SUMMARY
There is profound knowledge that sleep restriction increases tonic (event-unrelated) electroencephalographic (EEG) activity. In the present study we focused on time-locked activity by means of phasic (event-related) EEG analysis during a psychomotor vigilance task (PVT) over the course of sleep deprivation. Twenty healthy subjects (10 male; mean age ± SD: 23.45 ± 1.97 years) underwent sleep deprivation for 24 h. Subjects had to rate their sleepiness hourly (Karolinska Sleepiness Scale) and to perform a PVT while EEG was recorded simultaneously. Tonic EEG changes in the δ (1–4 Hz), θ (4–8 Hz) and α (8–12 Hz) frequency range were investigated by power spectral analyses. Single-trial (phase-locking index, PLI) and event-related potential (ERP) analyses (P1, N1) were used to examine event-related changes in EEG activity. Subjective sleepiness, PVT reaction times and tonic EEG activity (delta and theta spectral power) significantly increased over the night. In contrast, event-related EEG parameters decreased throughout sleep deprivation. Specifically, the ERP component P1 diminished in amplitude, and delta and theta PLI estimates decreased progressively over the night. It is suggested that event-related EEG measures (such as the amplitude of the P1 and especially delta/theta phase-locking) serve as a complimentary method to track the deterioration of attention and performance during sleep loss. As these measures actually reflect the impaired response to specific events rather than tonic changes during sleep deprivation they are a promising tool for future sleep research.
Keywords: attention, EEG, PVT, phase locking, sleep deprivation, sleep restriction
INTRODUCTION
It is well known that changes in tonic electroencephalographic (EEG) activity are related to fluctuations in alertness (Aeschbach et al., 1999; Cajochen et al., 2001). While theta and alpha power increase, neurobehavioural performance during prolonged wakefulness decreases (Drapeau and Carrier, 2004; Galliaud et al., 2008; Philip and Akerstedt, 2006). In addition, several studies demonstrated strong positive correlations between the Karolinska Sleepiness Scale (KSS) and EEG theta as well as alpha activity (Akerstedt and Gillberg, 1990; Horne and Baulk, 2004). Most of the former investigations of brain functions related to performance in a psychomotor vigilance task (PVT) have either not collected EEG and PVT performance data simultaneously (Cajochen et al., 1999; Galliaud et al., 2008), or have not reported correlations between EEG and PVT performance measures (Caldwell et al., 2003). However, there are some earlier studies reporting simultaneous EEG recordings during PVT. As an example, Corsi-Cabrera et al. (1996) found a relationship between slowing of reaction times and increase in absolute EEG power (4–20 Hz) during the performance of a visual discriminative task as a function of sleep deprivation. The present study now intended to investigate the effects of sleep deprivation on neural mechanisms involved in stimulus processing by combining three measures: tonic EEG, phasic EEG and PVT performance. Therefore, we recorded EEG simultaneously during eight hourly PVT sessions between 23:30 and 06:30 h. Different quantitative EEG measures were applied to analyse the EEG. Besides rather unspecific power spectral analysis by means of Fast Fourier Transformation, we additionally used more specific event-related measures: event-related potentials (ERPs) and phase-locking index (PLI). Whereas power spectral estimates lack any time information, ERP and PLI are measures directly related to the timing of neural activity and therefore allow new insights into the mechanisms of stimulus-related cognitive processing during sleep deprivation.
ERPs, calculated by additive averaging, are scalp-recorded brain potentials representing a measure of neural activity and providing information about the temporal course of information processing in the human brain. P1 (a positive component peaking at about 100 ms after stimulus onset) and N1 (a negative component peaking at about 160 ms after stimulus onset) are ERP components being strongly related to selective, reflexive and visual-spatial attention as well as feature detection (Heinze et al., 1994; Hillyard and Anllo-Vento, 1998). Previous work on event-related activity and performance in a visual discriminative task during total sleep deprivation (Corsi-Cabrera et al., 1999) could demonstrate a progressive amplitude reduction and increase in latency of almost all late components of the ERP (P180, N242, P281, N382, N500, P718).
