Background: Alternative pathway (AP) of complement is involved in proteinuria-derived tubular injury.
Results: Factor H and properdin recognize different nonoverlapping epitopes on tubular heparan sulfates.
Conclusion: Tubular heparan sulfate play an important role in renal AP regulation.
Significance: Low anticoagulant heparinoids can control AP-derived tubular injury.
Keywords: Complement System, Heparan Sulfate, Inflammation, Kidney, Proteoglycan
Abstract
During proteinuria, renal tubular epithelial cells become exposed to ultrafiltrate-derived serum proteins, including complement factors. Recently, we showed that properdin binds to tubular heparan sulfates (HS). We now document that factor H also binds to tubular HS, although to a different epitope than properdin. Factor H was present on the urinary side of renal tubular cells in proteinuric, but not in normal renal tissues and colocalized with properdin in proteinuric kidneys. Factor H dose-dependently bound to proximal tubular epithelial cells (PTEC) in vitro. Preincubation of factor H with exogenous heparin and pretreatment of PTECs with heparitinase abolished the binding to PTECs. Surface plasmon resonance experiments showed high affinity of factor H for heparin and HS (KD values of 32 and 93 nm, respectively). Using a library of HS-like polysaccharides, we showed that chain length and high sulfation density are the most important determinants for glycosaminoglycan-factor H interaction and clearly differ from properdin-heparinoid interaction. Coincubation of properdin and factor H did not hamper HS/heparin binding of one another, indicating recognition of different nonoverlapping epitopes on HS/heparin by factor H and properdin. Finally we showed that certain low anticoagulant heparinoids can inhibit properdin binding to tubular HS, with a minor effect on factor H binding to tubular HS. As a result, these heparinoids can control the alternative complement pathway. In conclusion, factor H and properdin interact with different HS epitopes of PTECs. These interactions can be manipulated with some low anticoagulant heparinoids, which can be important for preventing complement-derived tubular injury in proteinuric renal diseases.
Introduction
The complement system consists of a set of inactive liver-derived plasma components that are linked and activated in a cascading manner. Activation of the complement system occurs via three major pathways: (i) the alternative pathway (AP),2 which is spontaneously and constantly activated on biological surfaces); (ii) the classical pathway, which is triggered by immune complexes; and (iii) the lectin pathway, which is initiated by carbohydrates on microbial surfaces. Activation of each of these pathways results in assembly of the so-called C3 convertase, followed by formation of the C5 convertase, and finally the terminal C5b-9 membrane attack complex (MAC). Basically, the complement system has three major functions: via C3b opsonization the elimination of cells/microbes by phagocytosis, via the terminal C5b-9 complex lysis of cells/microbes, and via the anaphylactic C3a and C5a the recruitment of neutrophils and macrophages. Novel aspects are the local production of complement that function as danger signals to initiate and amplify inflammatory reactions and the production of complement by immune cells, having implications for transplantation and autoimmunity (1). The AP of the complement system has been shown to play an important role in various renal diseases (2). Over- or underactivation and mutations of the complement system can result in renal injury. It has been shown that complement-mediated tubular injury upon proteinuria occurs via two mechanisms: (i) the presence of complement factors in ultrafiltrate activates the AP on tubular cells or (ii) filtered serum proteins stimulate tubular cells to produce complement factors (3, 4).
The AP of complement is initiated by spontaneous generation of C3 convertase and has no discriminatory nature to recognize host cells from pathogens. Therefore, the AP needs a precise regulatory system. To this end, at least four membrane bound and two soluble regulatory proteins have been described in the AP (5). Factor H is one of the most important fluid phase as well as surface bound regulators of the AP, which is known to play a role in self-recognition by binding to negatively charged oligosaccharides on host cells (5, 6). Factor H is an abundant (0.3–0.5 mg/ml) 155-kDa glycoprotein composed of 20 complement control protein (CCP) modules (7). In electron microscopic images, factor H has the appearance of flexible “beads on a string” with the ability to fold back on itself (7, 8). There are three binding sites for C3b in factor H molecule, namely in CCP1–4, CCP7–15, and CCP19–20 (9), as well as two heparin-binding sites in CCP6–8 and CCP19–20. A third possible heparin-binding site in CCP12–14 has been suggested, although some studies have challenged this (9). N-terminal CCP1–4 has been shown to have decay accelerating and cofactor activity, whereas C-terminal CCP19–20 with binding sites for both C3b and heparins is known to be important for the interaction of factor H with the cell surface (7). Some microbes express ligands for CCP19–20 and CCP6–8 of factor H to recruit factor H and escape the AP (7).
Interaction of factor H (specially CCP19–20) with proteoglycans (PGs) and polyanions on cell surface is known to be the key for host cell recognition, and as such it is of great importance in tissues that lack the cell membrane-bound regulators of the AP (like basement membrane of glomeruli in kidney) (7, 9). To date, three diseases have been associated with mutations or polymorphisms of the two factor H heparin-binding sites, namely dense deposit disease (membranoproliferative glomerulonephritis type II), atypical hemolytic uremic syndrome, and age-related macular degeneration (10). In these diseases, overactivation of AP caused by nonfunctioning factor H results in tissue damage. Each of the heparin-binding sites of factor H has unique specificities; thus it has been suggested that cell type recognition by factor H depends on the PGs and polyanions expressed on the cells (7).
PGs are comprised of a core protein with glycosaminoglycan (GAG) side chains. GAGs are linear polysaccharides composed of a repetitive disaccharide structure. Based on the composition of GAG chains, PGs are categorized as heparan sulfate (HS), chondroitin sulfate, dermatan sulfate, and keratan sulfate proteoglycans (11, 12). PGs can be found on almost all mammalian cell surfaces and within extracellular matrices, whereas HS proteoglycans (HSPG) are predominantly found in basement membranes. They play an important role in cell activities via their interaction with chemokines, growth factors, and cytokines (13). The sulfation pattern on GAG chains can influence the activity of PGs by changing the negative charge of these molecules. The expression and sulfation pattern of GAG chains vary in different tissues, as well as in different pathologic conditions (14). The interaction of a number of complement factors with GAGs has been known for a long time. Heparins, HS, and other GAGs have been shown to mostly inhibit the three complement activation pathways via interaction with several complement factors like C1, C1q, C2, C4, C3b, factor H, factor B, and properdin (7, 15–19). Although there are data on the heparin-binding sites of factor H, little is known about the properties of heparins and GAG chains of PGs that interact with factor H. Previously, we showed that in proteinuria, properdin, which is the only positive regulator of the AP, interacts with HS on tubular cells and activates the AP (17). Furthermore, it has been shown that protein overload on renal tubular cells reduces their HS density and the binding of factor H to tubular epithelial cells (4). During proteinuria both properdin and factor H are readily available in urine for tubular cells. Because both proteins interact with GAGs on the tubular cells, the composition of tubular GAGs might be important in the regulation of the AP. Thus a better understanding of the nature of GAG-factor H interaction can lead to more specific therapeutic strategies in AP-mediated renal diseases.
In this study, we show that factor H binds to tubular epithelial cells via the HS polysaccharide side chains of HS proteoglycans and that this binding is partially dependent on the composition and sulfation pattern of those HS. We also show that factor H and properdin recognize different and nonoverlapping HS epitopes on renal tubular cells. In addition, we demonstrate selective inhibition of properdin binding to proximal tubular epithelial cells, without loss of factor H binding to these cells by some heparinoids with low anticoagulant activity. This finding might be of great importance for potential therapeutic interventions.
EXPERIMENTAL PROCEDURES
Tissues
Proteinuria was induced in male Wistar rats with a single injection of adriamycin as described previously (20). Protein overload nephropathy was also induced in male Wistar rats as described previously (21). Healthy male Wistar rats were used as the source of normal control rat renal tissue. The kidneys of rats were collected, quickly frozen in liquid nitrogen, and stored at −80 °C. Sections were cut and used for immunohistochemistry and immunofluorescent staining (see below).
