Background: In expression systems diacylglycerol (DAG) produced during AT1 angiotensin receptor signaling can be converted to 2-arachidonoylglycerol.
Results: Inhibition of CB1 receptors and DAG lipase augmented angiotensin II-induced vasoconstriction in resistance arteries.
Conclusion: Angiotensin II-induced vasoconstriction is attenuated via 2-arachidonoylglycerol release and consequent CB1 receptor activation.
Significance: This is the first demonstration that angiotensin II-induced endocannabinoid release can modulate vasoconstriction.
Keywords: Angiotensin II, Cannabinoids, G Protein-coupled Receptors (GPCR), Vascular Biology, Vascular Smooth Muscle Cells, Diacylglycerol Lipase
Abstract
In the vascular system angiotensin II (Ang II) causes vasoconstriction via the activation of type 1 angiotensin receptors. Earlier reports have shown that in cellular expression systems diacylglycerol produced during type 1 angiotensin receptor signaling can be converted to 2-arachidonoylglycerol, an important endocannabinoid. Because activation of CB1 cannabinoid receptors (CB1R) induces vasodilation and reduces blood pressure, we have tested the hypothesis that Ang II-induced 2-arachidonoylglycerol release can modulate its vasoconstrictor action in vascular tissue. Rat and mouse skeletal muscle arterioles and mouse saphenous arteries were isolated, pressurized, and subjected to microangiometry. Vascular expression of CB1R was demonstrated using Western blot and RT-PCR. In accordance with the functional relevance of these receptors WIN55212, a CB1R agonist, caused vasodilation, which was absent in CB1R knock-out mice. Inhibition of CB1Rs using O2050, a neutral antagonist, enhanced the vasoconstrictor effect of Ang II in wild type but not in CB1R knock-out mice. Inverse agonists of CB1R (SR141716 and AM251) and inhibition of diacylglycerol lipase using tetrahydrolipstatin also augmented the Ang II-induced vasoconstriction, suggesting that endocannabinoid release modulates this process via CB1R activation. This effect was independent of nitric-oxide synthase activity and endothelial function. These data demonstrate that Ang II stimulates vascular endocannabinoid formation, which attenuates its vasoconstrictor effect, suggesting that endocannabinoid release from the vascular wall and CB1R activation reduces the vasoconstrictor and hypertensive effects of Ang II.
Introduction
Angiotensin II (Ang II)2 is the key effector molecule of the renin-angiotensin system. It plays a crucial role in physiologic and pathologic control processes such as aldosterone secretion, vasoconstriction, cell proliferation, inflammation, atherosclerosis, and vascular remodeling (1–4). Ang II exerts its biological actions by activating type 1 (AT1R) and type 2 (AT2R) angiotensin receptors. The most important short and long term physiological and pathophysiological actions of the hormone are mediated by AT1R (1–4). AT1R is a G protein-coupled receptor, and its signal transduction is characterized by diverse, G protein-dependent and independent signaling mechanisms (2–5). The main signal transduction mechanism of AT1Rs is activation of Gq/11 proteins, which elevates intracellular calcium levels, stimulates diacylglycerol (DAG) formation, and activates downstream signaling molecules such as tyrosine kinases and mitogen-activated protein kinases (1–6). Calcium signal generation has been linked to endocannabinoid formation in neurons and other cells (7, 8).
Endocannabinoids serve as endogenous ligands for cannabinoid receptors and participate in tissue-specific paracrine regulatory mechanisms such as retrograde control of neurotransmitter release (7). To date several endocannabinoid compounds have been identified including arachidonoyl ethanolamide (anandamide), 2-arachidonoylglycerol (2-AG), and 2-arachidonylglyceryl ether (7, 9–11). Cannabinoid receptors were originally named after their affinity for Δ9-tetrahydrocannabinol, the main active ingredient of the extracts of Cannabis sativa. Endocannabinoid receptors include CB1 cannabinoid receptors (CB1Rs), which are characteristically present in neural tissues, and CB2 receptors (CB2Rs), which occur mostly in immune cells (7). However, both receptors were identified in a number of other tissues, and the existence of other cannabinoid receptors has also been proposed (10, 12). CB1Rs in the central nervous system typically occur in presynaptic locations, and they modulate synaptic transmission. During stimulation by neurotransmitters such as glutamate and acetylcholine, endocannabinoid-mediated CB1R activation mediates important physiological functions such as depolarization-induced retrograde synaptic inhibition (7, 9, 13).
In addition to the key functions of endocannabinoids in the central nervous system, their roles in peripheral tissues are also increasingly in the focus of interest (12, 14). It has been shown that the cannabinoid system plays a role in cardiovascular, inflammatory, gastrointestinal, and peripheral metabolic regulatory mechanisms (10, 12, 14). In the cardiovascular system, negative inotropic, vasodilator, and hypotensive actions of cannabinoids have been reported (14). The growing number of studies with compounds that modulate the endocannabinoid system may lead to novel therapeutic approaches in a number of metabolic and cardiovascular disorders (10, 12, 14). Although CB1 receptors have key roles in the central neural control of appetite and evidence for the tonic activity of the endocannabinoid/CB1 receptor system in obesity provide a rationale for the use of CB1 receptor antagonists as anti-obesity agents, these molecules were withdrawn from the market due to their central side effects (12). However, there is still hope that selective targeting of peripheral CB1 receptors has potential therapeutic value in metabolic and other diseases (12).
We have reported recently in transient expression systems that Ang II-induced activation of AT1R can lead to CB1R activation in cells coexpressing both AT1 and CB1receptors (8, 15). Moreover, we have shown that stimulation of AT1Rs and other Gq/11 protein-coupled G protein-coupled receptors can cause 2-AG-mediated paracrine transactivation of CB1Rs (8). The Ang II-induced CB1R activation was inhibited by DAG lipase inhibitors, suggesting that DAG generated from phosphoinositides can be converted to 2-AG by DAG lipase during the signaling of Ca2+-mobilizing hormones and neurotransmitters (8, 15). Although the physiological relevance of this mechanism has not been fully established, our preliminary data suggest that Ang II-induced endocannabinoid release may mediate the central hypertensive effect of Ang II in the paraventricular nucleus (16).
Based on our earlier findings on the mechanism of Ang II-induced paracrine transactivation of CB1 receptors, we hypothesized that the vasoconstrictor effect of Ang II may be attenuated by vascular endocannabinoid formation and consequent CB1R activation, and this mechanism can have a significant modulatory effect during the regulation of the vascular tone. This hypothesis was tested in this study.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar rats were used (300–350 g, Charles River Laboratories, Semmelweis University, Budapest, Hungary). C57BL/6J (21–25 g) mice were obtained from the animal facility of the Department of Physiology. CB1R knock-out (−/−, CB1R-KO) and wild type (+/+, C57BL/6J, Cnr1tm1zim) mice (21–25 g) were kindly provided by Professor Andreas Zimmer, University of Bonn (9). Animals were anesthetized with pentobarbital sodium (Euthasol, ASTfarma, 50 mg/kg intraperitoneally) and immediately sacrificed by fast bleeding. All procedures conform with the Guide for the Care and Use of Laboratory Animals (NIH, 1996) legal and institutional guidelines for animal care and were approved by the Animal Care Committee of Semmelweis University, Budapest and by Hungarian authorities (no. 263/003/2008).
Chemicals
Angiotensin II, norepinephrine, sodium nitroprusside, acetylcholine (Ach), WIN55212 (R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone mesylate (a CB1R agonist), tetrahydrolipstatin (a diacylglycerol lipase inhibitor) and Nω-nitro-l-arginine (LNA) (a nitric oxide (NO)-synthase inhibitor) were purchased from Sigma. CB1R antagonists O2050 (6aR,10aR)-3-(1-methanesulfonylamino-4-hexyn-6-yl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H dibenzo [b,d]pyran), a silent antagonist, and inverse agonist AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-car boxamide) were purchased from Tocris Bioscience (Elliswille, MI), and SR141716 (rimonabant, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide) was purchased from Cayman Chemicals (Tallinn, Estonia). AT1R antagonist candesartan was purchased from Toronto Research Chemicals (Toronto, Canada).
Isolation of Vessels for Pressure Video Microangiography
Rat and mouse skeletal muscle (gracilis) arterioles (∼120 μm in diameter) and mouse saphenous arteries (∼180 μm in diameter, Table 1) were prepared under microscopic guidance. For the preparation of gracilis arterioles the adductor muscles were removed, placed into cold Krebs solution that contained 110 mm NaCl, 5 mm KCl, 2.5 mm CaCl2, 1 mm MgSO4, 1 mm KH2PO4, 24 mm NaHCO3, and 10 mm glucose, pH 7.4, and prepared as described before (17, 18). For the preparation of saphenous arteries, the surface of the leg was cleared from the skin, and the arterial segment was further isolated in situ using a microscope (19). Segments were cannulated in a vessel chamber (Experimetria) and subjected to pressure microarteriography (Living Systems, Burlington, VT) as also described previously (20–22). The cannulated vessel was visualized by digital videomicroscopy, and the inner diameter was measured using Leica DFC 320 digital camera and LeicaQWin software (Leica, Wetzlar, Germany) (20). Arterial segments developed substantial (10–18%) vascular tone (Table 1).
