Abstract
The transcription factor growth arrest and DNA damage-inducible gene 153 (GADD153), also known as CHOP, is considered to function as a proapoptotic molecule. Overexpression of GADD153 leads to cell cycle arrest and/or apoptosis. However, its clinical implications in non-small cell lung cancer (NSCLC) remain controversial. Therefore, we investigated the expression of GADD153 in stage I NSCLC using immunohistochemistry. Paraffin-embedded tissue sections from 76 patients, who were diagnosed with primary stage I NSCLC and had undergone a curative lung resection, were stained using an anti-GADD153 antibody. The intensity of GADD153 immunostaining was evaluated within the cell membrane and cytoplasm of invasive cancer components. The correlation between the intratumoral expression of GADD153 and various clinical parameters were explored. GADD153 was detected in 29 (38.2%) cases. No statistically significant difference in expression was demonstrated between stage IA and stage IB tumors (35.0 vs. 39.3%; P=0.735). The expression of GADD153 was not affected by histological subtypes or histological grades of differentiation. The intratumoral expression of GADD153 did not influence the overall survival rate (53.29 vs. 52.18 months; P=0.743) or disease-free survival rate (46.97 vs. 54.19 months; P=0.084) of stage I NSCLC patients. However, patients with GADD153 expression demonstrated an improved disease-specific survival rate (28.80 vs. 53.85 months; P=0.020). No patients with GADD153 expression demonstrated distant metastasis (P=0.029). These data suggest that GADD153 expression may be a valuable prognostic factor of early-stage NSCLC in patients who have undergone curative lung resection.
Keywords: non-small cell lung cancer, growth arrest and DNA damage-inducible gene, immunohistochemistry, prognosis
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 75–80% of all lung cancers and is the leading cause of cancer-related mortality worldwide (1,2). Twenty percent of stage I and 30% of stage II NSCLC patients (3) who are treated with curative intent experience recurrence of cancer, which is often incurable at the time of discovery, and have a 5-year survival rate of less than 50% (1). Despite years of intense efforts to control lung cancer mortality with surgical resection, chemotherapy and radiotherapy, NSCLC remains the leading cause of cancer-related mortality. This clearly indicates an urgent requirement for elucidating the mechanisms of lung cancer carcinogenesis, as well as discovering new approaches for its prevention and treatment.
The transcription factor growth arrest and DNA damage-inducible gene 153 (GADD153), also known as CHOP (4,5), is considered to function as a proapoptotic molecule. GADD153 belongs to the CCAAT/enhancer binding protein (C/EBP) family of transcription factors. It forms heterodimers with other C/EBP family proteins and changes their transcriptional activity (5,6).
GADD153 is ubiquitously expressed at a low level in several cell types, and its expression is induced by a variety of stress factors (5,7), including genotoxic stress, endoplasmic reticulum (ER) stress and nutrient depletion. Stress induces GADD153 expression at transcriptional and post-transcriptional levels (4,8–11). ER stress is induced by environmental conditions that are frequently encountered in cancer growth; therefore, the role of ER stress in carcinogenesis and tumor progression is being actively researched (12,13). Overexpression of GADD153 has been reported to lead to cell cycle arrest and/or apoptosis (14,15). Disruption of the GADD153 gene has been identified to render cells more resistant to ER stress-induced apoptosis, while exogenous GADD153 is capable of inducing growth arrest and/or apoptosis (14–16). GADD153 is one of the important factors in the death of cancer cells and in the anticarcinogenic process, where it downregulates cell growth and survival rate. Studies have suggested that GADD153 triggers the critical early events leading to the initiation of apoptosis, which are considered to be of significance in the prognosis of early NCSLC (17). In this study, we aimed to determine whether the expression of GADD153 is an indicator of good prognosis in stage I NSCLC patients. Consequently, we evaluated GADD153 expression in 76 stage I NSCLC tissue samples and investigated the correlation between expression and clinical and pathological findings.