Immunofluorescent Staining
Frozen sections (5-μm thickness) were cut from rat renal tissues with cryostat Leica CM1950. First, the sections were fixed in acetone for 10 min. The sections were then rehydrated in TBS, and endogenous peroxidase activity was blocked with 0.1% H2O2. After washing with TBS, the slides were incubated with 10 μg/ml goat anti-rat factor H antibody (prepared as described before (22)) or with a mixture of 10 μg/ml polyclonal rabbit anti-human properdin (prepared as described previously (23)) and 10 μg/ml goat anti-rat factor H antibodies diluted in 1% BSA for 1 h. The sections were incubated with HRP-labeled donkey anti-goat immunoglobulins (Jackson ImmunoResearch, West Grove, PA) (for single factor H staining) or a mixture of DyLight 488-conjugated donkey anti-rabbit antibody and HRP-labeled donkey anti-goat immunoglobulins (for properdin and factor H double staining) (1:100; diluted in 1% normal rat serum) for 45 min. The HRP antibody was detected by the TSATM tetramethylrhodamine system (PerkinElmer Life Sciences) (10 min, 1:50). For double staining of factor H and brush borders, additional incubation of sections with phalloidin-FITC (Sigma) for 15 min (1.5 μg/ml) was performed. The slides were washed again with TBS and mounted in VECTASHIELD mounting medium with DAPI for nuclear staining (Brunschwig Chemie, Amsterdam, The Netherlands). The whole staining procedure was performed at room temperature. The photographs in Fig. 1 were taken with a Leica DM 4000B microscope. The photographs shown in Fig. 2 were taken at the Imaging and Microscopy Center at the University Medical Center (Groningen, The Netherlands) with confocal microscopy (Zeiss LSM 780, Jena, Germany).
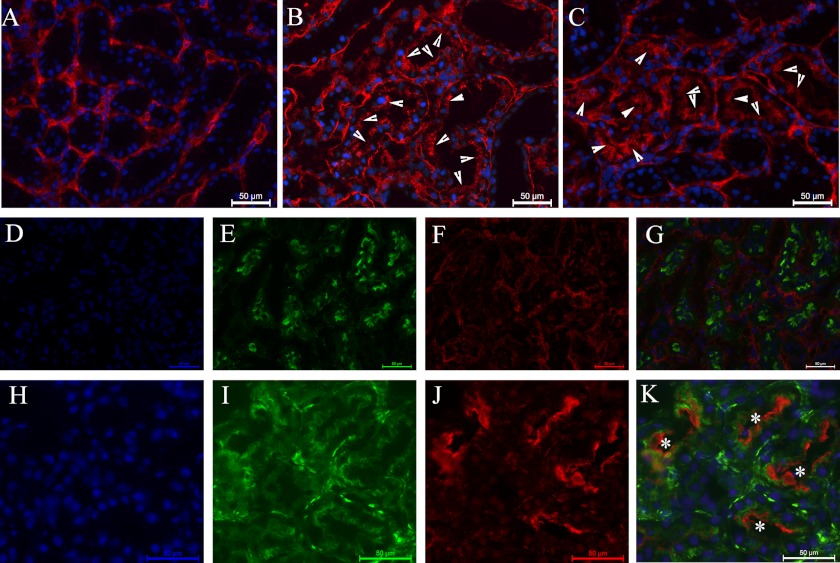
FIGURE 1.
Renal expression of factor H. A, factor H (in red) is present in interstitium of normal rat renal tissue. B and C, factor H is present in interstitium and luminal side of tubular cells (arrowheads) in protein overload (B) and adriamycin nephropathy (C). D–G, factor H (in red) is absent in luminal side of the tubuli in normal rat tissue. Phalloidin-FITC (in green) stains the brush border of the proximal tubuli. H–K, presence of factor H (in red) on top of phalloidin-FITC (in green) shows the luminal presence of factor H (white asterisks) in protein overload proteinuric rat renal tissue. Each scale bar shows 50 μm. The nuclei are stained with DAPI in blue.
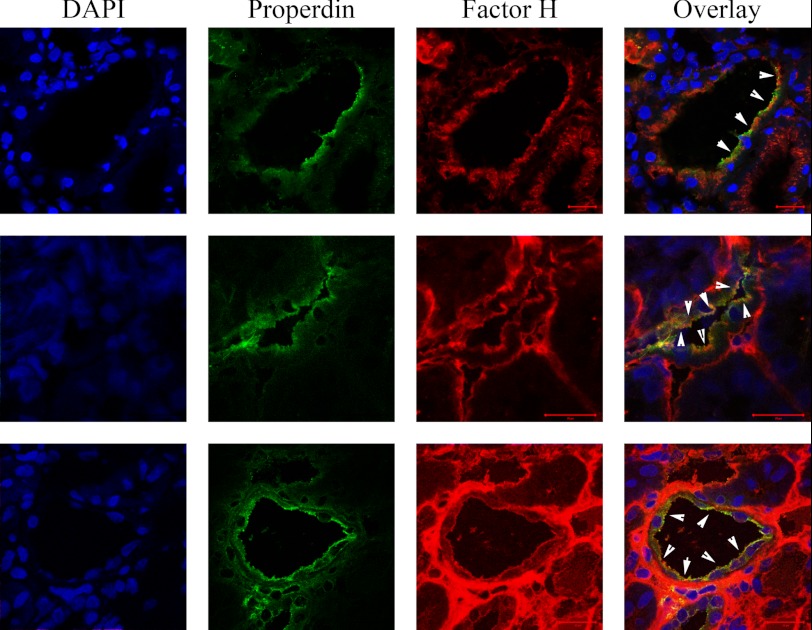
FIGURE 2.
Factor H and properdin colocalization on luminal side of tubuli. Factor H and properdin are both present on the luminal side of tubular cells in adriamycin-induced nephrosis. The nuclei are shown in blue, properdin is in green, and factor H is in red. The white arrowheads show colocalization areas of factor H and properdin on the luminal side of tubular cells. The scale bars represent 20 μm in all images.
HK-2 Cells
The immortalized human kidney proximal epithelial cell line HK-2 was provided by Dr. M. van der Toorn (Laboratory of Allergology and Pulmonary Diseases, University Medical Center Groningen, The Netherlands). The cells were cultured in DMEM/F-12 medium (Invitrogen) supplemented with 2 mm l-glutamine, 25 mm HEPES, 50 units/ml penicillin, 50 μg/ml streptomycin (all purchased from Invitrogen), and also 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, 36 ng/ml hydrocortisone, and 10 ng/ml EGF (all purchased from Sigma). For factor H staining on HK-2 cells, the cells were grown on cover glass in wells, in medium as described above. The medium was removed, and the cells were washed with TBS and incubated with 5% normal goat serum for 15 min. After washing with TBS, the cells were incubated with 10 μg/ml polyclonal rabbit anti-human factor H antibody (prepared as described previously (24, 25)). Bound anti-factor H antibody was detected by FITC-labeled goat anti-rabbit immunoglobulins (Southern Biotech, Birmingham, AL). The whole staining procedure was done on ice without fixation and permeabilization. For evaluating the binding sites for factor H on HK-2 cells, the binding assay was performed by incubation of the cells with 150 μg/ml human factor H (prepared as described previously (24, 25)) before incubation with anti-factor H antibody. Pretreatment of the cells with heparitinase I (from flavobacterium, 0.05 units/ml; Seikagaku Corporation, Tokyo, Japan) and chondroitinase ABC (from Proteus vulgaris, 5 units/ml; Seikagaku Corporation) was done for 1 h at 37 °C to cleave HS and chondroitin/dermatan sulfate side chains of proteoglycans on HK-2 cells, respectively. The heparitinase I and chondroitinase ABC were diluted in acetate buffer (50 mm C2H3O2Na, 5 mm CaCl2·H2O, 5 mm MgCl2·6H2O, pH 7.0 for heparitinase I or pH 8.0 for chondroitinase ABC). The heparin competitions on HK-2 cells were done by preincubation of human factor H (150 μg/ml) with various concentrations of porcine intestinal mucosal heparin (starting from 300 μg/ml with 2× dilutions) for 30 min at room temperature, followed by incubating the mixture for 1 h on cells and detecting factor H binding to the cells as described above.