TABLE 1.
Geometric values of isolated arteries
Control (CD) and passive (PD, relaxed) diameter (μm) and spontaneous tone values of rat and mouse gracilis arterioles and mouse saphenous arteries (mouse a. saphena) are shown. Mean ± S.E. values and number of segments (n) are indicated for each vessel type. Vascular tone was calculated as percent contraction related to passive diameter. Values for rat saphenous arteries were separated as of wild type (control) and CB1R knockout mice.
| Prepared segments | CD | PD | Tone | n |
|---|---|---|---|---|
| μm | μm | % | ||
| Rat gracilis arteriole | 123.7 ± 5.4 | 152.9 ± 6.6 | 18.5 ± 1.7 | 38 |
| Mouse gracilis arteriole | 119.4 ± 7.0 | 135.8 ± 5.4 | 12.3 ± 1.8 | 5 |
| Mouse a. saphena (control mice) | 185.4 ± 15.0 | 216.7 ± 17.0 | 13.7 ± 3.3 | 10 |
| Mouse a. saphena (CB1 ko mice) | 179.4 ± 17.2 | 210.1 ± 21.4 | 13.9 ± 2.2 | 5 |
Experimental Protocols for Pressure Microangiography
Pressurized segments were allowed to equilibrate for 30 min at 50 mm Hg intraluminal pressure, and pharmacological responses of the arterial segments were tested according to the specific protocols. Pharmacological agonists were administered in a concentration-dependent manner into the chamber, and steady-state diameter was recorded for each concentration or in a single (submaximal) concentration. 10-Min washout periods were applied between drugs. Precontraction of segments before vasodilator treatment was made with norepinephrine (50–100 nm). Endothelial integrity was tested by Ach (10 μm, NO-dependent vasodilator) in all experiments. The experiments were terminated by obtaining passive (relaxed) vascular diameter in calcium-free Krebs solution.
According to specific protocols on rat gracilis arterioles, concentration-responses to Ang II were obtained in vehicle or with CB1R inhibitors O2050 (neutral antagonist, 1 μm), SR141716, and AM251 (inverse agonists 1–1 μm) in three different experimental sets for each inhibitor (nine, five, and five animals, respectively). Ang II-induced concentration-responses were repeated on separate segments to prevent desensitization (data not shown), which can be attributed to the rapid agonist-induced AT1R-internalization (3). Because AM251, an inhibitor of CB1Rs was reported to inhibit also constitutive activity of CB1Rs previously (8), we also applied the neutral antagonist of CB1R, O2050. Inhibitor was applied for at least 10 min before and during agonist administration. In these experiments CB1R agonist WIN55212 (1 μm) was also applied. Endothelial integrity was tested by Ach, and vasodilator capacity of rat gracilis vessels was also tested by sodium nitroprusside (up to 10 μm). In similar protocols, responses to Ang II were also obtained with DAG lipase inhibition by THL (1 μm, n = 6). DAG lipase inhibition was applied to clarify the role of endogenously produced endocannabinoids in the vascular CB1R activity. In separate experiments, simultaneous administration of THL and O2050 was also applied (additional three rats), and effects of Ang II were also obtained. In additional experiments, the effects of WIN55212 and Ang II were also obtained with LNA (10 μm, n = 5) and with endothelial disruption (performed with intraluminal administration of a bubble for 10 minutes, n = 6) to test the NO/endothelial dependence of the cannabinoid effects. In additional experiments (n = 4), AT1R blocker candesartan (10 μm) was also applied to repeat Ang II concentration-responses to test the AT1R dependence of the Ang II response on rat gracilis arterioles (0.1–100 nm). The CB1R inhibitor O2050 was also tested on mouse gracilis arterioles (n = 4). To clarify the role of CB1Rs in the control of vascular tone, a similar protocol was also performed on saphenous arteries from CB1R knock-out (n = 4) and wild type (n = 4) mice with inhibition of CB1Rs (O2050). In an additional set of experiments (n = 6) the effect of THL on the vasoactive responses was also tested on mouse saphenous arteries of control mice of the CB1R KO tribe (C57BL/6J). Control and passive diameter and spontaneous tone of the prepared segments are summarized (Table 1). The number of animals used in each set is given (n), and 1–2 segments were prepared from each animal. In all experimental settings vehicles were also tested, and time control was also applied.
RNA Extraction and Real-time PCR
Segments of thoracic aorta were removed and gracilis arterioles were removed under microscopic guidance as described above from 4 rats. Samples were washed and placed in cold sterile phosphate buffer solution (PBS, with 137 mm NaCl, 2.7 mm KCl, 10.1 mm Na2HPO4, 1.8mm KH2PO4, pH 7.4). RNA was extracted and further processed for real-time PCR (Roche Applied Science) as described (23). RT-PCR assays were performed using the SYBR Green method. Primers were designed and synthesized by Sigma. Cycling conditions were: 10 min of preincubation at 95 °C, 45–50 cycles of 95 °C for 10 s, 62 °C for 5 s, and 72 °C for 15 s. Fluorescence data including melting curves were obtained. For normalization, glyceraldehyde-3-phosphate dehydrogenase (Gapdh), a housekeeping gene, was used (ENSRNOG00000004253). Relative messenger RNA levels of cannabinoid receptor type 1 gene (Cnr1) were calculated (ENSRNOG00000008223). Primers for Cnr1 were forward (GGACTCAGACTGCCTGCACA) and reverse (ACAAAAGCAGCAGGCTCACA) and for Gapdh were forward (CCTGCACCACCAACTGCTTAG) and reverse (CAGTCTTCTGAGTGGCAGTGATG). Tissue gene expression levels were plotted against Gapdh expression levels. Amplicons obtained from gel electrophoresis and relative CB1R mRNA values are shown in Fig. 1B.
FIGURE 1.
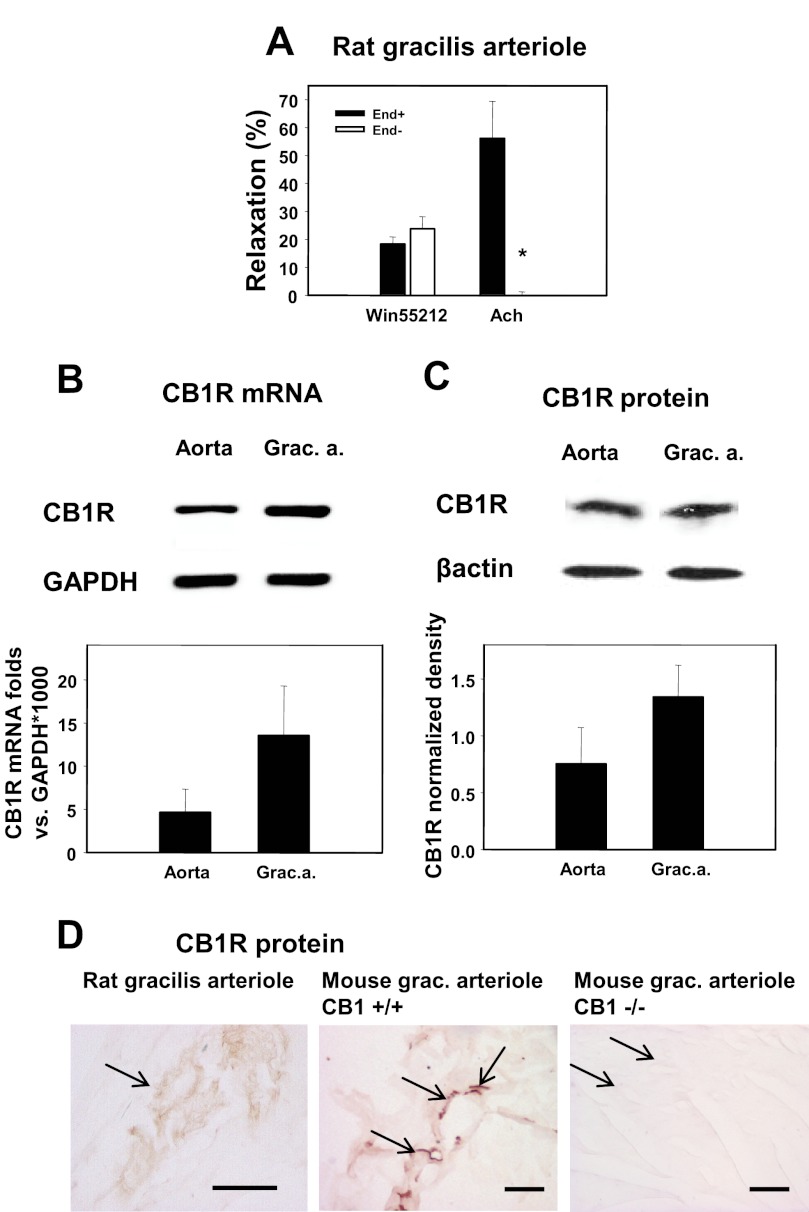
CB1R receptor expression and function in rat skeletal muscle arterioles. A, relaxations induced in rat gracilis arteriole segments by the CB1R agonist WIN 55212 (1 μm) and by the endothelial relaxant Ach (10 μm) as well as the effects of de-endothelization (5–5 segments) are shown. Values were calculated as percent change of diameter compared with control. Mean ± S.E. values are shown. The asterisk indicates significant change of agonist-induced tone in response to inhibitor treatment (p < 0.05). B, shown is gel electrophoresis of the mRNA amplicons and relative quantification of mRNA expression. Messenger RNA expression of CB1R (Cnr1 gene) was normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh, n = 4). Grac. a., gracilis arterioles. C, shown is Western blot detection of CB1R protein from tissue homogenates. Expression of CB1R protein in rat aorta and gracilis arterioles was quantitatively detected by densitometry (n = 4) of HRP-induced fluorescence. D, immunohistochemical localization of the CB1R protein (arrows) in rat gracilis arterioles and in mouse gracilis vessels is shown. No staining was detected in CB1R knock-out (−/−) mice. Left bar, 50 μm. Bars in middle and right, 100 μm.