Materials and methods
Study population and samples
Tissue specimens were obtained from 76 patients who were diagnosed with stage I primary NSCLC and underwent curative surgical removal of a primary lesion at the Yonsei Medical Center (Seoul, Republic of Korea) between 1995 and 1998. Pathological evaluation was established for histological classification and staging in all patients. No patient underwent radiotherapy or chemotherapy prior to or after surgery until the disease recurred. Of the 76 patients, 56 (73.7%) were male and 20 (26.3%) were female. The age of the patients ranged from 31 to 83 years, with a mean age of 61.8±9.97 years, which was similar to the age distribution in our institution’s large database of patients with stage I NSCLC (data not shown). All the clinical and pathological information and follow-up data were based on studies from our tumor registry service. The study was reviewed and approved by the institution’s Surveillance Committee and their permission was obtained to use tissue blocks and other pertinent information from patient files.
Immunohistochemical staining for GADD153
Paraffin-embedded 4 μm-thick tissue sections from 76 primary stage I NSCLC samples were stained with mouse monoclonal antibody against human GADD153 (Abcam, Ltd., Cambridge, UK). All sections were deparaffinized in a series of xylene baths and rehydrated using a graded alcohol series. Paraffin sections were retrieved via microwave treatment and treated with 0.3% hydrogen peroxidase to block endogenous peroxidase activity. The sections were then incubated with the primary anti-GADD153 antibody.
Following incubation, the sections were processed using standard avidin-biotin immunohistochemical techniques according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA, USA). Diaminobenzidine was used as a chromogen and hematoxylin was used for counterstaining. Adjacent normal-appearing bronchial epithelium within each tissue section served as an internal reference. The intensity of GADD153 immunostaining was evaluated within the cell membrane and cytoplasm of invasive cancer components. Cell staining was regarded as positive or negative and all slides were independently evaluated by two pathologists who were unaware of the clinical and pathological information of the subjects. Cancer cells in at least 4 fields were counted at ×200 magnification. The GADD153 immunoreactivity level was classified by the proportion of positive cells: 0, <5% positive cells; 1+, 5–30% positive cells; 2+, 30–50% positive cells; 3+, >50% positive cells. The intensity of GADD153 expression was also scored; 0, negative to weak; 1, moderate; 2, strong. The score was the sum of the intensity and the percentage of positive cells. A score of ≤1 was applied as a cut-off point for loss of GADD153 expression.
Statistical analysis
Survival data of all patients were obtained from the Korea National Statistical Office. A two-sample t-test for independent samples and an χ2-test were used for continuous and categorical variables, respectively. The Kaplan-Meier estimator was used to compute survival rate probability as a function of time and the log-rank test was used to compare survival time between the groups. All statistical tests were two-sided. SPSS software (version 15.0) was used throughout and P<0.05 was considered to indicate a statistically significant difference.
Results
NSCLC patient characteristics
The NSCLC cases included 56 (73.7%) male and 20 (26.3%) female patients (age range, 31–83 years; mean age, 61.8±9.97 years). The demographic characteristics of the patients are shown in Table I.
Table I.
Stage I NSCLC patient characteristics relative to GADD153 expression.
| GADD153 expression in tumor tissues | |||
|---|---|---|---|
|
|
|||
| Patient characteristics | Positive (n=29) | Negative (n=47) | P-value |
| Gender | |||
| Male | 18 | 38 | 0.07 |
| Female | 11 | 9 | |
| Smoking status | |||
| Smoker; packs/year, mean ± SD | 21; 45.0±28.73 | 33; 35.2±19.69 | 0.112; 0.311 |
| Non-smoker | 6 | 4 | |
| Unknown | 2 | 10 | |
| Pathology | 0.674 | ||
| Adenocarcinoma | 16 | 21 | |
| Squamous cell carcinoma | 12 | 24 | |
| Other | 1 | 2 | |
| Histological grade | 0.305 | ||
| Well-differentiated | 12 | 10 | |
| Moderately differentiated | 7 | 17 | |
| Poorly differentiated | 6 | 12 | |
| Unclassified | 4 | 8 | 0.735 |
| TNM stage | |||
| T1N0M0 | 7 | 13 | |
| T2N0M0 | 22 | 34 | |
| Distant metastasis | 0 | 8 | 0.029 |
| Mean survival time (months, 95% CI) | |||
| Disease-free | 54.19 (50.736–57.652) | 46.97 (38.062–55.881) | 0.084 |
| Disease-specific | 53.85 (50.702–57.002) | 28.80 (13.895–43.705) | 0.020 |
| Overall | 52.18 (46.735–57.624) | 53.29 (49.123–57.447) | 0.743 |
GADD153, growth arrest and DNA damage-inducible gene 153; NSCLC, non-small cell lung cancer; CI, confidence interval.