Competition ELISA
Maxisorp 96-well plates (U96 from VWR International, Amsterdam, The Netherlands) were coated overnight in PBS with 5 μg/ml heparin-albumin (Sigma). After washing in PBS with 0.05% Tween 20, the wells were blocked with 3% skimmed milk powder in PBS for 1 h. In a separate microtiter plate, factor H (2 μg/ml diluted in PBS) was incubated with a dilution range of different HS-like heparinoids (see below) for 30 min, then transferred to the ELISA plate washed wells, and incubated for 1 h. The wells were washed again, and monoclonal mouse anti-human factor H antibody (2 μg/ml; Abcam, Cambridge, UK) diluted in PBS was added for 1 h. Secondary antibody was added after a washing step (HRP-labeled goat anti-mouse immunoglobulins, 1:250; DAKO, Glostrup, Denmark). Substrate reaction was done with 3,3′,5,5′-tetramethylbenzidine substrate (Sigma) for 15 min in the dark, and the reaction was stopped by adding 1.5 n H2SO4. Absorbance was measured at 450 nm in a microplate reader. All of the incubations were done at room temperature in a volume of 100 μl/well. For properdin and factor H competition ELISA, factor H was biotinylated as described before (26). Biotin-labeled factor H (10 μg/ml) was preincubated with various concentrations of properdin for 30 min before incubation on heparin-albumin-coated wells. Furthermore, increasing concentrations of properdin were incubated on heparin-albumin-coated wells followed by washing and incubation with 10 μg/ml biotin-factor H. The factor H-biotin complex was detected by streptavidin HRP. Substrate reaction and plate measurement were done in the same fashion as described above.
Polysaccharides
Heparin from porcine intestinal mucosa, dextran, dextran sulfate, fucoidan, and chondroitin sulfates A, B, and C were from Sigma. Keratan sulfate, isolated from bovine cartilage, was obtained from Fluka Biochemicals (Buchs, Switzerland). HS isolated from bovine kidney or from Engelbreth-Holm-Swarm (EHS) sarcoma and N-sulfated K5 were obtained from Seikagaku Corp. Escherichia coli capsular polysaccharide K5, with the same (GlcUA→GlcNAc)n structure as the nonsulfated HS/heparin precursor polysaccharide (27); O-sulfated K5; low molecular weight O-sulfated K5; and N-desulfated reacetylated heparin were kindly provided by Dr. G. van Dedem (Diosynth, Oss, The Netherlands). HS from human aorta was isolated as described by Iverius (28). Completely N- and O-desulfated heparin was prepared according to Bäckström et al. (29) and reacetylated as described above. N+O-sulfated K5 was produced by N-deacetylation (hydrazinolysis), subsequent N-sulfation with sulfur trioxide-trimethylamine (30, 31), followed by N-acetylation. Same N-sulfation procedure was also done with completely desulfated heparin to make O-desulfated, N-sulfated heparin (31).
Surface Plasmon Resonance-based Binding Assay
Surface plasmon resonance experiments were performed on a BIAcore 3000, using a CM5 sensor chip, HBS-P buffer (10 mm HEPES, 150 mm NaCl, 0.005% surfactant P20, pH 7.4), and the corresponding reagents from BIAcore. GAG functionalized surfaces were prepared as described before (32). Briefly, the sensor chip of four flow cells were activated with 50 μl of 0.2 m 1-ethyl-3-(dimethylaminopropyl) carbodiimide, 0.05 m N-hydroxy-succinimide and functionalized with streptavidin (0.2 mg/ml in 10 mm acetate buffer, pH 4.2). After quenching of the 1-ethyl-3-(dimethylaminopropyl) carbodiimide/0.05 m N-hydroxy-succinimide-activated groups with 1 m ethanolamine, pH 8.5, biotinylated heparin, HS, or dermatan sulfate were individually injected on three of the surfaces (immobilization levels of 50, 67, and 269 RU, respectively), the fourth surface was left as a negative control surface. Binding assays were performed at 10 μl/min by injecting 2-fold dilution series of factor H (0–500 nm) for 5 min, followed by a 5-min washing with HBS-P buffer. Regeneration was achieved by sequential injections of 0.05% SDS (0.5 min) and 2 m NaCl (2.5 min). The sensorgrams shown correspond to signals obtained after online subtraction of the negative control. The data were processed using the BIAevaluation 3.1 software to determine the on and off rate constants (kon and koff) from which the affinity (KD value) was calculated (KD = koff/kon). For competition assays, factor H (125 nm) was preincubated with polysaccharides (1 μg/ml) for 45 min at room temperature, prior to analysis.
FACS Analysis
Properdin and factor H binding to HK-2 cells, its dependence on tubular HS, and functional consequence of binding were assessed by flow cytometry. HK-2 cells were cultured in 48-well culture plates. The cells were incubated with heparitinase I (from flavobacterium, 0.05 units/ml) or chondroitinase ABC (5 units/ml) for 30 min at 37 °C. After washing, the cells were incubated with human properdin (10 μg/ml) and factor H (100 μg/ml) for 30 min at 37 °C. In addition, properdin and factor H were preincubated with various heparinoids (300 μg/ml) for 15 min at room temperature before incubation on untreated cells. Functional consequences of properdin and factor H binding were assessed by additionally washing and incubating cells with 5% pooled human serum for 60 min at 37 °C to allow complement activation. All dilutions and washing procedures so far were performed in serum-free culture medium. After washing, the cells were harvested using a cell scraper and subsequently stained for properdin, factor H, C3d, or C5b-9 using a rabbit polyclonal to human properdin (made in-house) or a mouse monoclonal to human factor H (Abcam, Cambridge, UK), C3d (Quidel, San Diego, CA), or C5b-9 (AE11; Hycult Biotech, Uden, Netherlands), followed by R-Phycoerythrin (RPE)-conjugated polyclonal goat anti-rabbit immunoglobulin (Southern Biotech Associates, Birmingham, AL) or Allophycocyanin (APC)-conjugated goat-anti-mouse (Beckman Coulter, Woerden, The Netherlands), respectively. All antibody incubations and wash steps were performed on ice for 30 min in FACS buffer (1% BSA, 0.02% sodium azide in PBS). Cell surface staining was assessed using a FACScalibur flow cytometer (BD Biosciences).
RESULTS
Factor H Is Present on the Luminal Side of Tubuli during Proteinuria
Normal rat kidney tissue showed the presence of endogenous factor H in glomeruli and interstitium, mostly localized in interstitial extracellular matrix, without any tubular staining for factor H. In both proteinuric models (protein overload nephropathy and adriamycin-induced nephrosis) factor H staining was not only observed interstitially but also on the luminal side of the tubular cells (arrowheads in Fig. 1). Luminal localization of factor H is evidenced by double staining with phalloidin-FITC, which binds to F-actin in tubular brush borders of proximal tubuli. As shown in Fig. 1 (D–G), in normal rat renal tissue, factor H was absent on the brush borders, whereas in protein overload nephropathy renal tissue (Fig. 1, H–K), factor H was localized on top of the brush borders (stained for phalloidin-FITC and shown in green). This suggested to us tubular binding of ultrafiltrate-derived factor H during proteinuria (Fig. 1, D–K).
As we showed previously (17), properdin can also be present on the apical side of tubular cells during proteinuria. Therefore, we investigated the presence of properdin and factor H in adriamycin induced nephropathy, using a double staining approach. We found colocalization of properdin and factor H on the apical sides of tubular cells in 20–25% of the tubuli. However, some tubuli were also observed to be positive for either factor H or properdin. Fig. 2 shows the colocalization of properdin and factor H on apical sides of tubuli in adriamycin-induced nephropathy. Because both properdin and factor H are known to interact with proteoglycans on tubular cells, we decided to further investigate factor H binding to renal tubular cells, the binding properties of GAGs for factor H, and the modulation of the AP of complement activation on tubular cells by GAGs.
Factor H Binds to Tubular Epithelial Cells via Heparan Sulfates
Because under proteinuric conditions, renal tubular cells become exposed to serum proteins including complement factors, and factor H has been found in the urines of proteinuric patients (33–35), we evaluated whether renal tubular cells can bind factor H. Renal proximal tubular epithelial cells (HK2 cells) did not express factor H in normal conditions as shown by negative FACS staining for factor H on these cells. Binding assay showed dose-dependent binding of factor H to HK2 cells (Fig. 3A). Binding of exogenous factor H to HK2 cells was inhibited by preincubation of factor H with heparin. Furthermore, heparitinase I pretreatment of HK2 cells abolished factor H binding, whereas chondroitinase ABC pretreatment did not show any effect on factor H binding to cells (Fig. 3B). Both heparin competition and enzyme (heparitinase I and chondroitinase ABC) digestion results were confirmed and visualized by immunofluorescent staining (Fig. 3, C–F). These data show HS-dependent binding of factor H to HK2 renal proximal tubular cells.
