that IgG binding of either FcRn or protein A at the Cγ2-Cγ3 interface does not affect binding of FcγRIIa at the hinge region (30). Because IgE and CD23 both exist in membrane-bound and soluble forms, and soluble FcϵRIα has also recently been shown to exist at functionally relevant concentrations (31), there is considerable potential for receptor cross-regulation. Mutually exclusive receptor binding ensures independent functions for IgE-FcϵRI and IgE-CD23 interactions.
IgE is a clinically important drug target. An anti-IgE antibody (omalizumab) is an effective therapy, currently used in the treatment of moderate to severe asthma that is not controlled by corticosteroids. Omalizumab binds to the Cϵ3 domain of IgE and competitively inhibits FcϵRI binding, although its in vivo activity relies on more than just inhibition of this interaction (32). Results presented here demonstrate that IgE is amenable to allosteric inhibition, an approach that may have significant advantages over competitive inhibition (33) and lay the foundation for the development of allosteric modulators of IgE-receptor interactions.
Supplementary Material
Acknowledgments
We thank Dr. Rebecca Beavil (King's College London) for the gift of mAb 7.12 and Dr. Malcom Begg (GlaxoSmithKline, Stevenage, United Kingdom) for assistance with LAD-2 cell culture.
This work was supported by grants from the Wellcome Trust and the Medical Research Council.

This article contains supplemental Fig. S1.
- FcϵRI
- Fcϵ receptor I
- sFcϵRI
- soluble Fcϵ receptor I
- SPR
- surface plasmon resonance
- TR-FRET
- time-resolved FRET.
REFERENCES
- 1. Gould H. J., Sutton B. J., Beavil A. J., Beavil R. L., McCloskey N., Coker H. A., Fear D., Smurthwaite L. (2003) The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 21, 579–628 [DOI] [PubMed] [Google Scholar]
- 2. Aubry J. P., Pochon S., Graber P., Jansen K. U., Bonnefoy J. Y. (1992) CD21 is a ligand for CD23 and regulates IgE production. Nature 358, 505–507 [DOI] [PubMed] [Google Scholar]
- 3. Luo H. Y., Hofstetter H., Banchereau J., Delespesse G. (1991) Cross-linking of CD23 antigen by its natural ligand (IgE) or by anti-CD23 antibody prevents B lymphocyte proliferation and differentiation. J. Immunol. 146, 2122–2129 [PubMed] [Google Scholar]
- 4. Garman S. C., Wurzburg B. A., Tarchevskaya S. S., Kinet J. P., Jardetzky T. S. (2000) Structure of the Fc fragment of human IgE bound to its high-affinity receptor FcϵRIα. Nature 406, 259–266 [DOI] [PubMed] [Google Scholar]
- 5. Wan T., Beavil R. L., Fabiane S. M., Beavil A. J., Sohi M. K., Keown M., Young R. J., Henry A. J., Owens R. J., Gould H. J., Sutton B. J. (2002) The crystal structure of IgE Fc reveals an asymmetrically bent conformation. Nat. Immunol. 3, 681–686 [DOI] [PubMed] [Google Scholar]
- 6. Garman S. C., Kinet J. P., Jardetzky T. S. (1998) Crystal structure of the human high-affinity IgE receptor. Cell 95, 951–961 [DOI] [PubMed] [Google Scholar]
- 7. Hibbert R. G., Teriete P., Grundy G. J., Beavil R. L., Reljic R., Holers V. M., Hannan J. P., Sutton B. J., Gould H. J., McDonnell J. M. (2005) The structure of human CD23 and its interactions with IgE and CD21. J. Exp. Med. 202, 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wurzburg B. A., Tarchevskaya S. S., Jardetzky T. S. (2006) Structural changes in the lectin domain of CD23, the low-affinity IgE receptor, upon calcium binding. Structure 14, 1049–1058 [DOI] [PubMed] [Google Scholar]
- 9. Holdom M. D., Davies A. M., Nettleship J. E., Bagby S. C., Dhaliwal B., Girardi E., Hunt J., Gould H. J., Beavil A. J., McDonnell J. M., Owens R. J., Sutton B. J. (2011) Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcϵRI. Nat. Struct. Mol. Biol. 18, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi J., Ghirlando R., Beavil R. L., Beavil A. J., Keown M. B., Young R. J., Owens R. J., Sutton B. J., Gould H. J. (1997) Interaction of the low-affinity receptor CD23/FcϵRII lectin domain with the Fcϵ3–4 fragment of human immunoglobulin E. Biochemistry 36, 2112–2122 [DOI] [PubMed] [Google Scholar]
- 11. Dierks S. E., Bartlett W. C., Edmeades R. L., Gould H. J., Rao M., Conrad D. H. (1993) The oligomeric nature of the murine FcϵRII/CD23. Implications for function. J. Immunol. 150, 2372–2382 [PubMed] [Google Scholar]