Tissue Immunohistochemistry
Tissue perfusion was performed under anesthesia. Rat and mouse gracilis muscles were removed, washed in ice-cold PBS, fixed with 4% paraformaldehyde subjected to cryoprotection in sucrose solutions, then quickly frozen on dry ice as described previously (23, 24). Cryostat sections of gracilis muscles were mounted on Super Colorfrost slides (Fisher). Sections were blocked with 1% bovine serum albumin, and endogenous peroxidase activity was blocked with 3% H2O2 solution. Staining was performed with primary CB1R antibody (Cayman Chemicals, Tallinn, Estonia), and biotin-extravidin-peroxidase was developed by using the ABC method (Vector Laboratories, Burlington, CA) (24). Brain samples of CB1R knock-out and wild type mice were used to check immunoreactivity in a CB1R-specific tissue (data not shown).
Western Blotting of CB1R Protein
Five rats were anesthetized and sacrificed. Thoracic aortas and gracilis arterioles were removed as described above. Samples were washed twice in ice-cold PBS solution-modified Krebs-Ringer buffer (see above). Aortas were cut into pieces, and tissue samples weighing 20–30 mg were homogenized in glass tubes and lysed in SDS lysis buffer containing 10% mercaptoethanol and protease inhibitor cocktails (Sigma). In the lysis buffer samples were sonicated, boiled, and centrifuged. Proteins from both tissue, and cell samples were separated with SDS-polyacrylamide gel electrophoresis and were blotted onto PVDF membranes. Membranes were treated with antibodies against CB1R (Cayman Chemicals) and β-actin (Sigma) followed by the treatment with HRP-conjugated secondary antibodies. Visualization was made with SuperSignal West Pico reagent (Promega, Madison, WI), and results were quantitatively evaluated with densitometry.
Data Analysis
Data are presented as the mean ± S.E. values. Vascular responses are expressed as percent changes in vessel diameter from base-line values. Comparisons were made with one- or two-way analysis of variance tests followed by Tukey's and Holm-Sidak post hoc tests. Vasoconstrictor responses were also analyzed with nonlinear curve-fitting (four-parameter logistic curves). Effective maximal responses (Emax) and half-maximal responses (half-effective concentration, EC50) were calculated. Graphic presentations and statistical calculations were made using the Sigmaplot and Sigmastat program packages (Systat Software, Inc. San Jose, CA). For paired data, Student's t test was applied. Probability levels p < 0.05 were taken as statistically significant.
RESULTS
Activation of CB1R Exerts Vasodilatory Effects in Rat Gracilis Arterioles
In rat gracilis arterioles, WIN55212, a CB1R agonist, relaxed arterioles by 18.2 ± 2.5% (Fig. 1A), showing that CB1Rs are functionally present on these vessels and that their activation induces relaxation. Disruption of the endothelium did not interfere with the vasodilator effect of WIN55212, whereas it completely inhibited Ach-induced vasodilation (Fig. 1A), proving the presence of pharmacologically active endothelium in our preparations.
Vascular Expression of CB1R mRNA and Protein
The above-described functional data suggest that CB1Rs are present in rat gracilis arterioles. CB1R mRNA expression was detected in rat gracilis arterioles and was compared with expression in the rat aorta. The relative expression levels of CB1Rs and Gapdh are shown in Fig. 1B. The presence of CB1R protein and its localization have also been confirmed in the vascular tissue using a Western blot (Fig. 1C). Immunohistochemistry shows the localization of CB1R in rat and mouse gracilis muscle tissue. CB1R staining was not observed in the vessels prepared from CB1R knock-out mice, which confirms the specificity of the antibody used in our studies (Fig. 1D).
Effects of the CB1R Inhibitor O2050 (Neutral Antagonist) on Ang II-induced Vasoconstriction and the Effects of Vasodilators in Gracilis Arterioles
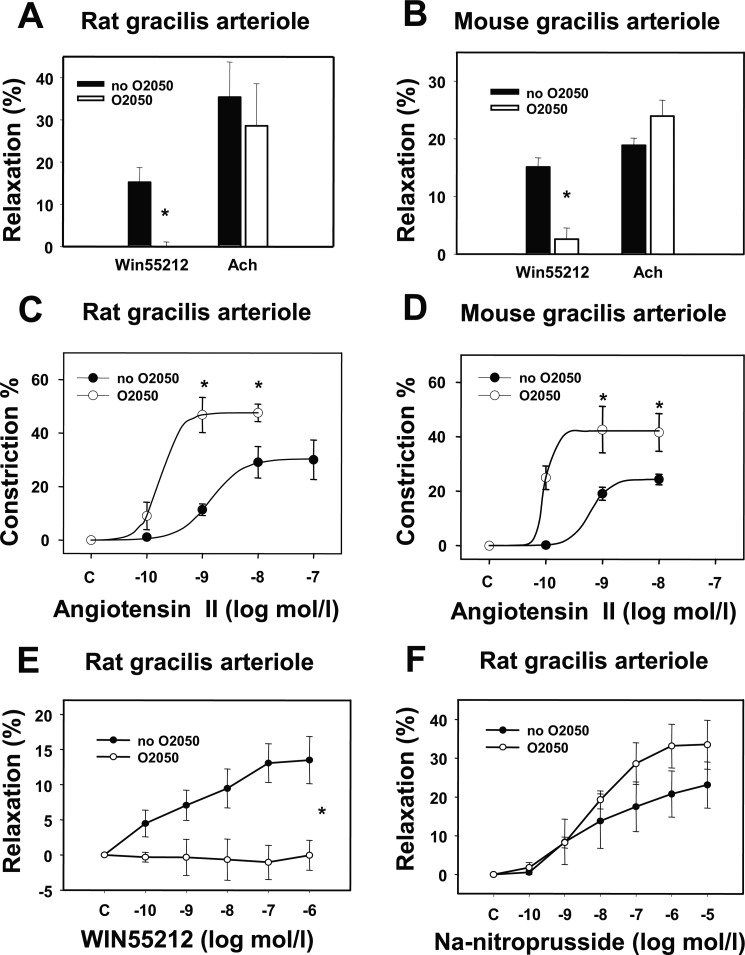
Both in rat and mouse gracilis arterioles, WIN55212, a CB1R agonist, relaxed arterioles by approximately 15% (Fig. 2, A and B). The neutral antagonist of CB1R O2050 had no significant effect on the basal tone of gracilis arterioles (Table 2) but completely inhibited the WIN55212-induced vasodilations (Fig. 2, A, B, and E). O2050 did not modulate endothelium-dependent and -independent vasodilatory responses; it had no significant effect on Ach-induced (Fig. 2, A and B) or sodium nitroprusside-induced (Fig. 2F) vasodilation of gracilis arterioles. Ang II caused concentration-dependent vasoconstriction (Fig. 2, C and D). CB1R inhibition by O2050 significantly enhanced Ang II-induced vasoconstrictions both in isolated rat and mouse gracilis arterioles (Fig. 2, C and D). At 1 nm Ang II, O2050 augmented the constriction from 11.4 ± 2.1 to 46.7 ± 6.5% in rat gracilis arterioles. In rat gracilis arterioles, nonlinear curve-fitting showed that in the presence of the CB1R inhibitor, O2050, the Emax value of Ang II-induced contraction significantly increased from 30.3 ± 4.4 to 47.5 ± 4.5% (p < 0.05, n = 5–6), whereas changes in log EC50 did not reach the level of statistical significance (Fig. 2C). In mouse gracilis arterioles, in the presence of O2050, the Emax value also significantly increased from 24.4 ± 1.5 to 42.1 ± 6.1% (p < 0.05, n = 4, Fig. 2D), and changes in logEC50 were not significant. These data show that endocannabinoid release reduces the maximum of the Ang II contraction effect.