GADD153 immunohistochemistry
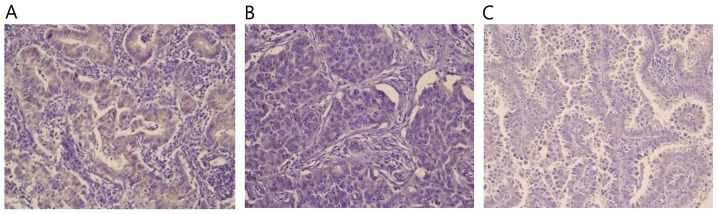
Immunohistochemical staining of GADD153 in normal and cancer tissue sections was conducted (Fig. 1). Immunoreactivity for the GADD153 antibody was identified primarily in the cytoplasm of cancer cells. However, in the normal lung tissue, bronchial epithelial cells demonstrated weak immunoreactivity. The intensity of GADD153 staining in the cytoplasm was strong, granular and distinct in the lung cancer tissue. The number of cases with GADD153 positive staining was 29 (38.7%). There was heterogeneity of staining inside the same tumor with sporadic, patchy, focal or diffused patterns. Such intramural heterogeneity made scoring difficult in certain cases, but immunohistochemical staining of GADD153 was stable and reproducible.
Figure 1.
GADD153 expression in NSCLC tissue. (A) Adenocarcinoma and (B) squamous cell carcinoma of the lung demonstrate strong immunoreactivity with GADD153. (C) Bronchioloalveolar carcinoma demonstrates positive GADD153 expression (magnification, ×200). GADD153, growth arrest and DNA damage-inducible gene 153; NSCLC, non-small cell lung cancer.
Correlation between GADD153 expression and various clinicopathological parameters
A total of 37 (48.7%) of the 76 stage I NSCLCs were adenocarcinomas, 36 (47.3%) were squamous cell carcinomas and 3 (3.9%) were other histological types of NSCLC. GADD153 expression was positive in 29 (38.2%) and negative in 47 (61.8%) cases. We were able to classify 64 tissue samples for histological grade of differentiation; 12 (54.5%) out of 22 were well-differentiated, 7 (29.2%) out of 24 were moderately differentiated and 6 (33.3%) out of 18 were poorly differentiated. Analysis of GADD153 expression revealed that expression was not influenced by histological type (P=0.674) or differential grade (P=0.305). In a subgroup analysis among subtypes of adenocarcinoma, no differences were observed between bronchioloalveolar carcinomas and other subtypes of adenocarcinoma. The smoking status of the patients did not influence the expression of GADD153, and none of the patients with GADD153 expression experienced distant metastasis of statistical significance (P=0.029) (Table I).