PLI investigates the oscillatory phase of an EEG signal and reflects the time jitter of ERP components in the frequency domain. A large PLI reflects a small jitter, and a small PLI a large jitter. Or, in other words the PLI reflects the intertrial variance of ERP components and thereby seems to be a direct measure of temporal synchronization, indicating an important aspect of the timing of neural activity (for more detail, see Fig. S1). In general there is increasing evidence for a close functional relationship between early ERP components and phase-locking of low-frequency oscillations, such as theta and alpha (Busch et al., 2009; Klimesch et al., 2004; Mathewson et al., 2009). For example, Freunberger et al. (2007) reported that during a visual priming task there are P2-related differences between congruent and incongruent targets, which are related to theta phase-locking in the time–frequency domain. Additionally, Hanslmayer et al. (2005) demonstrated a positive association between increased alpha phase-locking and enhanced P1 amplitude obtained for good visual perception performance. These findings provide evidence that oscillations reorganize their phases, as reflected in high PLI values, due to external events to establish good cognitive performance (cf. Klimesch et al., 2007). Furthermore, ERP components are now thought to be generated due to these phase realignments and superposition of different frequency bands (Gruber et al., 2005).
As tonic EEG changes related to sleep pressure are already well known, the question arises, whether similar, systematic changes in phasic EEG could be observed throughout sleep deprivation. The PVT task used in our study has a strong emphasis on visual attention. To reveal effects concerning the timing aspect of deterioration in visual attention, we therefore included two different phasic EEG measures: ERP and PLI. With respect to earlier findings we expect an increase in spectral power of low-frequency components (delta and theta oscillations), but a decrease of early visual ERP components and lower phase-locking in slow frequencies. Furthermore, we hypothesized that the phasic measures of EEG activity are a complimentary tool to describe behavioural decrements and sleepiness over the course of sleep deprivation as they are related more directly (than tonic spectral estimates) to brain activity in response to specific stimuli as usually of interest in a real world setting.
MATERIALS AND METHODS
Subjects
Twenty healthy subjects (10 male) with a mean age of 23.45 years (SD = 1.97; range 19–28 years) participated in the study. All subjects reported sleeping regularly for 7–9 h per night (bedtime: 22:30–23:30 h; rising-time: 06:30–07:30 h) and received financial compensation (70 €) for their participation. This study was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria
Subjects were selected on the basis of self-reported questionnaires according to the following criteria: no history of severe organic and mental illness, no medications, no drugs, no sleep disturbances, no extreme evening- or morning-type individuals, and no signs of mood disorders. Subjects who worked night shifts or went on a transatlantic trip 3 months prior to the study were excluded. We used actimetry recordings (Cambridge Neurotechnology Actiwatch®) to quantify the usual sleep duration and sleep efficiency for three nights, prior to sleep deprivation. Furthermore, subjects had to report daily sleep habits and sleep quality by sleep logs. During the three nights before sleep deprivation, actimetric recording indicated an estimated mean total sleep time of 477.62 min (SD = 45.65) and a sleep efficiency of 88.32% (SD = 4.72).
Procedure
To investigate physiological correlates of sleep deprivation EEG was recorded while subjects performed the PVT hourly between 23:30 and 06:30 h. Additionally, subjective sleepiness was assessed hourly using the KSS (Akerstedt and Gillberg, 1990). For the sleep deprivation session, participants had to wake between 07:00 and 08:00 h, and attended the sleep laboratory at 19:00 h staying awake and being monitored all night (until 07:00 h). During the night subjects’ physical activity and light exposure (max. 8 lux) was maintained as low as possible. Because of metabolic influences food supply was restricted throughout the night. Any use of stimulants was prohibited. Subjects remained under constant supervision of the experimenter and were engaged in playing games (e.g. UNO®) between PVT testing sessions.