FIGURE 2.
Effects of CB1R inhibition on agonist-induced responses of gracilis arterioles. A and B, shown are the effects of the CB1R antagonist O2050 (1 μm) on the relaxations induced by the CB1R agonist WIN 55212 (1 μm) and by the endothelial relaxant Ach (10 μm) in rat gracilis arteriole segments (A, n = 4) and in mouse gracilis arteriole segments (B, n = 4). Values were calculated as percent change of diameter from control. Mean ± S.E. values are shown. The asterisk indicates significant change of agonist-induced tone in response to inhibitor treatment (p < 0.05). C and D, cumulative log-concentration-effect curves for Ang II were taken by pressure arteriography in rat (C, n = 5–6) and mouse (D, n = 4) gracilis arterioles. Effects of the CB1R antagonist O2050 (1 μm) are shown. E and F, shown are cumulative log-concentration-effect curves induced by WIN 55212 (0.1 nm–1 μm) and by sodium nitroprusside (0.1 nm--10 μm) in rat gracilis arterioles (n = 4–7). Effects of the CB1R antagonist O2050 (1 μm) are shown. Values were calculated as percent change of diameter from the incubated control. Mean ± S.E. values are shown. The asterisk indicates significant change of vasoconstrictor-induced contraction in response to inhibitor treatment (p < 0.05). Data obtained with curve fitting method showed that in rat gracilis arterioles Emax values were 30.3 ± 4.4 and 47.5 ± 4.5% (p < 0.05, n = 5–6) in the absence and in the presence of O2050, respectively. Log EC50 values were −8.8 ± 0.2 and −9.7 ± 0.29 in the absence and presence of O2050, respectively. In mouse arterioles Emax values were 24.3 ± 1.5 and 42.1 ± 6.1% in the absence and presence of O2050, respectively (p < 0.05, n = 4). Log EC50 values were −9.2 ± 0.2 and −10.0 ± 3.4 in the absence and presence of O2050. Changes in EC50 values were not significant.
TABLE 2.
Effects of specific inhibitors on the basal vascular tone
Summary of the effects of specific inhibitors, CB1 receptor antagonists O2050 (n = 9, 1 μm), AM251 (n = 7, 1 μm), and SR141716 (SR, n = 5, 1 μm), diacylglycerol lipase inhibitor THL (n = 7, 1 μm), AT1R inhibitor candesartan (Cand, n = 4, 10 μm), and nitric-oxide synthase inhibitor LNA (n = 4, 10 μm) on the basal vascular tone of rat gracilis arterioles and effects of O2050 (n = 4, 1 μm) and THL, n = 7, 1 μm) on mouse saphenous arteries. Note that CB1 receptor antagonist (inverse agonist) AM251 and NO synthase inhibitor LNA cause a significant elevation of the basal vascular tone, whereas the other inhibitors do not change the tone of these vessels in the applied doses. Data are the mean ± S.E. values. Differences in diameter values with inhibitors from control were considered as significant if p < 0.05. NS, not significant.
| Prepared segments | O2050 | AM251 | SR | THL | Cand | LNA |
|---|---|---|---|---|---|---|
| Gracilis arterioles | ||||||
| Contraction % | 0.06 ± 2.3 | 4.82 ± 1.2 | 2.17 ± 2.1 | 7.36 ± 5.2 | 2.11 ± 1.4 | 11.0 ± 5.1 |
| n | 9 | 7 | 5 | 7 | 4 | 4 |
| Statistics | NS | p < 0.05 | NS | NS | NS | p < 0.05 |
| Saphenous arteries | ||||||
| Contraction % | −3.3 ± 1.3 | 4.71 ± 3.9 | ||||
| n | 4 | 7 | ||||
| Statistics | NS | NS | ||||
Effects of O2050 on Ang II-induced Vasoconstrictor Responses in CB1R Knock-out and Wild Type Mice
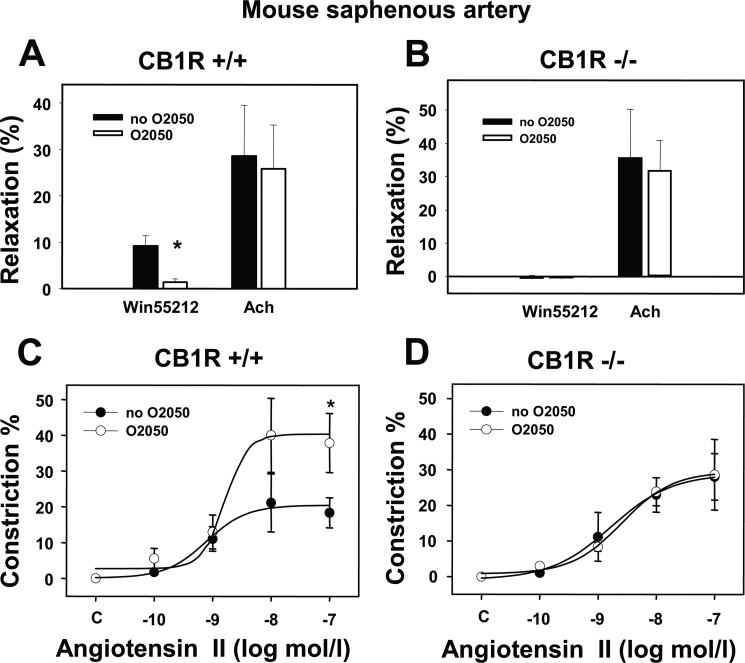
To provide independent genetic evidence for the role of CB1Rs in the modulation of Ang II-induced vasoconstriction, the above angiometric tests were also performed in saphenous arteries of CB1R-KO and corresponding wild type mice. Mouse saphenous arteries, similar in size to downstream rat gracilis arterioles, were prepared to study this effect on segments from CB1R-deficient and wild type mice. In saphenous arteries of wild type mice, O2050 alone did not change the basal tone (Table 2) but inhibited WIN55212-induced vasodilation (Fig. 3A, p < 0.05). This result is in accordance with data obtained in rat gracilis arterioles (shown above). However, WIN55212-induced vasodilation was entirely absent in CB1R-deficient mice (Fig. 3B), suggesting that these vessels of wild type mice contain functionally relevant CB1Rs. Furthermore, in saphenous arteries of both CB1R-deficient and wild type mice Ach-induced vasodilation was unaffected by CB1R inhibition (Fig. 3, A and B), which is also in agreement with the above observations. Similar to the results obtained in gracilis arterioles (see Fig. 2, A and B), O2050 significantly increased Ang II-induced contractile responses in wild type mice by increasing Ang II-induced contraction (Emax value was increased from 20.4 ± 3.7 to 40.3 ± 6.9%, p < 0.05, n = 4–5). However, O2050 had no significant effect on the logEC50 values of the Ang II response (−9.1 ± 0.3 and −8.8 ± 0.4 m, respectively; not significant, Fig. 3C). However, in contrast to wild type mice, the Ang II-induced vasoconstriction was not affected by the CB1R inhibitor in CB1R-KO mice (Emax values 28.7 ± 7.1 and 29.4 ± 6.4% in the absence and presence of O2050, respectively; Fig. 3D). These data provide genetic evidence for the role of CB1R in the attenuation of Ang II-induced vasoconstriction in mouse saphenous arteries.
FIGURE 3.
Comparison of agonist-induced responses in CB1R knock-out and wild type mice. Vasodilations induced by the CB1R agonist WIN55212 (1 μm) and by the endothelial vasorelaxant Ach (10 μm) as well as the effects of CB1R inhibition by the O2050 (1 μm) are shown in saphenous arteries of wild type (A, +/+) and of CB1R-deficient (B, −/−) mice (n = 4–6). C and D, shown is modulation of Ang II concentration-response curves by O2050 (1 μm) in the two mice strains (n = 4–5). Mean ± S.E. values are shown. The asterisk indicates significant effect of agonist or antagonist, p < 0.05. Data obtained with curve-fitting method showed that in wild type mice Emax values were 20.4 ± 3.7 and 40.3 ± 6.9% (p < 0.05, n = 4–5), and log EC50 values were −9.1 ± 0.3 and −8.8 ± 0.4 m in the absence and in presence of O2050, respectively. In CB1R-KO mice Emax values were 28.7 ± 7.1 and 29.4 ± 6.4% in the absence and presence of O2050, respectively. Changes in EC50 values were not significant.