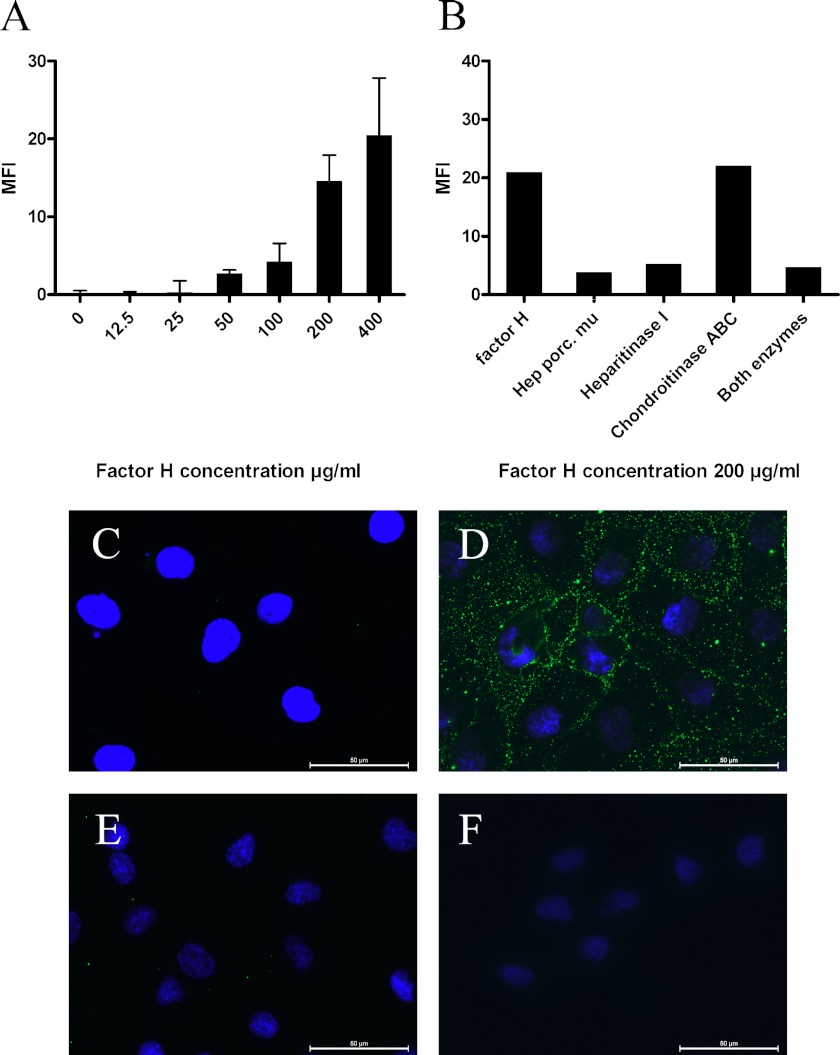
FIGURE 3.
HS-dependent binding of factor H to HK2 cells. A, factor H binds to HK2 cells in a dose-dependent manner in flow cytometry analysis; this graph shows the result of one (performed in duplicate) of four experiments. The results are expressed as mean fluorescence intensity (MFI) ± S.E. B, in a representative FACS experiment of three, HK-2 cells were incubated either with heparitinase I, chondroitinase ABC, or both heparitinase I and chondroitinase ABC before factor H incubation (100 μg/ml). Factor H binding was detected on the cells by FACS staining. The results are expressed as mean fluorescence intensity. C–F, immunofluorescent staining of HK2 cells. C, cultured HK2 cells do not express factor H. D, exogenous factor H (150 μg/ml) binds to HK2 cells. E, preincubation of exogenous factor H (150 μg/ml) with heparin (300 μg/ml) impairs the binding of factor H to these cells. F, heparitinase I pretreatment of HK2 cells abolish factor H binding to the cells. The scale bars represent 50 μm.
GAG Binding Properties of Full-length Factor H
Full-length Factor H interacted dose-dependently with immobilized heparin-albumin in ELISA, whereas there was no binding detected in the wells coated with albumin alone (Fig. 4A). In a series of competition ELISA experiments, various preparations of GAGs were used to inhibit the factor H interaction with coated heparin-albumin. The purpose of these ELISA experiments was to compare the binding properties of full-length factor H for different heparinoids and GAGs (Table 1). Not all the GAGs and HS preparations had the same potential for inhibiting factor H interaction with heparin-albumin. Unfractionated heparin dose-dependently inhibited the binding of factor H to heparin-albumin. Among HS, only one preparation from human aorta was able to inhibit factor H binding to heparin-albumin by 50% at the concentration of 25 μg/ml (Fig. 4B and Table 1). The E. coli K5 capsular polysaccharide has the same (Glc-GlcNAc)n structure as the unmodified biosynthetic precursor of heparin/HS. No inhibition was found with unmodified K5 (Fig. 4B). These data suggest that the degree of sulfation and GAG chain modification is important for its interaction with factor H. No inhibition was found with N-sulfated K5. However, with O-sulfated K5, a strong inhibition was detected (IC50 = 1.6 μg/ml). Depolymerization of full-length O-sulfated K5 into LMW fragments (mean molecular mass of ∼3000 Da), strongly reduced binding with factor H (Table 1). Surprisingly, introducing an additional sulfation group to O-sulfated K5 yielded a N+O-sulfated K5 preparation that had lost its ability to compete with heparin for binding to factor H. In other GAG category, only the highly sulfated preparations (dextran sulfate and fucoidan) inhibited the binding of factor H to immobilized heparin. (Table 1) The interaction of factor H with heparin-albumin was inhibited by exogenous heparin from porcine intestinal mucosa. However N- or O-desulfation of heparin resulted in the loss of inhibitory capacity, as well as depolymerization of heparin into LMW heparin (Table 1).
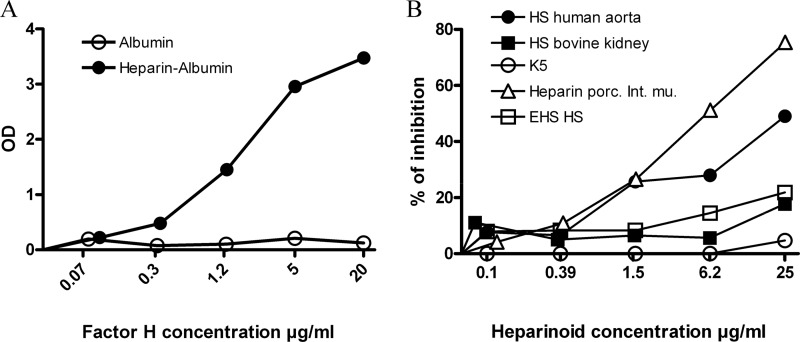
FIGURE 4.
Glycosaminoglycans interaction with full-length factor H in solid phase ELISA assay. A, factor H interacts with heparin side chains of heparin-albumin (closed circle) in a dose-dependent manner, whereas there is no interaction with albumin (open circle). B, in a representative solid phase ELISA assay, three preparation of HS, heparin from porcine intestinal mucosa, and K5 competed with coated heparin albumin (5 μg/ml) for binding to fluid phase factor H (2 μg/ml). All the GAG preparations have been tested in our assay two or more times. This graph shows the importance of sulfation degree on glycosaminoglycans chain for interaction with factor H. Optical density (OD) was measured at 450 nm.
TABLE 1.