Survival analysis
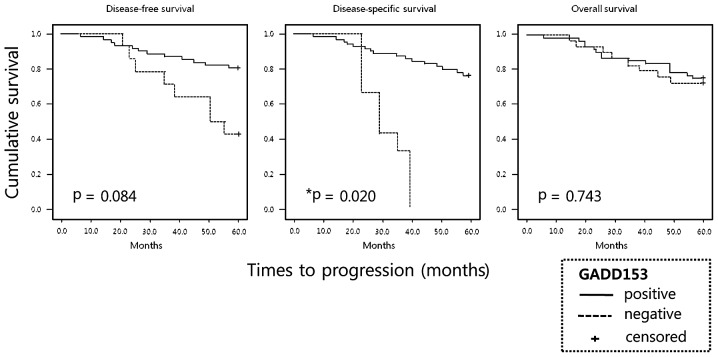
The total follow-up period of the patients who were alive at the time of analysis was 5 years. A total of 21 (27.6%) of the 76 patients died during the follow-up period; 11 (14.5%) succumbed to cancer-related events. The 5-year survival rate probability of the patient population was 72.3%, which is similar to previous results from a large-scale study (18). Overall survival and disease-free survival rate curves did not demonstrate statistically significant differences between NSCLC patients with and without GADD153 expression (Fig. 2). However, the patients with GADD153 expression had a significantly improved disease-specific survival rate compared to patients with negative GADD153 expression (28.80 vs. 53.85 months; P=0.020) (Table I; Fig 2). Subgroup analyses classified according to pathological diagnosis, grade of differentiation or TNM stages demonstrated that GADD153 expression status did not influence disease-free, disease-specific and overall survival rate in NSCLC patients (data not shown).
Figure 2.
Survival analysis of stage I NSCLC patients according to GADD153 expression. Patients whose tumors expressed GADD153 had a significantly improved disease-specific survival compared to those without GADD153 expression (P=0.02). GADD153, growth arrest and DNA damage-inducible gene 153; NSCLC, non-small cell lung cancer.
Discussion
It has been well-established that mutational activation of the Ras gene is a key factor in human cancer development (19). Oncogenic Ras proteins transform cells via multiple downstream signaling cascades, which lead to the phosphorylation and activation of proliferation-inducing transcription factors, including Elk-1, Ets-2 and c-Myc (19,20). Oncogenic Ras has also been identified to downregulate the expression of proapoptotic proteins, including the Bcl-2 family protein Bak and the transcriptional repressor Par-4 (21–24). One study also demonstrated that oncogenic Ras downregulated GADD153 expression at protein and mRNA levels (25).
GADD153, which belongs to the C/EBP family of transcription factors, forms heterodimers with other members of the C/EBP family, resulting in the inhibition of transcriptional activities (5,17). The GADD153 gene is typically induced in response to cellular stress (17). Previously, it has been reported that GADD153 expression may be regulated through various MAP kinase signaling pathways, and that the particular signaling pathway involved is dependent upon the type of stimuli (16,26,27). There is considerable evidence indicating that GADD153 is directly involved in the apoptosis pathway. It has been demonstrated that GADD153 upregulates the proapoptotic factor BH-3 (BIM) and downregulates the antiapoptotic Bcl-2 (28,29). Therefore, GADD153 increases cellular sensitivity to apoptosis by suppressing the transcription of antiapoptotic Bcl-2 (29). Previous studies have demonstrated that oncogenic Ras downregulates GADD153 expression and exogenous GADD153 inhibits Ras-induced cellular transformation (25). Therefore, GADD153 is important not only in killing cancer cells, but also in the anticarcinogenic process, where it downregulates cell growth and survival rate. A previous study has suggested that GADD153 may act as a critical marker of early response to cell injury, and each molecule is known to function in a different signal transduction pathway responsive to cell injury (30). The main role of the GADD153 gene is to block proliferation at G1 and 2 checkpoints in response to DNA damage. Transfection of the GADD153 gene into various cancer cell lines induces apoptosis without any stress-inducing factors, indicating that GADD153 is directly involved in the regulation of apoptosis (16). Furthermore, the GADD153 protein plays a significant role in the induction of apoptosis of cancer cells treated with N-(4-hydroxyphenyl)retinamide, a synthetic retinoid, in a retinoic acid receptor-independent pathway (31,32). These results strongly suggest that the expression of the GADD153 gene is a new molecular mechanism of antitumor activity. However, studies on the clinical relevance of GADD153 expression in human cancer are extremely limited. In previous studies, increased expression of GADD153 correlated with a lower tumor stage in colon cancer and with a higher survival rate in melanoma (33,34). A further study on lung cancer demonstrated that GADD153 expression correlated with a larger tumor size, higher pathological T stage, higher TNM stage and shorter overall survival rate (35). Therefore, we focused on the expression of GADD153 in patients with stage I (3) primary NSCLC and its association with clinical outcome. The aim of this study was to examine the expression of GADD153 in stage I NSCLC with respect to prognosis.