PVT
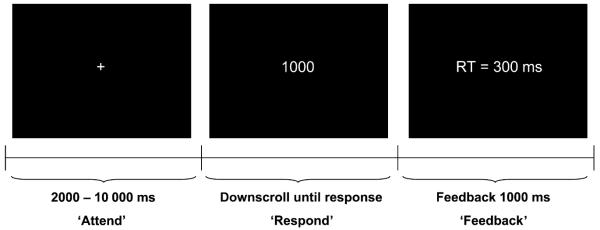
The PVT (adapted version of Dinges and Powell, 1985), a simple cued reaction time task, was originally developed in order to measure sustained attention. During the task subjects were seated 1.2 m in front of a computer monitor (refresh rate = 75 Hz) and fixated on a white cross (Fig. 1; ‘Attend’). At random intervals (2000–10 000 ms) a millisecond counter started to scroll and a trigger was sent to the EEG amplifier. The subject’s task was to respond as quickly as possible by pressing a button to stop the counter (Fig. 1; ‘Respond’). After pressing the button, the achieved reaction time was displayed on the screen for 1000 ms, providing the subject with feedback on performance (Fig. 1; ‘Feedback’). If no response was given within 2000 ms, subjects were alerted by the experimenter and those trials were excluded from analysis. The task contained 120 trials and lasted about 12 min according to individual performance.
Figure 1.
PVT. Schematic representation of one trial within the sustained attention task. RT, reaction time.
EEG recordings
For EEG recording a 32-channel NeuroScan Synamp amplifier (Compumedics Neuroscan, Charlotte, NC, USA) was used. All signals were filtered (0.10 Hz high-pass filter; 70 Hz low-pass filter; 50 Hz notch filter) and digitized online with 500 Hz sampling rate. Electrodes were attached according to the international electrode (10–20) placement system. Twenty-three EEG channels (Fp1, Fpz, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, Oz, O2, as well as A1 and A2 for later re-referencing), four electrooculogram (EOG) channels to control for eye artefacts, one submental electromyogram channel, one electrocardiogram channel and one respiratory channel (chest wall movements) were placed. Data were recorded against a common reference placed at Fcz and re-referenced offline to linked earlobes (A1, A2). Impedances were kept below 5 kΩ. EOG correction was applied and data were visually inspected for eye or muscle artefacts as well as micro sleeps. Data analyses were performed using Brain Vision Analyser (BrainProducts, Gilching, Germany) and Matlab7.0.1 (MathWorks, Natick, MA, USA).
EEG analyses
Spectral power
To investigate tonic EEG changes, spectral power analyses were calculated. Data recorded during PVT were segmented into consecutive, event-unrelated epochs of 1000 ms duration. To obtain power spectral estimates (amplitude, μV) in the frequency domain, Fast Fourier Transformation was applied. Spectral power estimates were then averaged within the δ (1–4 Hz), θ (4–8 Hz) and α (8–12 Hz) frequency range.
ERP
For ERP analyses raw data were first band-pass filtered within 0.5–30 Hz and then epoched in 1000-ms time windows ranging from −500 to 500 ms around stimulus onset. A baseline correction was applied for the pre-stimulus range from −200 to 0 ms preceding the appearance of the millisecond counter (Fig. 1: ‘Respond’). On average, 88.87 (SD = 21.47) artefact-free trials were included for ERP calculations. We investigated the P1 and N1 components by performing a peak-detection, semi-automatically. The time frame for the P1 was set between 70 and 130 ms, and for the N1 between 130 and 200 ms.
PLI
The PLI reflects the extent of inter-trial phase variability for a given frequency across time (for more detail, see Fig. S1). Analogue to a correlation coefficient, PLI ranges between zero and one, reflecting maximal phase-locking, i.e. minimal phase variability over the trials, when the PLI tend towards 1. For our analyses PLI values were estimated on the basis of Garborwavelet transformation for an epoch length of −500 to 500 ms around stimulus onset for each time point and each frequency (1 < fn > 20 Hz; frequency resolution = 1 Hz) for all recording sites, separately per subject.
Statistical analyses
Statistical analyses were performed using SPSS 16.0.0 software (SPSS, Chicago, IL, USA). One subject was excluded because of too abundant ocular artefacts. Nineteen subjects remained for final data analyses. Kolmogorov–Smirnov tests were applied to test for the normality of the distribution of the data, which was given in all cases. The significance level was set to P < 0.05 and Greenhouse–Geisser correction was applied when necessary. For post hoc comparisons paired-sample t-tests were performed.
