Abstract
In this study we report the pharmacokinetics and severe adverse effects of sunitinib in a woman with a gastrointestinal stromal tumor (GIST). A 60-year-old woman with small intestinal GIST developed severe thrombocytopenia (1.7×104/μl) following 1 week of treatment with sunitinib at 50 mg/day. Although the dose of sunitinib was reduced to 25 mg/day, platelet levels remained low. On day 7, the trough concentration of sunitinib plus SU12662 was 46.1 ng/ml and the area under the curve (AUC) was 1,393.0 ng·h/l. The dose was again reduced to 12.5 mg/day. However, the day after resumption of treatment, the patient developed symptoms of left heart failure due to myocardosis caused by sunitinib. Sunitinib has been reported to inhibit platelet-derived growth factor receptor (PDGFR) phosphorylation at concentrations over the range of 50–100 ng/ml (sunitinib plus SU12662) in vivo. In this case, the plasma concentration was sufficient to inhibit PDGFR at 25 or 50 mg/day. However, thrombocytopenia appeared at both dosages. Although the results in this case did not suggest a correlation between thrombocytopenia and plasma concentration, the degree of thrombocytopenia was decreased by reduction of the dose. In conclusion, the findings reported here indicate that the plasma concentration of sunitinib plus SU12662 is an important indicator to reduce adverse effects.
Keywords: sunitinib, SU12662 (N-desethyl sunitinib), gastrointestinal stromal tumor, thrombocytopenia
Introduction
Most gastrointestinal stromal tumors (GIST) have activating mutations in stem cell factor receptor (KIT) or platelet-derived growth factor receptor-α (PDGFRA) (1). Approximately 85% of GIST cases have activating mutations in KIT, and 5% have activating mutations in PDGFRA (1). Although the KIT gene contains 21 exons, KIT in GIST only has mutations within exons 8, 9, 11, 13, 14, 17 and 18. Most mutations (70%) in the KIT gene have been reported in the juxtamembrane domain (exon 11), and 15% of cases have mutations in the extracellular domain (exon 9) (2).
Imatinib mesylate (Glivec®; Novartis Pharma, Basel, Switzerland) selectively inhibits KIT and PDGFRA and is the first-line treatment in adult patients with KIT-positive non-resectable malignant GIST. The standard dose of imatinib is 400 mg daily. Recently, it was reported that a high dose of imatinib (800 mg daily) resulted in a significantly superior progression-free survival rate in patients with GIST harboring a KIT gene exon 9 mutation (3). It was suggested that KIT exon 9 mutation caused resistance to imatinib.
Sunitinib malate (Sutent®; Pfizer, New York, NY, USA) is a multi-target tyrosine kinase inhibitor and the second-line treatment of GIST following disease progression in cases intolerant to imatinib. Sunitinib inhibits KIT, PDGFRA, PDGFRB, vascular endothelial growth factor receptor (VEGFR), FMS-like tyrosine kinase 3 (FLT3), colony-stimulating factor 1 (CSF-1) and glial cell line-derived neurotrophic factor receptor rearranged during transfection (RET) (4–5). Sunitinib is metabolized by cytochrome P450 (CYP) 3A4, and produces N-desethyl metabolite, SU12662. SU12662 is an active metabolite that inhibits KIT, PDGFR and VEGFR in a similar manner to sunitinib. Previous animal studies demonstrated that target plasma concentrations of sunitinib plus SU12662 for the inhibition of PDGFRB and fetal liver kinase-1/kinase-insert domain-containing receptor (Flk-1/KDR)/VEGFR-2 phosphorylation were in the range of 50–100 ng/ml (4). In addition, it is more crucial to maintain the effective concentration (50–100 ng/ml) than to obtain a high maximum plasma concentration (Cmax).
The metabolic capability of CYP3A4 varies greatly among individuals (6). Although most medications have various metabolic pathways, the plasma concentration of sunitinib varies greatly among individuals as sunitinib is metabolized only by CYP3A4.