Effects of Inverse Agonists of CB1R (SR141716 and AM251) on Ang II-induced Vasoconstriction and the Vasodilatory Responses in Gracilis Arterioles
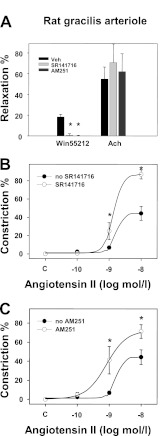
SR141716 (rimonabant) and AM251 effectively inhibited WIN55212-induced vasodilation of rat gracilis arterioles (Fig. 4A). These inhibitors of CB1R are “inverse agonists,” because they can inhibit the constitutive activity of CB1Rs (15). In gracilis vessels, AM251 slightly elevated the basal tone (Table 2). This can indicate a slight vascular constitutive activity of the CB1Rs in this preparation (on the other hand, the neutral antagonist, O2050, had no effect on the basal tone, Table 2). However, similar to the effect of O2050 (Fig. 2, C and D), CB1R inhibition with SR141716 and AM251 significantly enhanced Ang II-induced vasoconstrictions in isolated rat gracilis arterioles (Fig. 4, B and C). Nonlinear curve-fitting shows that SR141716 significantly increased the Emax value of the Ang II response from 44.3 ± 4.9 to 87.1 ± 5.1% (p < 0.05, n = 5–6), whereas logEC50 varied non-significantly from −8.8 ± −0.9 to −8.9 ± −0.3 m (Fig. 4B). Similar to the effect of SR141716, AM251 also significantly increased the Emax value of the Ang II response to 72.3 ± 6.4% (p < 0.05, n = 4–6) without affecting its logEC50 value (Fig. 4C). These data show that CB1R antagonists consequently augment the vasoconstrictor response of Ang II by increasing the efficacy of the agonist response without significantly affecting its potency. The differences in the kinetics of Ang II response in the presence of the different inhibitors may reflect their differential effects on constitutive CB1R activity.
FIGURE 4.
Effects of CB1R inhibition on agonist-induced responses of gracilis arterioles. A, shown are the effects of the CB1R antagonists AM251 (1 μm) and SR141716 (1 μm) on the relaxations induced by the CB1R agonist WIN 55212 (1 μm) and by the endothelial relaxant Ach (10 μm) in rat gracilis arteriole segments (A, n = 4–6). Values were calculated as percent change of diameter from control. Mean ± S.E. values are shown. Asterisks indicate significant change of agonist-induced tone in response to inhibitor treatment (p < 0.05). SR141716, rimonabant. B and C, shown are cumulative log-concentration-effect curves for Ang II taken by pressure arteriography in rat gracilis arterioles and the effects of the CB1R antagonists SR141716 (B, n = 5–6) and AM251 (C, n = 4–6) on them. Values were calculated as percent change of diameter from incubated control. Mean ± S.E. values are shown. Asterisks indicate significant changes in vasoconstrictor-induced contraction after inhibitor treatment (p < 0.05). Data obtained with the curve-fitting method showed that Emax values were 44.3 ± 4.9 and 87.1 ± 5.1% (p < 0.05, n = 5–6), and log EC50 values were −8.8 ± −0.9 and −8.9 ± −0.3 m in the absence and presence of SR141716, respectively. In the presence of AM251, the Emax value of the Ang II response was 72.3 ± 6.4% (p < 0.05 from control, n = 4–6). Changes in EC50 values were not significant.
Effects of DAG Lipase Inhibition on Ang II-induced Vasoconstrictor Responses
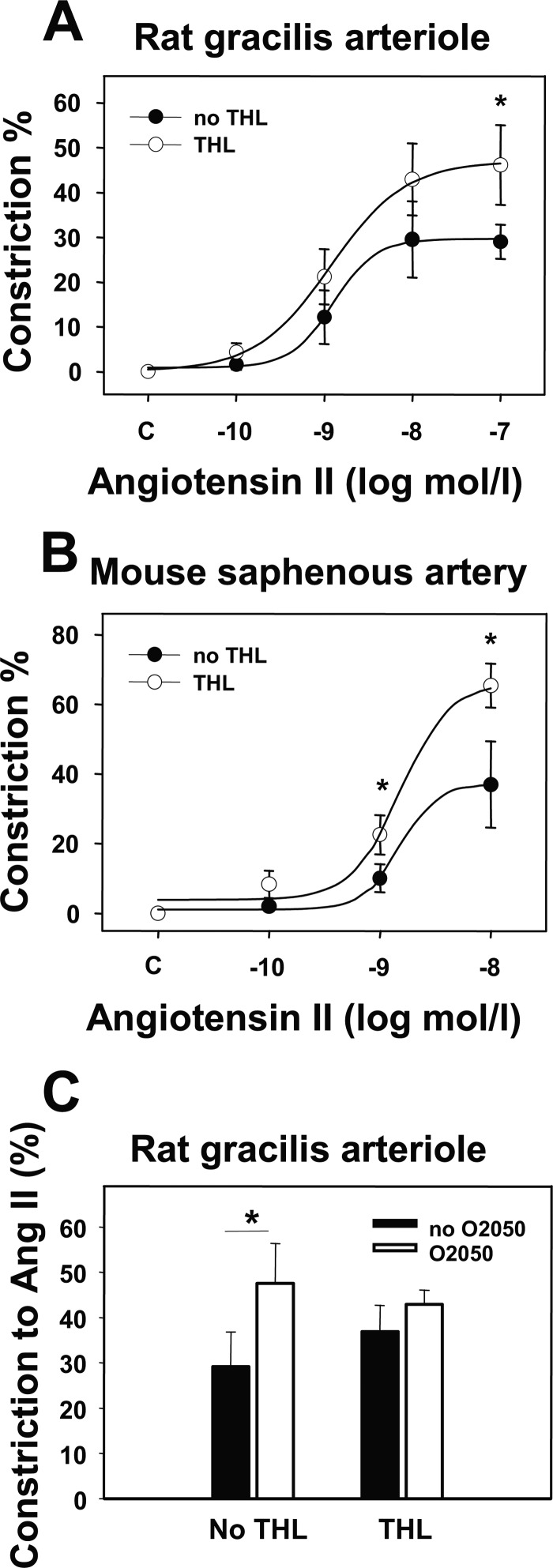
DAG lipase inhibition using THL induced a slight (4–7%), but not significant, increase in the basal vascular tone (Table 2) and caused a significant enhancement of Ang II-induced vasoconstriction in rat gracilis arterioles (Fig. 5A, p < 0.05 at 100 nm Ang II) without significantly modulating the Ach-induced vasodilation (data not shown). These data suggest the role of DAG lipase in Ang II-induced endocannabinoid formation in these vessels. Nonlinear curve-fitting showed that in the presence of the THL, Emax value significantly increased from 29.7 ± 4.9 to 47.0 ± 7.6% (p < 0.05, n = 5–6), and log EC50 varied from −8.9 ± 0.2 to −8.9 ± 0.2 m (statistically not significant, Fig. 5A). A similar effect of THL was observed in mouse saphenous arteries, where the Emax value significantly increased from 37.1 ± 6.8 to 66.1 ± 5.1% (p < 0.05, n = 4–5) and log EC50 varied from −8.8 ± 0.5 to −8.8 ± 0.2 M (not significant, Fig. 5B). The effect of THL and O2050 on Ang II-induced vasoconstriction was not additive in rat gracilis arterioles (Fig. 5C), which is consistent with the role of DAG lipase in Ang II-induced endocannabinoid formation.
FIGURE 5.
Effects of DAG lipase inhibition on Ang II-induced arteriolar tone. A and B, shown is the effect of the DAG lipase inhibitor THL (1 μm) on the Ang II-induced vasoconstriction in rat gracilis arterioles (A, n = 5–6) and on mouse saphenous arteries (B, n = 4–5), taken by pressure arteriography. Values were calculated as percent change of diameter compared with control. Mean ± S.E. values are shown. Asterisks indicate significant changes of vasoconstrictor-induced tone after inhibitor treatment (p < 0.05). C, shown is the effect of CB1R inhibitor (O2050, 1 μm) on the Ang II-induced (10 nm) vasoconstriction with and without THL pretreatment in rat gracilis arterioles (4–5 segments of each treatment). Mean ± S.E. values are shown. Asterisks indicate significant changes of the vasoconstrictor effect in the presence of the inhibitor (p < 0.05). Data obtained with the curve-fitting method showed that Emax values were 29.7 ± 4.9 and 47.0 ± 7.6% (p < 0.05, n = 5–6), and log EC50 values were −8.9 ± 0.2 and −8.9 ± 0.2 m in the absence and presence of THL, respectively. In mouse saphenous arteries Emax values were 37.1 ± 6.8 and 66.1 ± 5.1% (p < 0.05, n = 4–5), and log EC50 values were −8.8 ± 0.5 and −8.8 ± 0.2 m in the absence and presence of THL, respectively. Changes in EC50 values were not significant.