Changes in PVT performance [mean reaction time (RT), median RT, SD of RT, mean of the fastest 10% RT, mean of the 10% slowest RT and number of lapses (RT > 500 ms)] and sleepiness (KSS score) throughout sleep deprivation were evaluated by one-factor anovas for repeated-measures with the within-subject factor SESSION (23:30, 00:30, 01:30, 02:30, 03:30, 04:30, 05:30, 06:30 h). Bonferroni-corrected significance level P = 0.007 (0.05/6) was used because of multiple testing.
For statistical analyses of the EEG measures we used occipital electrode sites (O1, Oz, O2) where all ERP components could be clearly detected. To analyse tonic EEG changes at different locations over the night, two-factor anovas were run with factors SESSION (23:30, 06:30 h) and ELECTRODE (O1, Oz, O2). The dependent variables were delta, theta or alpha spectral power estimates, respectively. For ERP analyses we performed two-way anovas with the factor SESSION (23:30, 06:30 h) and ELECTRODE (O1, Oz, O2) for amplitude and latency values separately. To investigate changes in phase-locking, frequencies were averaged to obtain PLI estimates for the delta, theta or alpha frequency range. Two post-stimulus time windows were chosen to analyse PLI changes after stimulus presentation (0–250 ms, 250–500 ms). Additionally, a prestimulus time window (−400 to −150 ms) served as reference interval. For PLI estimates two-factor anovas with the within-subject factors SESSION (23:30, 06:30 h) and TIME (−400 to −150 ms, 0–250 ms, 250–500 ms) were calculated for every frequency band (delta, theta, alpha) and for each electrode (O1, Oz, O2).
Note that due to technical problems regarding EEG measures of two subjects during the first PVT session, the number of valid subjects varies throughout the different analyses between 17 and 19.
RESULTS
Performance
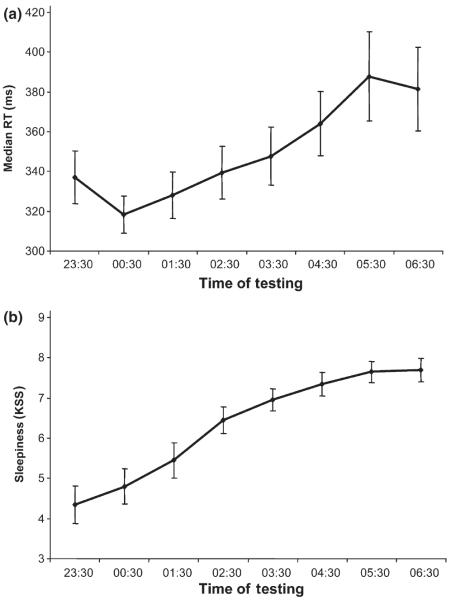
Subjects became slower and experienced more lapses with prolonged wakefulness. All one-factor repeated anovas – except the mean of the fastest 10% RT (F7,126 = 14.424, P = 0.253) – showed a significant effect for the factor SESSION (mean RT: F7,126 = 9.462, P = 0.004; median RT: F7,126 = 11.387, P < 0.001, cf. Fig. 2a; SD of RT: F7,126 = 11.820, P < 0.001; mean of the 10% slowest RT: F7,126 = 14.459, P < 0.001; number of lapses: F7,126 = 12.100, P = 0.001), indicating that performance was deteriorating over time. Pairwise comparisons between RT [mean RT, median RT, SD of RT, mean of the 10% slowest RT and number of lapses (RT > 500 ms)] during the first (23:30 h) and last PVT session (06:30 h) revealed a significant increase in all RT measures over the night (mean RT: t18 = −2.815, P = 0.011; median RT: t18 = −2.817, P = 0.011; SD of RT: t18 = −4.050, P = 0.001; 10% slowest RT: t18 = −4.209, P = 0.001; number of lapses: t18 = −3.421, P = 0.003).
Figure 2.
Time course of (a) overall median reaction times (±SEMs) while performing the PVT, and (b) sleepiness (±SEMs) over the course of sleep deprivation. KSS, Karolinska Sleepiness Scale; RT, reaction time.