Here, we describe a patient who developed thrombocytopenia while taking sunitinib. We assayed the plasma concentrations of sunitinib and SU12662 to avoid thrombocytopenia.
Patients and methods
Case report
A 60-year-old Japanese woman took sunitinib 50 mg once daily after breakfast. The patient's height and weight were 150 cm and 36.45 kg, respectively, resulting in a body surface area of 1.25 m2. Her medical history consisted of small intestinal GIST, which was immunohistochemically positive for KIT, smooth muscle actin, CD34 and vimentin. The tumor metastasized to the liver. She then took imatinib 400 mg once daily after breakfast for 3 years. The dose of imatinib was reduced to 300 mg once daily due to the appearance of adverse effects. However, the dose of imatinib was increased to 400 mg once daily due to liver metastasis.
She was admitted to Shinshu University Hospital for one week for the first administration of sunitinib since there was no decrease in liver metastases following the change to the higher dose of imatinib. Fourteen days after the first administration of sunitinib, the patient experienced nosebleeds, stomatitis and malaise. The platelet (PLT) count was decreased to 1.7×104/μl, which was categorized as grade 4 thrombocytopenia according to the National Cancer Institute criteria version 4.0. Sunitinib was then discontinued and the patient was admitted to our hospital. The PLT count was increased following administration of PLT and adverse effects were eliminated (Fig. 1). The administration of sunitinib was resumed at 25 mg once daily and continued for 3 weeks. However, sunitinib was discontinued as the PLT count again decreased.
Figure 1.
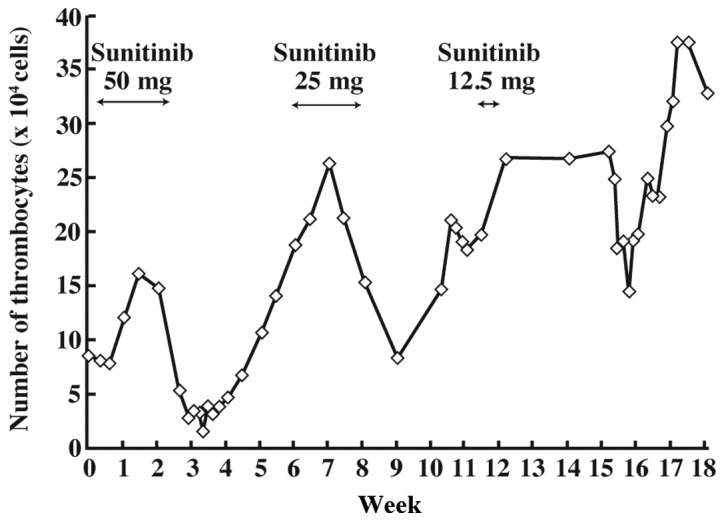
Thrombocytopenia following administration of sunitinib.
The PLT count normalized approximately 9 days after the discontinuation of sunitinib. Sunitinib was resumed at a dose of 12.5 mg once daily and the plasma concentrations of sunitinib and its metabolite, SU12662, were analyzed (Fig. 2). However, the patient developed a dry cough the day after resumption of sunitinib. A computed tomography scan revealed interstitial pneumonia. Echocardiography revealed hypokinesis of the left ventricle, which was shown to be drug-induced heart failure.
Figure 2.