Fluid phase inhibition of factor H binding to immobilized heparin-albumin by K5-derived polysaccharides, HS from different sources, different GAGs, and (chemically modified) heparins
Dose-response inhibition curves were generated as described under “Experimental Procedures,” and IC50 values were calculated.
| IC50 | |
|---|---|
| K5-derived polysaccharides | |
| K5 | >25 μg/ml |
| O-Sulfated K5 | ≈1.6 μg/ml |
| N-Sulfated K5 | >25 μg/ml |
| LMW O-sulfated K5 | >25 μg/ml |
| N+O-Sulfated K5 | >25 μg/ml |
| HS from different sources | |
| EHS HS | >25 μg/ml |
| HS-II human aorta | ≈25 μg/ml |
| HS bovine kidney | >25 μg/ml |
| Glycosaminoglycans | |
| Chondroitin sulfate A | >25 μg/ml |
| Chondroitin sulfate B | >25 μg/ml |
| Chondroitin sulfate C | >25 μg/ml |
| Keratan sulfate | >25 μg/ml |
| Dermatan sulfate | >25 μg/ml |
| Dextran T40 | >25 μg/ml |
| Dextran sulfate | <0.1 μg/ml |
| Fucoidan | ≈0,39 μg/ml |
| Heparins | |
| Heparin porcine intestinal mucosa | ≈6,5 μg/ml |
| LMW heparin porcine intestinal mucosa | >25 μg/ml |
| O-Desulfated heparin | >25 μg/ml |
| N-Desulfated heparin | >25 μg/ml |
| N-Desulfated reacetylated heparin | >25 μg/ml |
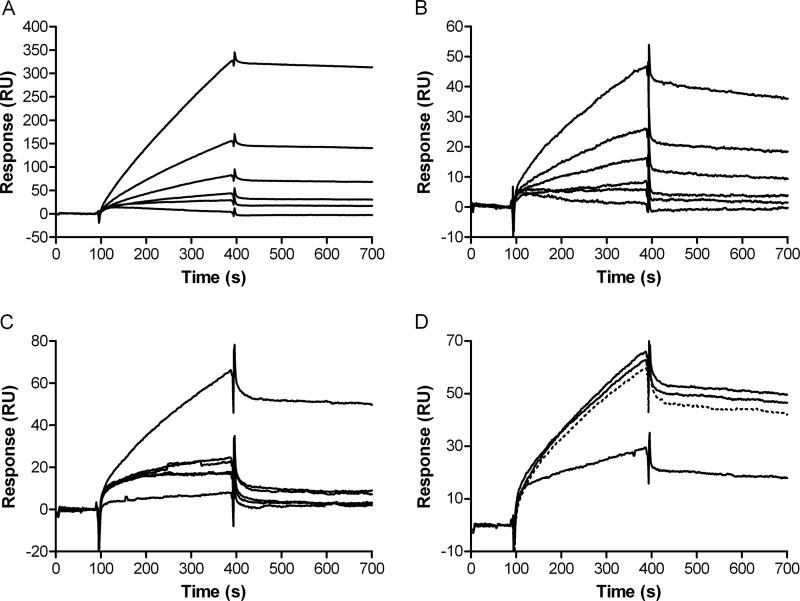
Next to ELISAs, surface plasmon resonance (BIAcore) experiments were performed to show the kinetics of factor H interaction with heparin, HS, and dermatan sulfate. Full-length factor H showed no binding to dermatan sulfate (data not shown), whereas a KD of 32 ± 2 nm (χ2 = 1.42) was calculated for factor H binding to heparin (Fig. 5A). Factor H binding to HS (from porcine intestinal mucosa) was observed in BIAcore; however, the affinity for HS was lower than that for heparin (KD 93 ± 5 nm, χ2 = 0.2) (Fig. 5B). Furthermore, BIAcore experiments demonstrated that fluid phase heparin dose-dependently prevented factor H from interacting with immobilized heparin or HS (Fig. 5C). In contrast, N-desulfated heparin and N-desulfated N-reacetylated heparin had no effect on binding of factor H to heparin-coated sensor chips (Fig. 5D). Based on these data, we conclude that factor H recognizes highly sulfated domains in full-length heparin and some HS isoforms. This suggests that high charge density is a crucial determinant for factor H binding to GAGs. Comparison of LMW O-sulfated K5 with full-length O-sulfated K5 and LMW heparin with unfractionated heparin reveals that next to sulfation, chain length is also an important determinant for factor H binding to GAGs. Because highly O-sulfated K5 preparations were also recognized by factor H, we concluded that C5 epimerization of GlcA units in the HS/heparin backbone is not important for factor H binding. Apparently, GAG backbone composition and the exact position of the sulfate groups are of less importance than sulfation density for factor H interaction, because unrelated GAGs (such as fucoidan and dextran sulfate) were also recognized by factor H.
FIGURE 5.
BIAcore analysis of factor H binding to heparin and HS. A and B, injection of (from bottom to top) 0, 31, 62, 125, 250, and 500 nm factor H on heparin-coated sensor chip (A) and HS-coated sensor chip (B). C, factor H (125 nm) was preincubated (from bottom to top) with 10, 5, 2.5, 1.25, 0.6, and 0 μg/ml heparin from porcine intestinal mucosa for 45 min at room temperature prior to injection on heparin-coated sensor chip. D, factor H (125 nm) preincubated with (solid lines from bottom to top) 1 μg/ml heparin from porcine intestinal mucosa, N-desulfated heparin, N-desulfated, N-reacetylated heparin, or factor H alone (dotted line) for 45 min at room temperature prior to injection on heparin-coated sensor chip.
Properdin and Factor H Recognize Different Epitopes in HS and Heparin
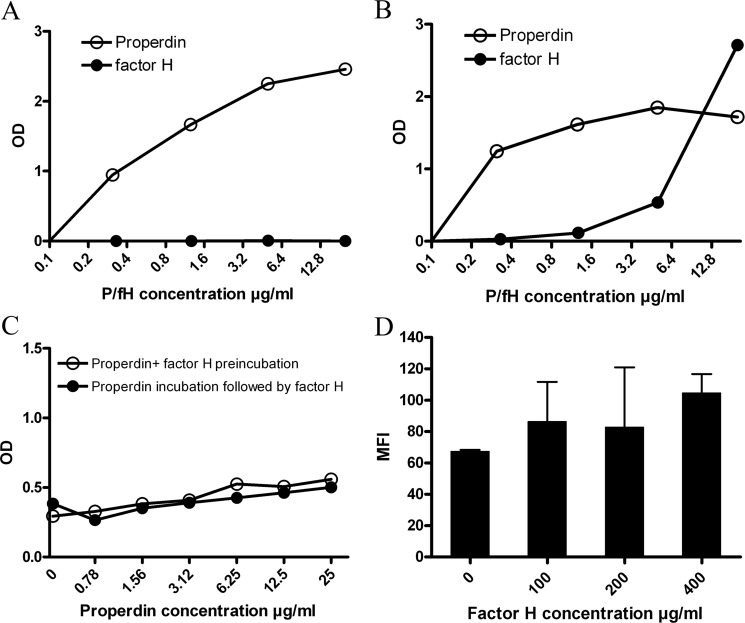
Previously, we showed that the major activator of the alternative complement pathway, properdin, binds to HS proteoglycans and heparin in various binding assays and to HS on tubular cells (17). Above, we showed that factor H, the main inhibitor of the AP, only binds to highly sulfated GAGs. Furthermore, we show that factor H is not able to bind to immobilized mouse EHS-perlecan HSPG in ELISA assay, whereas properdin interacts with EHS-perlecan dose-dependently (Fig. 6A). This may be explained by the absence of highly sulfated domains in EHS-perlecan HS (36). Although both properdin and factor H bind to immobilized heparin albumin, properdin interacts with heparin-albumin in lower concentrations compared with factor H (Fig. 6B). Previously, we used a library of heparinoids in inhibition ELISAs to show that properdin binds to both highly sulfated GAGs (e.g., heparin) and lower sulfated heparinoids, HS and GAGs (e.g., N-desulfated heparin) (17). The same inhibition ELISA assays with the same library of heparinoids was used for factor H (Table 1). These results showed that factor H in contrast to properdin interacts with highly sulfated heparinoids and HS and GAG preparations (Table 1 and Ref. 17).
FIGURE 6.
Properdin and factor H have different binding requirements on HS/heparin. A, properdin (open circles) interacts dose-dependently with HSPG in solid phase ELISA, whereas factor H (closed circles) does not interact with HSPG. B, heparin-albumin is able to interact with both properdin (open circles) and factor H (closed circles) in ELISA. Optical density (OD) measured at 450 nm. C, binding of factor H (10 μg/ml) to coated heparin-albumin did not change with increased concentration of properdin. Preincubation of factor H (10 μg/ml) with various concentrations of properdin (open circles) and incubation of properdin, followed by washing and factor H (10 μg/ml) incubation (closed circles) did not interfere with factor H binding to heparin. D, properdin binding (10 μg/ml) to HK2 cells is not impaired by increasing concentrations of factor H on the cells. The error bars represent S.E. of duplicates. All the assays in A–D have been performed three or more times; the graphs shown here are representative examples.