- 12. Hunt J., Keeble A. H., Dale R. E., Corbett M. K., Beavil R. L., Levitt J., Swann M. J., Suhling K., Ameer-Beg S., Sutton B. J., Beavil A. J. (2012) A fluorescent biosensor reveals conformational changes in human immunoglobulin E Fc: implications for mechanisms of receptor binding, inhibition, and allergen recognition. J. Biol. Chem. 287, 17459–17470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price N. E., Price N. C., Kelly S. M., McDonnell J. M. (2005) The key role of protein flexibility in modulating IgE interactions. J. Biol. Chem. 280, 2324–2330 [DOI] [PubMed] [Google Scholar]
- 14. Wurzburg B. A., Garman S. C., Jardetzky T. S. (2000) Structure of the human IgE-Fc Cϵ3-Cϵ4 reveals conformational flexibility in the antibody effector domains. Immunity 13, 375–385 [DOI] [PubMed] [Google Scholar]
- 15. Hunt J., Beavil R. L., Calvert R. A., Gould H. J., Sutton B. J., Beavil A. J. (2005) Disulfide linkage controls the affinity and stoichiometry of IgE Fcϵ3–4 binding to FcϵRI. J. Biol. Chem. 280, 16808–16814 [DOI] [PubMed] [Google Scholar]
- 16. Kanowith-Klein S., Hofman F., Saxon A. (1988) Expression of Fcϵ receptors and surface and cytoplasmic IgE on human fetal and adult lymphopoietic tissue. Clin. Immunol. Immunopathol. 48, 214–224 [DOI] [PubMed] [Google Scholar]
- 17. Myszka D. G. (1999) Improving biosensor analysis. J. Mol. Recognit 12, 279–284 [DOI] [PubMed] [Google Scholar]
- 18. Hammond G., Koffer A. (2006) Secretion Assays, in Cell Biology (Celis J. E., ed) Third Ed., pp. 221–227, Elsevier, Amsterdam [Google Scholar]
- 19. Cooper A. M., Hobson P. S., Jutton M. R., Kao M. W., Drung B., Schmidt B., Fear D. J., Beavil A. J., McDonnell J. M., Sutton B. J., Gould H. J. (2012) Soluble CD23 controls IgE synthesis and homeostasis in human B cells. J. Immunol. 188, 3199–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulman B. A., Kim P. S., Dobson C. M., Redfield C. (1997) A residue-specific NMR view of the non-cooperative unfolding of a molten globule. Nat. Struct. Biol. 4, 630–634 [DOI] [PubMed] [Google Scholar]
- 21. Herr A. B., White C. L., Milburn C., Wu C., Bjorkman P. J. (2003) Bivalent binding of IgA1 to FcαRI suggests a mechanism for cytokine activation of IgA phagocytosis. J. Mol. Biol. 327, 645–657 [DOI] [PubMed] [Google Scholar]
- 22. Taylor A. I., Sutton B. J., Calvert R. A. (2010) Mutations in an avian IgY-Fc fragment reveal the locations of monocyte Fc receptor binding sites. Dev. Comp Immunol. 34, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin W. L., West A. P., Jr., Gan L., Bjorkman P. J. (2001) Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol. Cell 7, 867–877 [DOI] [PubMed] [Google Scholar]
- 24. Deisenhofer J. (1981) Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9 and 2.8 Å resolution. Biochemistry 20, 2361–2370 [PubMed] [Google Scholar]
- 25. Sauer-Eriksson A. E., Kleywegt G. J., Uhlén M., Jones T. A. (1995) Crystal structure of the C2 fragment of streptococcal protein G in complex with the Fc domain of human IgG. Structure 3, 265–278 [DOI] [PubMed] [Google Scholar]
- 26. Suemura M., Kikutani H., Sugiyama K., Uchibayashi N., Aitani M., Kuritani T., Barsumian E. L., Yamatodani A., Kishimoto T. (1991) Significance of soluble Fcϵ receptor II (sFcϵRII/CD23) in serum and possible application of sFcϵRII for the prevention of allergic reactions. Allergy Proc. 12, 133–137 [PubMed] [Google Scholar]
- 27. Kelly A. E., Chen B. H., Woodward E. C., Conrad D. H. (1998) Production of a chimeric form of CD23 that is oligomeric and blocks IgE binding to the FcϵRI. J. Immunol. 161, 6696–6704 [PubMed] [Google Scholar]
- 28. Selvin P. R. (2002) Principles and biophysical applications of lanthanide-based probes. Annu. Rev. Biophys. Biomol. Struct. 31, 275–302 [DOI] [PubMed] [Google Scholar]
- 29. Fersht A. (1999) Structure and Mechanism in Protein Science, pp. 103–131, W. H. Freeman, New York [Google Scholar]
- 30. Wines B. D., Powell M. S., Parren P. W., Barnes N., Hogarth P. M. (2000) The IgG Fc contains distinct Fc receptor (FcR) binding sites: the leukocyte receptors FcγRI and FcγRIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J. Immunol. 164, 5313–5318 [DOI] [PubMed] [Google Scholar]
- 31. Dehlink E., Platzer B., Baker A. H., Larosa J., Pardo M., Dwyer P., Yen E. H., Szépfalusi Z., Nurko S., Fiebiger E. (2011) A soluble form of the high-affinity IgE receptor, FcϵRI, circulates in human serum. PLoS One 6, e19098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Babu K. S., Arshad S. H., Holgate S. T. (2001) Anti-IgE treatment: an update. Allergy 56, 1121–1128 [DOI] [PubMed] [Google Scholar]
- 33. Schön A., Lam S. Y., Freire E. (2011) Thermodynamics-based drug design: strategies for inhibiting protein-protein interactions. Future Med. Chem. 3, 1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.