We examined the prognostic significance of GADD153 expression in formalin-fixed paraffin-embedded tissues and revealed that GADD153 expression was downregulated in a significant amount of patients with stage I NSCLC. Overall, 29 of 76 tumors (38.7%) expressed GADD153, and its expression was localized in the membrane and cytoplasm rather than in the nucleus of NSCLC cells. Notably, patients with GADD153 expression demonstrated a positive correlation with improved disease-specific survival rate (P=0.020). However, our data indicated that GADD153 expression was not associated with an improved overall survival rate and was only slightly associated with greater disease-free survival; no statistically significant differences were identified. We demonstrated that GADD153 expression was closely associated with and may have a role in the prevention of stage I NSCLC distant metastasis (P=0.029); thus, the greater disease-specific survival rate in patients with GADD153 expression. Subsequently, we analyzed the association of GADD153 expression with the clinicopathological parameters of stage I NSCLC patients. GADD153 was associated with distant metastasis, but none of the other clinicopathological features of stage I NSCLC patients, suggesting that GADD153 may be involved in apoptotic events preventing metastasis.
Since this study was a preliminary investigation, the number of patients with stage I NSCLC was relatively small (76 patients) and the longest follow-up time was 60 months. This study has revealed that GADD153 expression is a candidate marker that may aid in the stratification of patients according to prognosis following curative surgical removal of a primary lesion. Further comprehensive studies involving the mechanisms that induce expression of GADD153 in NSCLC are required to define the role of GADD153 in lung carcinogenesis. The significant association with survival rate observed in the present study is of particular relevance and should be confirmed in additional cohorts of patients.
Acknowledgements
This study was supported by the Institutional Grant from Yonsei University College of Medicine (6-2008-0198) provided to YS Chang through the Human Barrier Research Institute.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Sobin L, Wittekind Ch, editors. TNM Classification of Malignant Tumors. 6th edition. Wiley-Liss; New York: 2002. pp. 99–103. [Google Scholar]
- 4.Luethy JD, Fargnoli J, Park JS, Fornace AJ, Jr, Holbrook NJ. Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem. 1990;265:16521–16526. [PubMed] [Google Scholar]
- 5.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 6.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jousse C, Bruhat A, Carraro V, et al. Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5’UTR. Nucleic Acids Res. 2001;29:4341–4351. doi: 10.1093/nar/29.21.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackman J, Alamo I, Jr, Fornace AJ., Jr Genotoxic stress confers preferential and coordinate messenger RNA stability on the five gadd genes. Cancer Res. 1994;54:5656–5662. [PubMed] [Google Scholar]
- 9.Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem. 1997;272:17588–17593. doi: 10.1074/jbc.272.28.17588. [DOI] [PubMed] [Google Scholar]
- 11.Sato N, Urano F, Yoon Leem J, et al. Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nat Cell Biol. 2000;2:863–870. doi: 10.1038/35046500. [DOI] [PubMed] [Google Scholar]
- 12.Uramoto H, Sugio K, Oyama T, et al. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Hsu WM, Hsieh FJ, Jeng YM, et al. GRP78 expression correlates with histologic differentiation and favorable prognosis in neuroblastic tumors. Int J Cancer. 2005;113:920–927. doi: 10.1002/ijc.20693. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:143–147. doi: 10.1016/0014-5793(96)01016-2. [DOI] [PubMed] [Google Scholar]