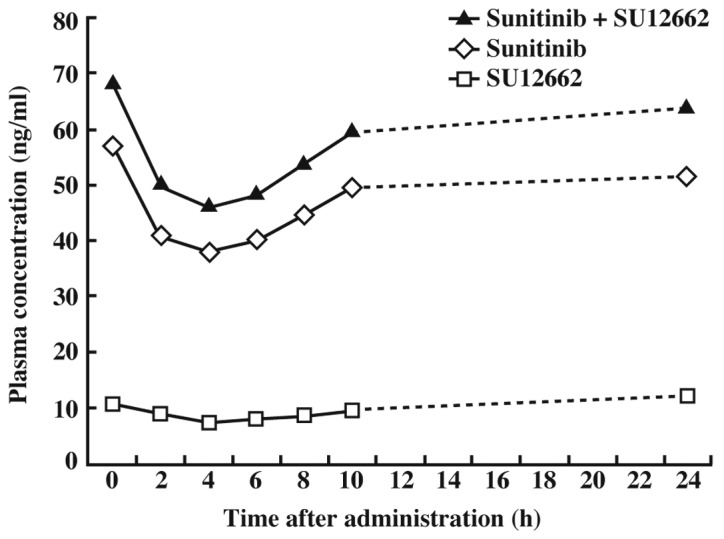
Diurnal variation of plasma concentration 7 days after starting sunitinib administration (25 mg/day).
Compounds
Sutent 12.5 mg was purchased from Pfizer Global Research and Development (Japan). Sunitinib malate and N-desethyl sunitinib (SU12662) were purchased from TRC (Toronto Research Chemicals, Ontario, Canada). The internal standard was 4-methyl-mexirethyn.
Pharmacokinetic sampling and assay
Blood sampling (pre-dose, and 2, 4, 6, 8, 10 and 24 h post-dose) was performed on day 7 of course 2 (sunitinib, 25 mg). Samples (0.5 ml) were collected in tubes containing ethylenediamine tetraacetic acid (EDTA). Samples were centrifuged at 3,500 rpm at 4°C for 10 min. NaOH (0.1 N) was added to the supernatants, and the compounds were extracted into 3 ml t-butyl methyl ether (TBME) and agitated for 5 min. The TBME phase was aspirated and evaporated to dryness (N2). Aliquots were subjected to high-performance liquid chromatography. The protocol was approved by the Medical Ethics Committee of Shinshu University and the patient provided informed consent prior to the study.
High-performance liquid chromatography conditions
The chromatographic system consisted of a mobile phase of mixture [0.05 M phosphoric buffer (pH 3), acetonitrile and B-7 low UV reagent (Waters, Milford, MA, USA) at a ratio of 695:300:5] with an ODS column pumped at a flow rate of 0.3 ml/min and UV/VIS detection at 431 nm (0–12 min) and 250 nm (12–20 min) (7). The retention times for N-desethyl sunitinib, sunitinib and internal control were 5.8, 8.3 and 14.8 min, respectively.
Results
The trough concentrations of sunitinib and SU12662 in plasma after 7 days at 25 mg were 38.0 and 7.4 ng/ml, respectively, and that of sunitinib plus SU12662 was 46.1 ng/ml. The average concentration was 56.0 ng/ml (Fig. 2). The area under the curve (AUC) of sunitinib plus SU12662 was 1,393.0 ng·h/l.
The concentrations of sunitinib, SU12662, and sunitinib plus SU12662 in plasma at 50 mg were 30.2, 9.2 and 40.1 ng/ml (3 days); 75.2, 28 and 105.3 ng/ml (7 days); and 102.3, 29.5 and 134.0 ng/ml (14 days), respectively (Fig. 3).
Figure 3.