Effects of NO Synthase Inhibition and Endothelial Disruption on Endocannabinoid Modulation of Ang II Vasoconstriction
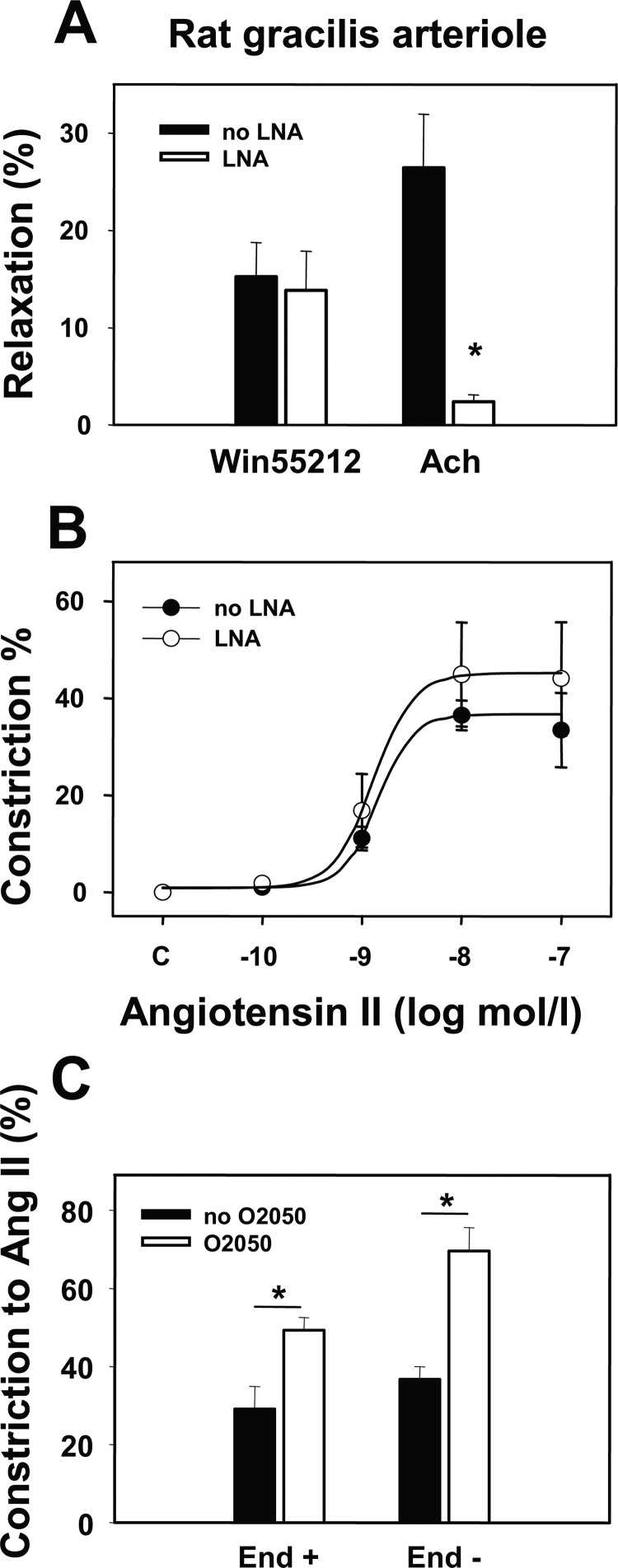
Inhibition of NO synthesis by Nω-nitro-l-arginine significantly increased the basal tone of gracilis arterioles (by ∼10%, Table 2, p < 0.05). LNA also effectively inhibited Ach-induced vasodilation (p < 0.05), but it did not significantly modified Ang II-induced vasoconstriction and did not affect WIN55212-induced vasodilation (Fig. 6, A and B). Disruption of the endothelium did not interfere with the augmentation of the Ang II-induced vasoconstriction by O2050 (Fig. 6C). These data suggest that the CB1R-mediated modulation of the Ang II-induced vasoconstriction is independent of NO production and endothelial function.
FIGURE 6.
Endothelial contribution to agonist-induced responses. Data were obtained by pressure arteriography on rat gracilis arterioles. A, shown is the effect of the NO synthesis inhibitor LNA (10 μm) on WIN55212 (1 μm) and Ach (10 μm)-induced vasodilations (n = 4–5). B, shown is the effect of pretreatment with LNA on the angiotensin II (Ang II)-induced vasoconstriction (n = 4–5). C, shown is the effect of the CB1R antagonist O2050 (1 μm) on the Ang II-induced (10 nm) vasoconstriction in segments with intact endothelium and after endothelial removal (n = 4–5). Values were calculated as percent change of diameter from control. Mean ± S.E. values are shown. Asterisks indicate significant changes of vessel tone in response to inhibitor treatment (p < 0.05).
Effect of AT1R Inhibitor Candesartan on Ang II-induced Vasoconstriction
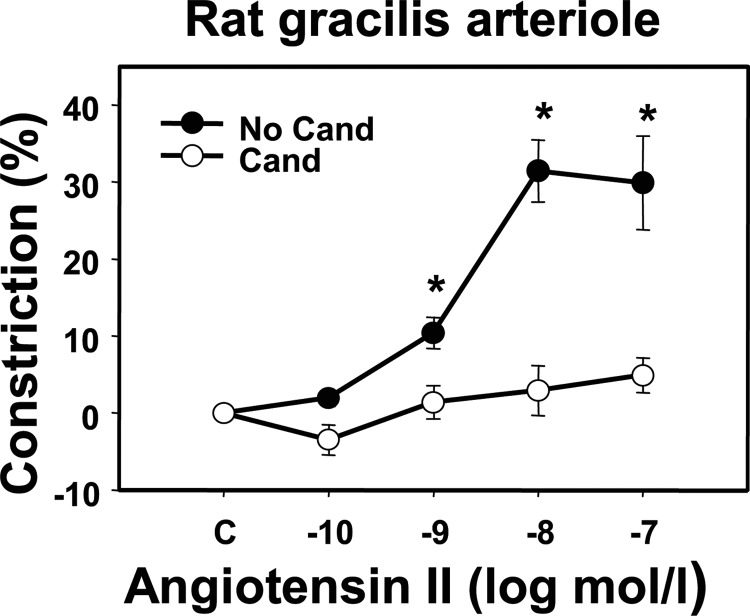
Presence of the specific AT1R blocker candesartan (10 μm) in the bath inhibited the Ang II-induced (0.1–100 nm) vasoconstriction of rat gracilis arterioles. Maximum responses (30.7 ± 2.4%) were reduced to 3.8 ± 1.9% (p < 0.05), indicating that the Ang II-induced vasoconstriction was mediated by AT1Rs (Fig. 7).
FIGURE 7.
AT1R dependence of the effect of Ang II on arterial tone. Effect of the specific AT1R inhibitor candesartan (Cand, 10 μm) on the Ang II-induced (0.1–100 nm) vasoconstriction in rat gracilis arterioles (n = 4–5). Mean ± S.E. values are shown. Asterisks indicate significant effects of the inhibitor on Ang II-induced vasoconstriction (p < 0.05).
DISCUSSION
We previously proposed that Ang II can cause the release of 2-AG, an endocannabinoid, followed by paracrine or autocrine transactivation of cannabinoid receptors. Phospholipase C activation generates diacylglycerol from inositol lipids enriched in arachidonic acid, which in turn can be converted to 2-AG by the DAG lipase (8, 15). This study provides the first direct demonstration of the physiological relevance of this mechanism in vascular tissues. It shows that endocannabinoid release can modulate the vasoconstrictor action of Ang II in resistance vessels by parallel activation of cannabinoid CB1Rs. A physiological mechanism, exerting an inherent braking effect on the vasoconstrictor and acute hypertensiogenic activity of this important physiological endocrine and local agonist, has thus been first identified.
Exogenously applied cannabinoids have been shown to have complex cardiovascular effects both by modulating the autonomic outflow and also through their direct cardiac and vascular effects (14, 25–28). Targets of cannabinoids in the vascular tissue have been identified as endothelial and vascular smooth muscle cells and perivascular neuronal fibers (14, 27, 29–34). In vivo studies and studies in isolated vessel segments have shown that natural cannabinoids (e.g. anandamide, 2-AG, Δ9-tetrahydrocannabinol) and synthetic cannabinoid agonists (e.g. WIN55212) cause vasodilation in many vascular beds, such as in aorta, coronary, cerebral, and mesenteric arteries (27, 29, 30, 32, 33, 35–40). The vascular effects of endocannabinoids in different vessel types may involve CB1R and Transient Receptor Potential Vanilloid (TRPV) receptors (26, 30, 37, 38) or other unidentified receptors (35, 36). In this study we observed substantial vasodilation to CB1R agonist WIN55212 in gracilis arterioles and saphenous arteries (Figs. 2, A and B, and 3A). The action of this agonist was fully inhibited by CB1R antagonists and was absent in the CB1R knock-out mice, demonstrating the presence of functionally relevant CB1Rs in these vessels (Figs. 3, A and B, and 4A). Similar to previous studies, we could confirm the vascular expression of the CB1R in rodents (26, 34, 41, 42). We also found that the neutral antagonist O2050, which does not influence constitutive activity of CB1Rs, did not modulate basal vascular tone. In contrast, the inverse agonist AM251, which also inhibits the constitutive activity of CB1Rs (8), slightly increased the basal vascular tone of gracilis vessels.