In the next experiments, we addressed the question whether factor H binding to heparin interferes with properdin binding (same or overlapping epitopes) or not (separated epitopes on heparin). To investigate this, a mixture of a constant amount of factor H was incubated with a dilution range of properdin on immobilized heparin-albumin (which was able to interact with both factor H and properdin) in ELISA plates, and factor H binding was detected. The interaction between factor H and heparin-albumin did not change with preincubation of factor H with dilutions of properdin (Fig. 6C). The same results were obtained, when properdin dilutions were incubated on heparin-albumin and washed, followed by incubation with a fixed concentration of factor H (Fig. 6C). These results show that the binding domains of properdin and factor H on heparin are distinct and not overlapping. Furthermore, properdin binding to HK2 cells did not change when 10 μg/ml of properdin was incubated with variable concentrations of factor H on HK2 cells in FACS staining (Fig. 6D). Taken together, these results confirm that properdin and factor H have different binding sites on HS of proximal tubular epithelial cells.
Low Anticoagulant Heparinoids Inhibit Properdin, but Not Factor H from Binding to HS/Heparin
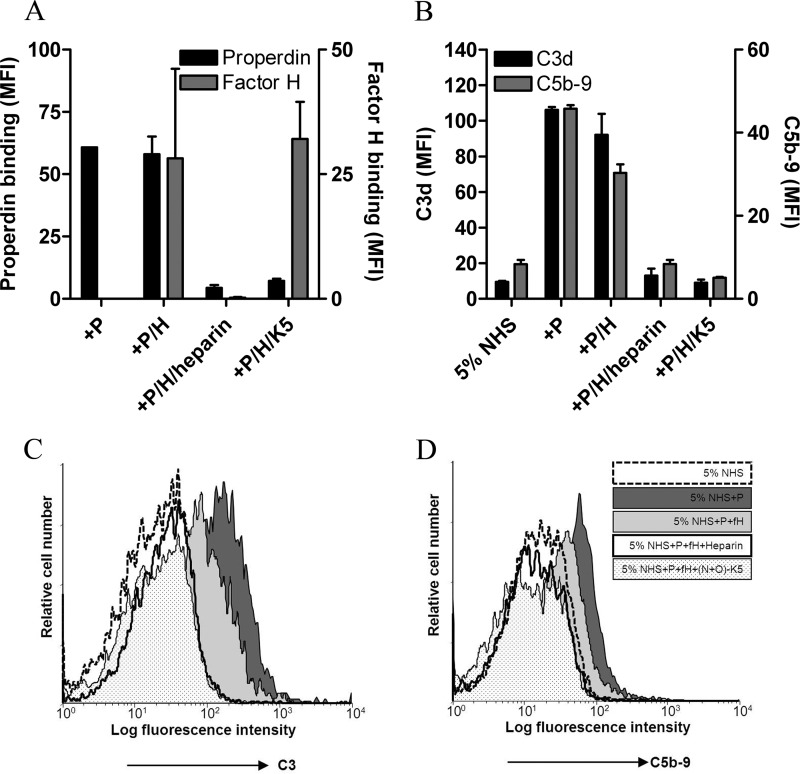
From a therapeutic point of view, we reasoned that competition with sulfated low anticoagulant heparinoids would interfere with properdin binding to HS/heparin, without affecting factor H binding to HS/heparin. It is known that N+O-sulfated K5 has a high degree of sulfation but a low anticoagulant activity (2–4-fold lower than heparin) (37). To this end, we performed FACS experiments in which we incubated HK-2 cells with either properdin or factor H in the presence of N+O-sulfated K5 and compared that with normal heparin incubation. From Fig. 7A, it becomes clear that properdin binding to HK2 cells is inhibited by both heparin and N+O-sulfated K5. Interestingly, factor H binding to HK2 cells is efficiently abolished by intact heparin, but not by N+O-sulfated K5.
FIGURE 7.
Heparinoids (low anticoagulant) can inhibit the AP activation on HK2 cells. A, binding of factor H (100 μg/ml) to HK2 cells (right y axis) is not prevented by preincubation of factor H with N+O-sulfated K5 (300 μg/ml), whereas unfractionated heparin fully inhibited factor H binding to the cells. Binding of properdin (10 μg/ml) to HK2 cells (left y axis) is inhibited by unfractionated heparin as well as N+O-sulfated K5 (300 μg/ml). B, C3 deposition (left y axis) and MAC formation (right y axis) on HK2 cells is hampered by preincubation of properdin and factor H with heparin and N+O-sulfated K5. The bars are the mean values of two independent FACS experiments ± S.E. (error bars). C and D, histograms show the same result for C3 deposition (C) and MAC formation (D) on HK2 cells by properdin (dark gray-shaded histogram). Both C3 deposition and MAC formation curves are reduced to some extent by factor H (light gray-shaded histogram), whereas preincubation of properdin and factor H with heparin (open bold histogram) and with N+O-sulfated K5 (dotted histogram) totally inhibited the AP activation on HK2 cells. The basal level of AP activation by 5% normal human serum (NHS) is shown in the dashed histogram. The histograms are shown from a representative FACS experiment.
Selective Blockade of Properdin Binding, but Not Factor H Binding, Inhibits AP Activation
As shown by competition ELISAs, FACS, and BIAcore experiments, factor H and properdin have different binding properties for GAGs. To mimic the physiologic situation, in which both properdin and factor H are present in the ultrafiltrate under proteinuric conditions, HK2 cells were incubated with a mixture of properdin and factor H, and the AP activation was measured (Fig. 7B; C3 and MAC). The addition of factor H to properdin reduced both C3 deposition and MAC formation. Moreover, preincubation of the properdin and factor H mixture with heparin and N+O-sulfated K5 reduced the AP activation to the basal level (Fig. 7B). The histograms of FACS experiments are shown in Fig. 7C (for C3d) and Fig. 7D (for C5b-9). Furthermore, we showed that heparin interacts with both properdin and factor H and leads to AP inhibition, whereas N+O-sulfated K5 inhibits properdin binding to HK2 cells without affecting factor H interaction with these cells, resulting in strong AP inhibition (Fig. 7).
DISCUSSION
Our study shows that properdin and factor H recognize and interact with nonoverlapping motifs of HS on the apical side of tubular cells and that these interactions can be targeted with certain low/nonanticoagulant heparinoids, resulting in AP inhibition on these cells. In the context of age-related macular degeneration, it has recently been shown that HS, including HS from Bruch's membrane, inhibit the AP of the complement system (38). Mutations in the CCP 7–9 GAG-binding domain of factor H have been associated with increased AP activation in age-related macular degeneration (39), whereas mutations in the CCP 19–20 GAG-binding domain of factor H have been associated to increased AP activation in atypical hemolytic uremic syndrome (40). These data emphasize the importance of the interaction of factor H with (surface-bound) HS to control alternative complement activation.
There is convincing evidence that the presence of complement factors in ultrafiltrate plays an important role in proteinuria-derived tubular injury (3, 41). We previously showed properdin to be involved in complement activation of renal tubular cells (23) and urinary properdin to be associated with a worsening of renal outcome in proteinuric patients (42). We then identified tubular HS to be the receptor for properdin during proteinuria (17). It has also been shown that adriamycin-induced proteinuria results in the AP activation on renal tubular cells (43). Recently, the critical role of factor H to prevent autologous complement activation on renal tubular epithelial cells has also been documented (44). Specific delivery of complement inhibitors to the renal tubular apical brush border zone in puromycin nephrosis protected the rats against tubulointerstitial injury and renal dysfunction (45). Protein overload reduces production of factor H by tubular epithelial cells in vitro as well as binding of exogenous factor H to these cells as shown by Buelli et al. (4). They also showed a reduction in HS density on HK2 cells after protein overload. We did not specifically study HS density on renal tubular cells under proteinuric conditions; however, our study confirms binding of factor H to HK2 cells via HS proteoglycans. Additionally, our findings emphasize the importance of HS composition/sulfation and its density for its interaction with factor H. We published firm expression of cell surface heparan sulfate proteoglycan syndecan-1 by HK2 cells in vitro (46) and also luminally by tubular cells in proteinuric conditions (17). FACS analysis showed that besides high syndecan-1, HK-2 cells express low levels of syndecans -2 and -4, and glypicans -1 and -5 (not shown). We therefore suggest that binding of factor H to living, nonfixed, nonpermeabilized cells (Fig. 3) is mainly via syndecan-1.