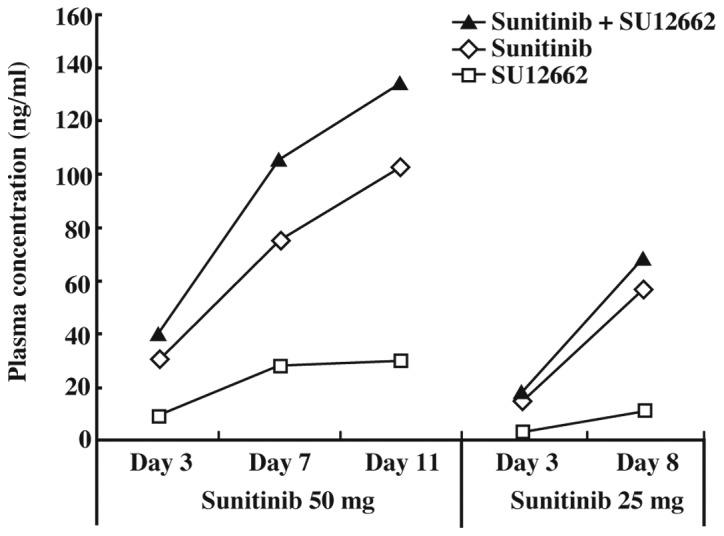
Progress of plasma concentration from the start of sunitinib administration (50 mg/day or 25 mg/day).
Discussion
A plasma concentration above 50 ng/ml of sunitinib plus SU12662 is required for the inhibition of tyrosine kinase phosphorylation. Fig. 3 shows that the plasma concentrations before administration of sunitinib and sunitinib plus SU12662 11 days after initiation of sunitinib treatment (50 mg/day) were approximately 100 and 120 ng/ml, respectively. These concentrations were not troughs since the trough concentration did not correlate with the plasma concentration prior to the administration of sunitinib, as shown in Fig. 2. The results shown in Fig. 2 indicated that the trough concentration of sunitinib (50 mg) plus SU12662 at 11 days would likely be over 50 ng/ml. Sunitinib inhibits Flk-1/KDR and PDGFR phosphorylation at concentrations over the range of 50–100 ng/ml (sunitinib plus SU12662) in vivo. It has been reported that plasma concentrations of sunitinib and SU12662 should be maintained within the range of 50–100 ng/ml (4). The results shown in Fig. 3 indicate that the plasma concentration of sunitinib was sufficient to inhibit Flk-1/KDR and PDGFR in this patient.
Pharmacokinetics in patients with GIST may be different from those in non-GIST patients. Notably, the concentration at 4 h after the administration of sunitinib was Cmin in Fig. 2. This patient underwent excision of the small intestine, and it was considered that this excision delayed the absorption of sunitinib. Many patients with small intestinal GIST undergo excision of the small intestine, and it is therefore predicted that sunitinib absorption will be delayed in such cases. Furthermore, the plasma concentrations of SU12662 on days 7 and 14 at 50 mg sunitinib were the same. This result was due to the reduced CYP3A4 activity caused by the tumor metastasis in the left side of the liver.
Reduction of sunitinib may not suppress thrombocytopenia at the effective concentration. Recently, it was reported that the adverse effects of sunitinib are correlated with plasma concentration (8). Diastolic blood pressure has been shown to be correlated with trough plasma concentration of total drug (sunitinib plus SU12662). Conversely, the absolute neutrophil count is correlated with AUCcum28tot (28-day cumulative AUC of total drug) (8). However, in this patient, the reduction in plasma concentration did not ameliorate thrombocytopenia.
This case report suggests that sunitinib at a dose of 50 mg could be an over-dosage in Asian women. Houk et al reported that the AUC and Cmax of both sunitinib and total drug are increased in Asians and in females (9). This was suggested to be associated with a reduction in CYP3A4 activity since both sunitinib and SU12662 are metabolized only by CYP3A4. Metabolism in the liver is associated with liver volume (10). If the liver volume is small, the metabolic capability is also small. The liver volume is small in Asians and females since the average weight and height are generally lower than those of non-Asians and males. Therefore, it is possible to calculate liver volume by body surface area (11). The results suggested that the metabolic capability of CYP3A4 is lower in Asian than non-Asian females.