- 15.Barone MV, Crozat A, Tabaee A, Philipson L, Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994;8:453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- 16.Maytin EV, Ubeda M, Lin JC, Habener JF. Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 kinase-dependent and -independent mechanisms. Exp Cell Res. 2001;267:193–204. doi: 10.1006/excr.2001.5248. [DOI] [PubMed] [Google Scholar]
- 17.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 18.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 19.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 20.Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- 21.Rosen K, Rak J, Jin J, Kerbel RS, Newman MJ, Filmus J. Downregulation of the pro-apoptotic protein Bak is required for the ras-induced transformation of intestinal epithelial cells. Curr Biol. 1998;8:1331–1334. doi: 10.1016/s0960-9822(07)00564-7. [DOI] [PubMed] [Google Scholar]
- 22.Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 1999;18:6362–6369. doi: 10.1093/emboj/18.22.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalca A, Qiu SG, El-Guendy N, Krishnan S, Rangnekar VM. Oncogenic Ras sensitizes cells to apoptosis by Par-4. J Biol Chem. 1999;274:29976–29983. doi: 10.1074/jbc.274.42.29976. [DOI] [PubMed] [Google Scholar]
- 24.Qiu SG, Krishnan S, el-Guendy N, Rangnekar VM. Negative regulation of Par-4 by oncogenic Ras is essential for cellular transformation. Oncogene. 1999;18:7115–7123. doi: 10.1038/sj.onc.1203199. [DOI] [PubMed] [Google Scholar]
- 25.Rong R, Montalbano J, Jin W, et al. Oncogenic Ras-mediated downregulation of Gadd153/CHOP is required for Ras-induced cellular transformation. Oncogene. 2005;24:4867–4872. doi: 10.1038/sj.onc.1208660. [DOI] [PubMed] [Google Scholar]
- 26.Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 27.Scott DW, Mutamba S, Hopkins RG, Loo G. Increased GADD gene expression in human colon epithelial cells exposed to deoxycholate. J Cell Physiol. 2005;202:295–303. doi: 10.1002/jcp.20135. [DOI] [PubMed] [Google Scholar]
- 28.Puthalakath H, O’Reilly LA, Gunn P, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 29.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman AD. GADD153/CHOP, a DNA damage-inducible protein, reduced CAAT/enhancer binding protein activities and increased apoptosis in 32D c13 myeloid cells. Cancer Res. 1996;56:3250–3256. [PubMed] [Google Scholar]
- 31.Kim DG, You KR, Liu MJ, Choi YK, Won YS. GADD153-mediated anticancer effects of N-(4-hydroxyphenyl)retinamide on human hepatoma cells. J Biol Chem. 2002;277:38930–38938. doi: 10.1074/jbc.M205941200. [DOI] [PubMed] [Google Scholar]
- 32.Xia Y, Wong NS, Fong WF, Tideman H. Upregulation of GADD153 expression in the apoptotic signaling of N-(4-hydroxyphenyl)retinamide (4HPR) Int J Cancer. 2002;102:7–14. doi: 10.1002/ijc.10664. [DOI] [PubMed] [Google Scholar]
- 33.Korabiowska M, Cordon-Cardo C, Betke H, et al. GADD153 is an independent prognostic factor in melanoma: immunohistochemical and molecular genetic analysis. Histol Histopathol. 2002;17:805–811. doi: 10.14670/HH-17.805. [DOI] [PubMed] [Google Scholar]
- 34.Rask K, Thorn M, Ponten F, et al. Increased expression of the transcription factors CCAAT-enhancer binding protein-beta (C/EBBeta) and C/EBzeta (CHOP) correlate with invasiveness of human colorectal cancer. Int J Cancer. 2000;86:337–343. doi: 10.1002/(sici)1097-0215(20000501)86:3<337::aid-ijc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Kim KM, Yu TK, Chu HH, et al. Expression of ER stress and autophagy-related molecules in human non-small cell lung cancer and premalignant lesions. Int J Cancer. 2011 Sep 27; doi: 10.1002/ijc.26463. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]