KSS
Subjective sleepiness changed over the course of sleep deprivation, with higher KSS scores in the morning (F7,126 = 28.207, P < 0.001; Fig. 2b). Pairwise comparisons between KSS score during the first (23:30 h) and last PVT session (06:30 h) revealed a significant increase of subjective sleepiness (t18 = −6.185; P < 0.001).
EEG
Spectral power
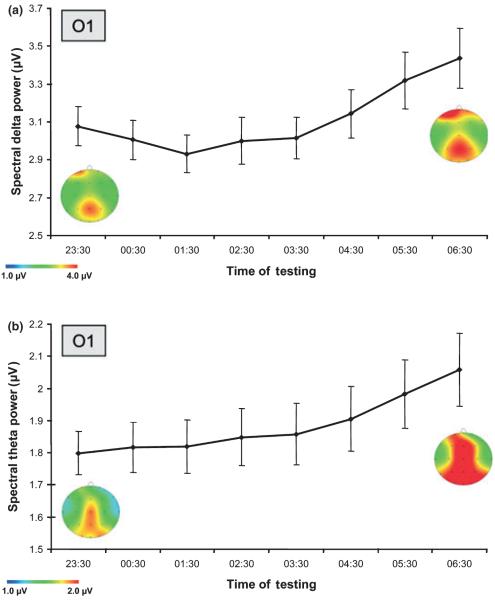
For tonic EEG activity an increase in spectral power estimates was revealed by a significant main effect for the factor SESSION within the delta and theta frequency band collapsed over all occipital electrodes (δ: F1,17 = 7.179, P = 0.016; θ: F1,17 = 14.430, P = 0.001). Pairwise comparisons between spectral power estimates during the first (23:30 h) and last PVT session (06:30 h) showed a significant increase of tonic delta (O1: t17 = −2.898, P = 0.010; Oz: t17 = −2.468, P = 0.024; O2: t17 = −3.330, P = 0.032; Fig. 3a) and theta power (O1: t16 = −3.842, P = 0.001; Oz: t16 = −3.772, P = 0.001; O2: t16 = −3.726, P = 0.002; Fig. 3b). The increase of spectral power estimates in the alpha band was only evident as statistical trend (F1,17 = 4.225, P = 0.056).
Figure 3.
Tonic spectral power over the course of the night. Shown is the significant increase in spectral power estimates (±SEMs) in the delta (a) and theta (b) frequency range. Note that these tonic increases mimic the behavioural decrements in cf. (depicted in Fig. 2).
ERP
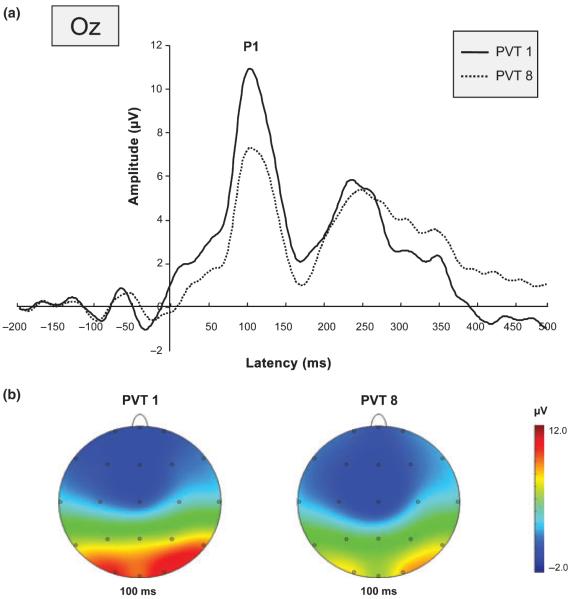
While tonic power increased the amplitude of the ERP component, P1 decreased over the night (F1,16 = 16.046, P = 0.001; cf. Fig. 4). Pairwise comparisons between the amplitude of P1 during the first (23:30 h) and last PVT session (06:30 h) revealed a significant decrease of amplitude (O1: t16 = 3.630, P = 0.002; Oz: t16 = 3.847, P = 0.001; O2: t16 = 4.316, P = 0.001). There was neither a significant P1 latency change, nor any significant modification of the N1 component over the course of sleep deprivation.