CYP3A5 is also likely to demonstrate differences in metabolic ability among individuals. It is difficult to separate the effects of CYP3A5 and CYP3A4 as they have similar spectra of substrates. CYP3A5 is the predominant isozyme among human liver CYP3As (12), and CYP3A5 may be responsible for the metabolism of sunitinib. However, it has been reported that there are ethnic and individual differences in the expression of CYP3A5. The activity of CYP3A5.3 is extremely low and this CYP3A5*3 genotype was 60% in Japanese or Chinese, 31% in Indian, 70% in Caucasians, and 35% in African-Americans (13–15). Thus, it is likely to be involved in the differences in expression of hepatic CYP3A between ethnicities and individuals. These differences suggested that plasma concentration of sunitinib differs between individuals.
We concluded that the monitoring of sunitinib plasma concentration is essential to determine the appropriate dose in individual patients. Furthermore, this study showed that the frequency of thrombocytopenia and hypothyroidism in Asians, including Japanese, with sunitinib is higher than in Europeans and Americans. This is because the plasma concentrations in Asians are higher than those in Europeans and Americans due to the liver volume being smaller and due to CYP3A5*3 being the major genotype in Asians.
Acknowledgements
We thank Dr Hajime Ichimura for providing clinical insights and taking blood samples.
Abbreviations
- GIST
gastrointestinal stromal tumor
- KIT
stem cell factor receptor
- Flk-1/KDR
fetal liver kinase-1/kinase-insert domain-containing receptor
- PDGFR
platelet-derived growth factor receptor-α
- VEGFR
vascular endothelial growth factor receptor
- FLT3
FMS-like tyrosine kinase 3
- CSF-1
colony-stimulating factor 1
- RET
glial cell line-derived neurotrophic factor receptor (rearranged during transfection)
- CYP
cytochrome P450
- AUC
area under curve
References
- 1.Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006;48:83–96. doi: 10.1111/j.1365-2559.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 2.Hormick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumours. Hum Pathol. 2007;38:679–687. doi: 10.1016/j.humpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Mendel DB, Laird AD, Xin X, et al. In vivo antitumour activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 5.Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Nafziger AN, Bertino JS., Jr Drug-metabolizing enzyme inhibition by ketoconazole does not reduce interindividual variability of CYP3A activity as measured by oral midazolam. Drug Metab Dispos. 2006;34:2079–2082. doi: 10.1124/dmd.106.011742. [DOI] [PubMed] [Google Scholar]
- 7.Blanchet B, Saboureau C, Benichou AS, et al. Development and validation of an HPLC-UV-visible method for sunitinib quantification in human plasma. Clin Chim Acta. 2009;404:134–139. doi: 10.1016/j.cca.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Moltzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemther Pharmacol. 2010;66:357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 9.Houk BE, Bello CL, Kang D, Amantea M. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res. 2009;15:2497–2506. doi: 10.1158/1078-0432.CCR-08-1893. [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa Y, Chisuwa H, Ikegami T, et al. Relationship between in vivo FK506 clearance and in vitro 13-demethylation activity in liver-related liver transplantation. Transplantation. 1998;66:1089–1093. doi: 10.1097/00007890-199810270-00020. [DOI] [PubMed] [Google Scholar]
- 11.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 12.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetics basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 13.Saeki M, Saito Y, Nakamura T, et al. Single nucleotide polymorphisms and haplotype frequencies of CYP3A5 in a Japanese population. Hum Mutat. 2003;21:653. doi: 10.1002/humu.9147. [DOI] [PubMed] [Google Scholar]
- 14.Balram C, Zhou Q, Cheung YB, Lee EJ. CYP3A5*3 and *6 single nucleotide polymorphisms in three distinct Asian populations. Eur J Clin Pharmacol. 2003;59:123–126. doi: 10.1007/s00228-003-0594-2. [DOI] [PubMed] [Google Scholar]
- 15.Fukuen S, Fukuda T, Maune H, et al. Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese population. Pharmacogenetics. 2002;12:331–334. doi: 10.1097/00008571-200206000-00009. [DOI] [PubMed] [Google Scholar]