Factor H has been shown to bind to other negatively charged cell surface polyanions like sialic acids. Therefore, we cannot exclude the possibility of additional binding of factor H to renal tubular cells via other polyanions or other heparin-binding proteins. However, our data suggest that the factor H binding to HK2 cells is largely mediated by tubular HS (Fig. 3).
We clearly show that a high sulfate density on a GAG of sufficient length is needed for proper binding of factor H, whereas the exact GAG backbone and occurrence of iduronate units is of less importance. Compared with factor H, properdin also recognizes lower sulfated GAGs. We show that renal tubular cells bind factor H mostly via HS proteoglycans and protect these cells against AP activation. Properdin activates the AP on tubular cells via HS, and factor H inhibits the AP on these cells also via HS. HS requires different epitopes for interacting with either properdin or factor H. Thus we can speculate that the HS fine composition on luminal side of the tubular cells determines the regulation of the AP on these cells during proteinuria. Although lower sulfated HS will only bind properdin and favor activation of the AP, higher sulfated HS isoforms also bind factor H besides properdin, leading to down-regulation of the AP of complement.
Urinary factor H has been shown to be elevated in patients with various renal diseases (33). An increase in urinary level of factor H has been correlated with higher urinary MAC levels, increased proteinuria, signs of tubular injury, and worsening of renal function (33–35). This suggests that during proteinuria, urinary factor H is insufficiently able to regulate tubular complement activation and warrants the identification of novel therapeutics aimed at reduction of tubular complement activation during proteinuria.
Previously we tested interaction of properdin with various GAG preparations (17), and in the current study we checked the same library of GAGs for their interaction with factor H. Because the experimental settings were different, we cannot compare the IC50 of GAGs for their interaction with properdin and factor H. However, it is clear that properdin interacts with a broader spectrum of GAG preparations and accommodates lower sulfation density, whereas factor H needs more sulfated motifs on GAGs for interaction. These findings can be important from a therapeutic point of view. As we showed in the FACS staining, there are low anticoagulant heparinoids, namely N+O-sulfated K5, which can inhibit properdin, while they are not influencing factor H binding to the cell surface. Although it has been known for years that heparinoids can inhibit complement activation in vivo (15, 47–49), selection of heparinoids based on properdin binding, but not factor H interaction, is new and promising for AP-driven diseases such as tubular activation secondary to proteinuria.
Our study demonstrates the inhibitory effects of some low anticoagulant heparinoids on the positive regulator of AP (properdin) with limited effects on the inhibitory regulator of the pathway (factor H). These heparinoids represent good candidates to be tested in vivo for their therapeutic effects in AP-derived renal pathologies. Taken together, usage of heparinoids for targeting AP activation seems a promising intervention. Our study might be of a great importance for reducing proteinuria-induced AP activation and tubular injury. The ultimate goal is to bring a new promising therapy to clinical nephrology, especially for proteinuric patients in which urine monitoring shows signs of complement activation.
Acknowledgments
We thank Isabelle Bally and Nicole Thielens from the Institut de Biologie Structurale platform of the Partnership for Structural Biology and the Institut de Biologie Structurale (Grenoble, France) for assistance and access to the Biacore facility. We thank Klaas Sjollema for help with confocal microscopy, which was performed at University Medical Center Imaging and Microscopy Center. We thank Atze van der Pol for useful editing comments.
This work was supported in part by NWO Grants 40-00506-98-9021 and 175-010-2009-023.
- AP
- alternative pathway
- HS
- heparan sulfate(s)
- CCP
- complement control protein
- PG
- proteoglycan
- GAG
- glycosaminoglycan
- LMW
- low molecular weight.
REFERENCES
- 1. Berger S. P., Roos A., Daha M. R. (2005) Complement and the kidney. What the nephrologist needs to know in 2006? Nephrol. Dial. Transplant. 20, 2613–2619 [DOI] [PubMed] [Google Scholar]
- 2. Vieyra M. B., Heeger P. S. (2010) Novel aspects of complement in kidney injury. Kidney Int. 77, 495–499 [DOI] [PubMed] [Google Scholar]
- 3. Abbate M., Zoja C., Corna D., Rottoli D., Zanchi C., Azzollini N., Tomasoni S., Berlingeri S., Noris M., Morigi M., Remuzzi G. (2008) Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J. Am. Soc. Nephrol. 19, 1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buelli S., Abbate M., Morigi M., Moioli D., Zanchi C., Noris M., Zoja C., Pusey C. D., Zipfel P. F., Remuzzi G. (2009) Protein load impairs factor H binding promoting complement-dependent dysfunction of proximal tubular cells. Kidney Int. 75, 1050–1059 [DOI] [PubMed] [Google Scholar]
- 5. Zipfel P. F., Heinen S., Józsi M., Skerka C. (2006) Complement and diseases. Defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 43, 97–106 [DOI] [PubMed] [Google Scholar]
- 6. Pangburn M. K. (2000) Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology. 49, 149–157 [DOI] [PubMed] [Google Scholar]
- 7. Ferreira V. P., Pangburn M. K., Cortés C. (2010) Complement control protein factor H. The good, the bad, and the inadequate. Mol. Immunol. 47, 2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aslam M., Perkins S. J. (2001) Folded-back solution structure of monomeric factor H of human complement by synchrotron x-ray and neutron scattering, analytical ultracentrifugation and constrained molecular modelling. J. Mol. Biol. 309, 1117–1138 [DOI] [PubMed] [Google Scholar]
- 9. Jokiranta T. S., Hellwage J., Koistinen V., Zipfel P. F., Meri S. (2000) Each of the three binding sites on complement factor H interacts with a distinct site on C3b. J. Biol. Chem. 275, 27657–27662 [DOI] [PubMed] [Google Scholar]
- 10. Schmidt C. Q., Herbert A. P., Kavanagh D., Gandy C., Fenton C. J., Blaum B. S., Lyon M., Uhrín D., Barlow P. N. (2008) A new map of glycosaminoglycan and C3b binding sites on factor H. J. Immunol. 181, 2610–2619 [DOI] [PubMed] [Google Scholar]
- 11. Esko J. D., Lindahl U. (2001) Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rienstra H., Katta K., Celie J. W., van Goor H., Navis G., van den Born J., Hillebrands J. L. (2010) Differential expression of proteoglycans in tissue remodeling and lymphangiogenesis after experimental renal transplantation in rats. PLoS One 5, e9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bishop J. R., Schuksz M., Esko J. D. (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 14. Whitelock J. M., Iozzo R. V. (2005) Heparan sulfate. A complex polymer charged with biological activity. Chem. Rev. 105, 2745–2764 [DOI] [PubMed] [Google Scholar]
- 15. Maillet F., Petitou M., Choay J., Kazatchkine M. D. (1988) Structure-function relationships in the inhibitory effect of heparin on complement activation. Independency of the anti-coagulant and anti-complementary sites on the heparin molecule. Mol. Immunol. 25, 917–923 [DOI] [PubMed] [Google Scholar]
- 16. Maillet F., Kazatchkine M. D., Glotz D., Fischer E., Rowe M. (1983) Heparin prevents formation of the human C3 amplification convertase by inhibiting the binding site for B on C3b. Mol. Immunol. 20, 1401–1404 [DOI] [PubMed] [Google Scholar]
- 17. Zaferani A., Vivès R. R., van der Pol P., Hakvoort J. J., Navis G. J., van Goor H., Daha M. R., Lortat-Jacob H., Seelen M. A., van den Born J. (2011) Identification of tubular heparan sulfate as a docking platform for the alternative complement component properdin in proteinuric renal disease. J. Biol. Chem. 286, 5359–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirschfink M., Blase L., Engelmann S., Schwartz-Albiez R. (1997) Secreted chondroitin sulfate proteoglycan of human B cell lines binds to the complement protein C1q and inhibits complex formation of C1. J. Immunol. 158, 1324–1331 [PubMed] [Google Scholar]