Figure 4.
ERP curves (a) and topographical maps (b) at beginning (bold) and end (dotted) of the night. (a) PVT1. Note that positive potentials are plotted upward in this figure. (b) Topographical plots of the P1 component at 100 ms for PVT1 and PVT8. Warm colours denote amplitude increases. Note that the P1 amplitude significantly decreased from the first (PVT1; 23:30 h) to the last PVT session (PVT8; 06:30 h) at occipital electrode sites.
PLI
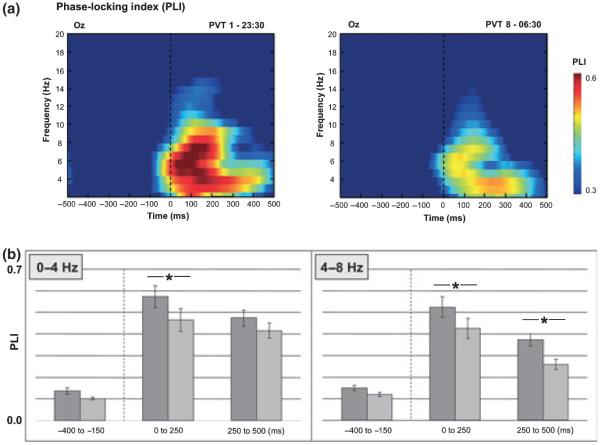
A two-way anova with repeated-measures for delta PLI estimates revealed a significant effect for the factor SESSION on every occipital electrode (O1: F1,16 = 9.694, P = 0.007; Oz: F1,16 = 9.231, P = 0.008; O2: F7,235 = 7.235, P = 0.016). Pairwise comparisons between delta PLI estimates during the first (23:30 h) and last PVT session (06:30 h) showed a significant decrease of delta phase-locking only within the time window 0–250 ms (O1: t16 = 3.156, P = 0.006; Oz: t16 = 2.873, P = 0.011; O2: t16 = 2.171, P = 0.045). For theta PLI a significant effect for the factor SESSION was found on O1 (F1,16 = 14.871, P = 0.001), Oz (F1,16 = 16.674, P = 0.001) and O2 (F1,16 = 19.420, P < 0.001). Pairwise comparisons between theta PLI estimates during the first (23:30 h) and last PVT session (06:30 h) showed a significant decrease of theta phase-locking within both post-stimulus time windows 0–250 ms (O1: t16 = 2.486, P = 0.024; Oz: t16 = 2.552, P = 0.021; O2: t16 = 2.573, P = 0.020) and 250–500 ms (O1: t16 = 4.169, P = 0.001; Oz: t16 = 4.192, P = 0.001; O2: t16 = 4.299, P = 0.001). For alpha PLI a significant effect for the factor SESSION was found on O1 (F1,16 = 4.984, P = 0.040), Oz (F1,16 = 6.594, P = 0.021) and O2 (F1,16 = 6.208, P = 0.024). Pairwise comparisons between alpha PLI estimates during the first (23:30 h) and last PVT session (06:30 h) showed a significant decrease of alpha phase-locking within the time windows 0–250 ms (O2: t16 = 2.873, P = 0.011) and 250–500 ms (O1: t16 = 2.183, P = 0.044). Colour-coded PLI matrices (Fig. 5a) indicate that phase-locking generally decreases during prolonged wakefulness – especially within the delta and theta frequency range (Fig. 5b).
Figure 5.
Phase-locking as revealed by the phase-locking index (PLI) of frequencies from 0 to 20 Hz over time. (a) Time–frequency plots of PLI estimates during psychomotor vigilance task PVT1 and PVT8 for the pre- and post-stimulus time window (−500 to 500 ms) at electrode site Oz. Warm colours indicate strong phase-locking, cold colours absent phase-locking. Note that the time window 500 ms prior to stimulus onset shows relatively low PLI values (~0.2), indicating a large variance in phase, whereas especially 0–250 ms post-stimulus PLI values above 0.5 indicate high phase locking. (b) Delta and theta PLI estimates (±SEMs) extracted from the time–frequency plots. Note that PLI in the delta and theta frequency range is significantly higher (P < 0.05) at the beginning (PVT1, dark gray) than at the end (PVT8, light gray) of the night.