Ang II is a potent vasoconstrictor (1, 18, 43–45), and its effect is mediated by AT1Rs, as it was demonstrated in isolated vessels from several vascular beds (1, 45). In accordance with previous studies in gracilis arterioles by others (45), the vasoconstrictor effect of Ang II observed in this study was mediated by AT1R, as candesartan, an AT1R blocker, prevented this effect (Fig. 7). Our data demonstrate that the Ang II-induced vasoconstriction was augmented by the concomitant blockade of CB1Rs in rat skeletal muscle resistance vessels, suggesting that activation of cannabinoid receptors substantially attenuates the vasoconstrictor effect of Ang II. This effect was shown in this study using three separate CB1R inhibitors (O2050, SR141716, AM251) but was absent in vessels from CB1R knock-out mice. The latter finding provided additional genetic evidence for the relevance of this mechanism. Although all three CB1R inhibitors had a marked effect on the Ang II-induced vasoconstriction, a neutral CB1R inhibitor (O2050) or inverse agonists (SR141716, AM251) had no or minimal effects on the basal vascular tone, respectively, demonstrating that they interfere with the effects of Ang II-induced endocannabinoid production in the vessels. 2-AG release may attenuate the calcium response via multiple mechanisms (16, 40).
The difference in the kinetics of the Ang II-induced contractile response observed between gracilis and saphenous arteries, as seen in Figs. 2 and 3, showing the concentration-response to Ang II in gracilis arterioles shifted to the left, can be attributed to their different individual properties. Gracilis arterioles are smaller in size and more peripheral in location compared with saphenous arteries. It has been observed that in the same vascular bed smaller resistance vessels may show augmented metabolic responses compared with larger and conduit vessels, which is in accordance with their physiological role in the control of tissue perfusion (e.g. Ref. 17, 21). The stronger effect of CB1R inhibition on the augmentation of the AT1R response in gracilis arterioles can be attributed to larger AT1R signaling-dependent augmentation of the contractile effects.
The inhibition of DAG lipase also enhanced the Ang II-induced vasoconstriction (Fig. 5) and diminished the modulating effect of the CB1R antagonist (O2050) in these vessels. These data specifically point at 2-AG being the endocannabinoid, which modulates Ang II-induced vasoconstriction in rat skeletal muscle resistance vessels. The effect of Ang II on 2-AG production was previously detected in CHO cells using mass spectrometry (8). In vascular tissue, several types of cells can be sources of endocannabinoids, such as vascular endothelial cells, perivascular neurons, platelets, leukocytes, monocytes, and macrophages, etc. (14, 27, 29, 46). Agonist-induced release of 2-AG endocannabinoid has been detected from vascular endothelial cells (47) from rat aorta (48) and from bovine coronaries (46). In bovine coronary arteries metacholine-stimulated 2-AG release was suspected to be derived from endothelium (46). Earlier studies have also suggested that NO release and endothelium-dependent hyperpolarization contributes to cannabinoid-induced vasodilation (14, 35, 39, 49), and anandamide-induced vasodilation was found to be endothelium-independent (36). Our results suggest that CB1R-mediated vasodilation in skeletal muscle resistance vessels is not mediated by the release of NO and independent of the endothelium. This conclusion is in accordance with earlier findings showing that endocannabinoid content of cerebral arteries was not influenced by endothelium denudation (40). Similar to the effect of Ang II observed by us, another G protein-coupled receptor agonist U46619 was shown to increase anandamide and 2-AG content of cerebral vessels (40) that was proposed to interfere with the vasoconstrictor effect of U46619, suggesting a possible physiological feedback mechanism exerted by endocannabinoids on the thromboxane receptor action (16, 40).
A growing number of studies suggest that vascular endocannabinoids may have important physiological control functions (16, 40, 46–48). However, our data provide the first evidence that in skeletal muscle resistance vessels, which have important functions in local blood flow and systemic blood pressure control processes, Ang II-induced endocannabinoid release significantly modulates the vascular tone. Additional studies are needed to elucidate the cellular mechanism of cannabinoid-induced CB1R-mediated vasodilatory action in vascular tissue. CB1R-mediated Gi/o signaling involves the inhibition of L-type calcium channels, which has been observed in cerebral vessels (11, 42). It has also been proposed that cannabinoid-induced vasodilation in coronary arteries involves the activation of calcium-activated K+ channels (36), which were also shown to be activated by atypical cannabinoid ligands in endothelial cells (50).
It is important to note that the mechanism observed by us in peripheral vessels shows an analogy with the retrograde synaptic inhibition exerted by the same substances on neuronal cells in the central nervous system (7, 13). This involves 2-AG-release induced by calcium signal generation and/or DAG formation, activation of CB1Rs, and concomitant Gi/o activation (11).
In conclusion, our study reveals that Ang II-stimulated release of endocannabinoids from the vascular wall exerts a substantial relaxing effect on the vessels through activation of vascular smooth muscle cell CB1Rs. This mechanism raises the possibility that, similar to endothelial NO-dependent vasodilation, endocannabinoid release from the vascular wall and CB1R activation counteracts the vasoconstrictor effect of Ang II. Thus, in skeletal muscle resistance arteries, endocannabinoid release can serve as an endothelium-independent, important control mechanism of the vascular tone by the agonist-induced attenuation of the contraction.
Acknowledgments
We are grateful to Drs. Csaba Ádori, Attila Mócsai, and Péter Várnai (Semmelweis University, Budapest), Andreas Zimmer (University of Bonn) and István Katona (Institute of Experimental Medicine of the Hungarian Academy of Sciences, Budapest, Hungary) for helpful discussions and to Judit Rácz, Ildikó Oravecz, Anikó Schulcz, and Edina Simon for expert assistance.
This work was supported by grants from the Hungarian National Science Foundation (OTKA NK-100883, NK-72661, OTKA TO32019), Hungarian Ministry of National Resources (ETT 495/09), and from the National Development Agency, Hungary (TÁMOP 4.2.1.B-09/1/KMR-2010-0001).
- Ang II
- angiotensin II
- Ach
- acetylcholine
- 2-AG
- 2-arachidonoylglycerol
- AT1R
- type 1 angiotensin receptor
- CB1R
- type 1 cannabinoid receptor
- DAG
- diacylglycerol
- LNA
- Nω-nitro-l-arginine
- THL
- tetrahydrolipstatin.