- 19. Yu H., Muñoz E. M., Edens R. E., Linhardt R. J. (2005) Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance. Biochim. Biophys. Acta 1726, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rook M., Lely A. T., Kramer A. B., van Goor H., Navis G. (2005) Individual differences in renal ACE activity in healthy rats predict susceptibility to adriamycin-induced renal damage. Nephrol. Dial. Transplant. 20, 59–64 [DOI] [PubMed] [Google Scholar]
- 21. van Timmeren M. M., Bakker S. J., Vaidya V. S., Bailly V., Schuurs T. A., Damman J., Stegeman C. A., Bonventre J. V., van Goor H. (2006) Tubular kidney injury molecule-1 in protein-overload nephropathy. Am. J. Physiol. Renal Physiol. 291, F456–F464 [DOI] [PubMed] [Google Scholar]
- 22. Daha M. R., van Es L. A. (1982) Isolation, characterization, and mechanism of action of rat β1H. J. Immunol. 128, 1839–1843 [PubMed] [Google Scholar]
- 23. Gaarkeuken H., Siezenga M. A., Zuidwijk K., van Kooten C., Rabelink T. J., Daha M. R., Berger S. P. (2008) Complement activation by tubular cells is mediated by properdin binding. Am. J. Physiol. Renal Physiol. 295, F1397–F403 [DOI] [PubMed] [Google Scholar]
- 24. Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. (1976) Control of the amplification convertase of complement by the plasma protein β1H. Proc. Natl. Acad. Sci. U.S.A. 73, 3268–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooimans R. A., Hiemstra P. S., van der Ark A. A., Sim R. B., van Es L. A., Daha M. R. (1989) Biosynthesis of complement factor H by human umbilical vein endothelial cells. Regulation by T cell growth factor and IFN-γ. J. Immunol. 142, 2024–2030 [PubMed] [Google Scholar]
- 26. Sadir R., Forest E., Lortat-Jacob H. (1998) The heparan sulfate binding sequence of interferon-γ increased the on rate of the interferon-γ-interferon-γ receptor complex formation. J. Biol. Chem. 273, 10919–10925 [DOI] [PubMed] [Google Scholar]
- 27. Vann W. F., Schmidt M. A., Jann B., Jann K. (1981) The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur. J. Biochem. 116, 359–364 [DOI] [PubMed] [Google Scholar]
- 28. Iverius P. (1971) Coupling of glycosaminoglycans to agarose beads (Sepharose 4B). Biochem. J. 124, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bäckström G., Höök M., Lindahl U., Feingold D. S., Malmström A., Rodén L., Jacobsson I. (1979) Biosynthesis of heparin. Assay and properties of the microsomal uronosyl C-5 epimerase. J. Biol. Chem. 254, 2975–2982 [PubMed] [Google Scholar]
- 30. Rej R. N., Ludwig-Baxter K. G., Perlin A. S. (1991) Sulfation of some chemically-modified heparins. Formation of a 3-sulfate analog of heparin. Carbohydr. Res. 210, 299–310 [DOI] [PubMed] [Google Scholar]
- 31. van den Born J., Gunnarsson K., Bakker M. A., Kjellén L., Kusche-Gullberg M., Maccarana M., Berden J. H., Lindahl U. (1995) Presence of N-unsubstituted glucosamine units in native heparan sulfate revealed by a monoclonal antibody. J. Biol. Chem. 270, 31303–31309 [DOI] [PubMed] [Google Scholar]
- 32. Vivès R. R., Sadir R., Imberty A., Rencurosi A., Lortat-Jacob H. (2002) A kinetics and modeling study of RANTES(9–68) binding to heparin reveals a mechanism of cooperative oligomerization. Biochemistry 41, 14779–14789 [DOI] [PubMed] [Google Scholar]
- 33. Onda K., Ohsawa I., Ohi H., Tamano M., Mano S., Wakabayashi M., Toki A., Horikoshi S., Fujita T., Tomino Y. (2011) Excretion of complement proteins and its activation marker C5b-9 in IgA nephropathy in relation to renal function. BMC Nephrol. 12, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamano M., Fuke Y., Endo M., Ohsawa I., Fujita T., Ohi H. (2002) Urinary complement factor H in renal disease. Nephron. 92, 705–707 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J. J., Jiang L., Liu G., Wang S. X., Zou W. Z., Zhang H., Zhao M. H. (2009) Levels of urinary complement factor H in patients with IgA nephropathy are closely associated with disease activity. Scand. J. Immunol. 69, 457–464 [DOI] [PubMed] [Google Scholar]
- 36. van den Born J., Salmivirta K., Henttinen T., Ostman N., Ishimaru T., Miyaura S., Yoshida K., Salmivirta M. (2005) Novel heparan sulfate structures revealed by monoclonal antibodies. J. Biol. Chem. 280, 20516–20523 [DOI] [PubMed] [Google Scholar]
- 37. Borgenström M., Wärri A., Hiilesvuo K., Käkönen R., Käkönen S., Nissinen L., Pihlavisto M., Marjamäki A., Vlodavsky I., Naggi A., Torri G., Casu B., Veromaa T., Salmivirta M., Elenius K. (2007) O-Sulfated bacterial polysaccharides with low anticoagulant activity inhibit metastasis. Semin. Thromb. Hemost. 33, 547–556 [DOI] [PubMed] [Google Scholar]
- 38. Kelly U., Yu L., Kumar P., Ding J. D., Jiang H., Hageman G. S., Arshavsky V. Y., Frank M. M., Hauser M. A., Rickman C. B. (2010) Heparan sulfate, including that in Bruch's membrane, inhibits the complement alternative pathway. Implications for age-related macular degeneration. J. Immunol. 185, 5486–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark S. J., Perveen R., Hakobyan S., Morgan B. P., Sim R. B., Bishop P. N., Day A. J. (2010) Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch's membrane in human retina. J. Biol. Chem. 285, 30192–30202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lehtinen M. J., Rops A. L., Isenman D. E., van der Vlag J., Jokiranta T. S. (2009) Mutations of factor H impair regulation of surface-bound C3b by three mechanisms in atypical hemolytic uremic syndrome. J. Biol. Chem. 284, 15650–15658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abbate M., Zoja C., Remuzzi G. (2006) How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17, 2974–2984 [DOI] [PubMed] [Google Scholar]
- 42. Siezenga M. A., van der Geest R. N., Mallat M. J., Rabelink T. J., Daha M. R., Berger S. P. (2010) Urinary properdin excretion is associated with intrarenal complement activation and poor renal function. Nephrol. Dial. Transplant. 25, 1157–1161 [DOI] [PubMed] [Google Scholar]
- 43. Lenderink A. M., Liegel K., Ljubanovi D., Coleman K. E., Gilkeson G. S., Holers V. M., Thurman J. M. (2007) The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am. J. Physiol. Renal Physiol. 293, F555–F564 [DOI] [PubMed] [Google Scholar]
- 44. Renner B., Ferreira V. P., Cortes C., Goldberg R., Ljubanovic D., Pangburn M. K., Pickering M. C., Tomlinson S., Holland-Neidermyer A., Strassheim D., Holers V. M., Thurman J. M. (2011) Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int. 80, 165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He C., Imai M., Song H., Quigg R. J., Tomlinson S. (2005) Complement inhibitors targeted to the proximal tubule prevent injury in experimental nephrotic syndrome and demonstrate a key role for C5b-9. J. Immunol. 174, 5750–5757 [DOI] [PubMed] [Google Scholar]
- 46. Celie J. W., Katta K. K., Adepu S., Melenhorst W. B., Reijmers R. M., Slot E. M., Beelen R. H., Spaargaren M., Ploeg R. J., Navis G., van der Heide J. J., van Dijk M. C., van Goor H., van den Born J. (2012) Tubular epithelial syndecan-1 maintains renal function in murine ischemia/reperfusion and human transplantation. Kidney Int. 81, 651–661 [DOI] [PubMed] [Google Scholar]
- 47. Weiler J. M., Edens R. E., Linhardt R. J., Kapelanski D. P. (1992) Heparin and modified heparin inhibit complement activation in vivo. J. Immunol. 148, 3210–3215 [PubMed] [Google Scholar]
- 48. Blondin C., Fischer E., Boisson-Vidal C., Kazatchkine M. D., Jozefonvicz J. (1994) Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol. Immunol. 31, 247–253 [DOI] [PubMed] [Google Scholar]
- 49. Türk H., Haag R., Alban S. (2004) Dendritic polyglycerol sulfates as new heparin analogues and potent inhibitors of the complement system. Bioconjug. Chem. 15, 162–167 [DOI] [PubMed] [Google Scholar]