REFERENCES
- 1. Mehta P. K., Griendling K. K. (2007) Angiotensin II cell signaling. Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 292, C82–C97 [DOI] [PubMed] [Google Scholar]
- 2. Higuchi S., Ohtsu H., Suzuki H., Shirai H., Frank G. D., Eguchi S. (2007) Angiotensin II signal transduction through the AT1 receptor. Novel insights into mechanisms and pathophysiology. Clin. Sci. 112, 417–428 [DOI] [PubMed] [Google Scholar]
- 3. Hunyady L., Catt K. J. (2006) Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 20, 953–970 [DOI] [PubMed] [Google Scholar]
- 4. Touyz R. M. (2005) Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension. Role of angiotensin II. Exp. Physiol. 90, 449–455 [DOI] [PubMed] [Google Scholar]
- 5. Haendeler J., Berk B. C. (2000) Angiotensin II mediated signal transduction. Important role of tyrosine kinases. Regul. Pept. 95, 1–7 [DOI] [PubMed] [Google Scholar]
- 6. Quignard J. F., Mironneau J., Carricaburu V., Fournier B., Babich A., Nurnberg B., Mironneau C., Macrez N. (2001) Phosphoinositide 3-kinase γ mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J. Biol. Chem. 276, 32545–32551 [DOI] [PubMed] [Google Scholar]
- 7. Freund T. F., Katona I., Piomelli D. (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 83, 1017–1066 [DOI] [PubMed] [Google Scholar]
- 8. Turu G., Várnai P., Gyombolai P., Szidonya L., Offertaler L., Bagdy G., Kunos G., Hunyady L. (2009) Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J. Biol. Chem. 284, 16914–16921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zimmer A., Zimmer A. M., Hohmann A. G., Herkenham M., Bonner T. I. (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 5780–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pacher P., Bátkai S., Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turu G., Hunyady L. (2010) Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 44, 75–85 [DOI] [PubMed] [Google Scholar]
- 12. Kunos G., Osei-Hyiaman D., Bátkai S., Sharkey K. A., Makriyannis A. (2009) Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol. Sci. 30, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katona I., Freund T. F. (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14, 923–930 [DOI] [PubMed] [Google Scholar]
- 14. Pacher P., Bátkai S., Kunos G. (2005) Cardiovascular pharmacology of cannabinoids. Handb. Exp. Pharmacol. 168, 599–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turu G., Simon A., Gyombolai P., Szidonya L., Bagdy G., Lenkei Z., Hunyady L. (2007) The role of diacylglycerol lipase in constitutive and angiotensin AT1 receptor-stimulated cannabinoid CB1 receptor activity. J. Biol. Chem. 282, 7753–7757 [DOI] [PubMed] [Google Scholar]
- 16. Gyombolai P., Pap D., Turu G., Catt K. J., Bagdy G., Hunyady L. (2012) Regulation of endocannabinoid release by G proteins. A paracrine mechanism of G protein-coupled receptor action. Mol. Cell. Endocrinol. 353, 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun D., Kaley G., Koller A. (1994) Characteristics and origin of myogenic response in isolated gracilis muscle arterioles. Am. J. Physiol. 266, H1177–H1183 [DOI] [PubMed] [Google Scholar]
- 18. Bagi Z., Hamar P., Kardos M., Koller A. (2006) Lack of flow-mediated dilation and enhanced angiotensin II-induced constriction in skeletal muscle arterioles of lupus-prone autoimmune mice. Lupus 15, 326–334 [DOI] [PubMed] [Google Scholar]
- 19. Várbíró S., Nádasy G. L., Monos E., Vajó Z., Acs N., Miklós Z., Tökés A. M., Székács B. (2000) Effect of ovariectomy and hormone replacement therapy on small artery biomechanics in angiotensin-induced hypertension in rats. J. Hypertens 18, 1587–1595 [DOI] [PubMed] [Google Scholar]
- 20. Nádasy G. L., Szekeres M., Dézsi L., Várbiró S., Székács B., Monos E. (2001) Preparation of intramural small coronary artery and arteriole segments and resistance artery networks from the rat heart for microarteriography and for in situ perfusion video mapping. Microvasc. Res. 61, 282–286 [DOI] [PubMed] [Google Scholar]
- 21. Szekeres M., Dézsi L., Nádasy G. L., Kaley G., Koller A. (2001) Pharmacologic inhomogeneity between the reactivity of intramural coronary arteries and arterioles. J. Cardiovasc. Pharmacol. 38, 584–592 [DOI] [PubMed] [Google Scholar]
- 22. Szekeres M., Nádasy G. L., Kaley G., Koller A. (2004) Nitric oxide and prostaglandins modulate pressure-induced myogenic responses of intramural coronary arterioles. J. Cardiovasc. Pharmacol. 43, 242–249 [DOI] [PubMed] [Google Scholar]
- 23. Szekeres M., Nádasy G. L., Turu G., Süpeki K., Szidonya L., Buday L., Chaplin T., Clark A. J., Hunyady L. (2010) Angiotensin II-induced expression of brain-derived neurotrophic factor in human and rat adrenocortical cells. Endocrinology 151, 1695–1703 [DOI] [PubMed] [Google Scholar]
- 24. Tóth Z. E., Mezey E. (2007) Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J. Histochem. Cytochem. 55, 545–554 [DOI] [PubMed] [Google Scholar]
- 25. Niederhoffer N., Schmid K., Szabo B. (2003) The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn. Schmiedebergs Arch. Pharmacol. 367, 434–443 [DOI] [PubMed] [Google Scholar]
- 26. Bátkai S., Pacher P., Osei-Hyiaman D., Radaeva S., Liu J., Harvey-White J., Offertáler L., Mackie K., Rudd M. A., Bukoski R. D., Kunos G. (2004) Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 110, 1996–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lípez-Miranda V., Herradón E., Martín M. I. (2008) Vasorelaxation caused by cannabinoids. Mechanisms in different vascular beds. Curr. Vasc. Pharmacol. 6, 335–346 [DOI] [PubMed] [Google Scholar]
- 28. Hiley C. R. (2009) Endocannabinoids and the heart. J. Cardiovasc. Pharmacol. 53, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hillard C. J. (2000) Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 294, 27–32 [PubMed] [Google Scholar]
- 30. Wagner J. A., Járai Z., Bátkai S., Kunos G. (2001) Hemodynamic effects of cannabinoids. Coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur. J. Pharmacol. 423, 203–210 [DOI] [PubMed] [Google Scholar]
- 31. Högestätt E. D., Zygmunt P. M. (2002) Cardiovascular pharmacology of anandamide. Prostaglandins Leukot. Essent. Fatty Acids 66, 343–351 [DOI] [PubMed] [Google Scholar]
- 32. Randall M. D., Harris D., Kendall D. A., Ralevic V. (2002) Cardiovascular effects of cannabinoids. Pharmacol. Ther. 95, 191–202 [DOI] [PubMed] [Google Scholar]
- 33. Randall M. D., Kendall D. A., O'Sullivan S. (2004) The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 142, 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tiyerili V., Zimmer S., Jung S., Wassmann K., Naehle C. P., Lütjohann D., Zimmer A., Nickenig G., Wassmann S. (2010) CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress, and improved endothelial function. Basic Res. Cardiol. 105, 465–477 [DOI] [PubMed] [Google Scholar]
- 35. Járai Z., Wagner J. A., Varga K., Lake K. D., Compton D. R., Martin B. R., Zimmer A. M., Bonner T. I., Buckley N. E., Mezey E., Razdan R. K., Zimmer A., Kunos G. (1999) Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 96, 14136–14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White R., Ho W. S., Bottrill F. E., Ford W. R., Hiley C. R. (2001) Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 134, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Sullivan S. E., Kendall D. A., Randall M. D. (2004) Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br. J. Pharmacol. 142, 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Sullivan S. E., Randall M. D., Gardiner S. M. (2007) The in vitro and in vivo cardiovascular effects of Δ9-tetrahydrocannabinol in rats made hypertensive by chronic inhibition of nitric-oxide synthase. J. Pharmacol. Exp. Ther. 321, 663–672 [DOI] [PubMed] [Google Scholar]
- 39. Dannert M. T., Alsasua A., Herradon E., Martín M. I., López-Miranda V. (2007) Vasorelaxant effect of Win 55,212-2 in rat aorta. New mechanisms involved. Vascul. Pharmacol. 46, 16–23 [DOI] [PubMed] [Google Scholar]
- 40. Rademacher D. J., Patel S., Ho W. S., Savoie A. M., Rusch N. J., Gauthier K. M., Hillard C. J. (2005) U-46619, but not serotonin, increases endocannabinoid content in middle cerebral artery. Evidence for functional relevance. Am. J. Physiol. Heart Circ. Physiol. 288, H2694–H2701 [DOI] [PubMed] [Google Scholar]
- 41. Daly C. J., Ross R. A., Whyte J., Henstridge C. M., Irving A. J., McGrath J. C. (2010) Fluorescent ligand binding reveals heterogeneous distribution of adrenoceptors and “cannabinoid-like” receptors in small arteries. Br. J. Pharmacol. 159, 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gebremedhin D., Lange A. R., Campbell W. B., Hillard C. J., Harder D. R. (1999) Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 276, H2085–H2093 [DOI] [PubMed] [Google Scholar]
- 43. Massett M. P., Ungvari Z., Csiszar A., Kaley G., Koller A. (2002) Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am. J. Physiol. Heart Circ. Physiol. 283, H2282–H2287 [DOI] [PubMed] [Google Scholar]
- 44. Phillips S. A., Olson E. B., Lombard J. H., Morgan B. J. (2006) Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. J. Appl. Physiol. 100, 1117–1123 [DOI] [PubMed] [Google Scholar]
- 45. Weber D. S., Lombard J. H. (2001) Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 280, H2196–H2202 [DOI] [PubMed] [Google Scholar]
- 46. Gauthier K. M., Baewer D. V., Hittner S., Hillard C. J., Nithipatikom K., Reddy D. S., Falck J. R., Campbell W. B. (2005) Endothelium-derived 2-arachidonylglycerol. An intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 288, H1344–H1351 [DOI] [PubMed] [Google Scholar]
- 47. Sugiura T., Kodaka T., Nakane S., Kishimoto S., Kondo S., Waku K. (1998) Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells. Is 2-arachidonoylglycerol a possible vasomodulator? Biochem. Biophys. Res. Commun. 243, 838–843 [DOI] [PubMed] [Google Scholar]
- 48. Mechoulam R., Fride E., Ben-Shabat S., Meiri U., Horowitz M. (1998) Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur. J. Pharmacol. 362, R1–3 [DOI] [PubMed] [Google Scholar]
- 49. Chataigneau T., Félétou M., Thollon C., Villeneuve N., Vilaine J. P., Duhault J., Vanhoutte P. M. (1998) Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric, and porcine coronary arteries. Br. J. Pharmacol. 123, 968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Begg M., Mo F. M., Offertaler L., Bátkai S., Pacher P., Razdan R. K., Lovinger D. M., Kunos G. (2003) G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J. Biol. Chem. 278, 46188–46194 [DOI] [PubMed] [Google Scholar]