For an overview concerning the time course of performance (RT), sleepiness (KSS) and the different EEG measures (spectral power, ERP, phase-locking) while performing the PVT during sleep deprivation, see also Fig. S2. Additionally, as supplementary Table S1, depicts Pearson product moment correlations between KSS, median RT, amplitude of P1, delta and theta spectral power estimates as well as delta and theta PLI estimates to highlight the relationship between changes in EEG, subjective sleepiness and performance.
DISCUSSION
Results indicate that cognitive impairment induced by sleep deprivation can be monitored directly during tasks of interest by focusing on EEG aspects reflecting the exact timing of neural activity. Specifically, we recorded the EEG hourly during PVT task completion over the course of sleep deprivation (23:30–06:30 h).
While our results confirm previous knowledge concerning changes during sleep deprivation: (i) increase in reaction time (PVT); (ii) increase in subjective sleepiness (KSS; Fig. 2); and (iii) increase in tonic spectral power estimates (Fig. 3), the present findings add a new aspect about event-related EEG changes throughout the course of sleep deprivation. With respect to the ERP component P1 we found that P1 amplitude (Fig. 4), and especially theta and delta PLI (Fig. 5), decrease with accumulating sleep pressure. This finding indicates that sleepiness increases the time jitter of ERP components in general, and of the P1 component in particular. We interpret this result in terms of a lack of precise neuronal timing, reflecting most likely a deficit to establish focused visual attention. Hanslmayer et al. (2005) found that good performance in a visual discrimination task is accompanied by significant higher P1 amplitudes. Additionally, Fründ et al. (2007) could demonstrate that in a speeded reaction time task, where subjects were instructed to respond with a button press as quickly as possible when a black square was presented, the P1 amplitude was significantly larger in trials with short response latencies. Our results show a significant decrease in the P1 amplitude throughout sleep deprivation, and thus possibly indicate a relationship with less cortical arousal, attention and neuronal timing during prolonged wakefulness. However, as it is unclear whether the reported reduction in the P1 component is directly due to increasing sleep pressure or due to a reduction of visual attention we compared ERP responses to the fastest 20% of trials (‘attended’ trials) with ERP responses to the slowest 20% (‘unattended’ trials). Results indicate that the observed reduction of P1 amplitude is mainly an effect of sleep deprivation and not merely inattention (Fig. S3).
Whereas the P1 component may reflect visual attention, delta and theta, in particular, may be associated with working memory demands. As an example, an increase in event-related theta (and delta) power (reflecting event-related synchronization, or ERS) can be observed consistently during the encoding of new information (for review, see Klimesch, 1999). This conclusion can be illustrated by the theta (or delta) old–new effect, which shows that during recognition ERS for old words is larger than for new words (Klimesch et al., 2006). It is also supported by the theta (or delta) subsequent memory effect, which is characterized by a brief increase in ERS (about 100–400 ms) during the encoding of items that can later successfully be recognized. With respect to the present study, the interesting fact is that this short-lasting increase in theta (or delta) ERS depends on tonic theta (or delta) power. Klimesch and colleagues (for review, see Klimesch, 1999) were able to demonstrate a double dissociation between cognitive performance and two types of power measures: tonic and event-related power (i.e. ERS). Good performance is associated with decreased tonic theta (or delta power), but with increased theta (or delta) ERS. Vice versa, bad performance is associated with increased tonic theta (or delta power), but with decreased theta (or delta) ERS. Thus, the finding of the present study, which shows an increase in tonic theta and delta power with an increase in sleep pressure and decreased PVT performance, is in line with the hypothesis that increased tonic theta and delta power reflects bad cognitive performance. This interactive relationship between tonic and event-related theta (or delta) power suggests that – in a physiological sense – both types of power represent different aspects of the same oscillatory activity. This interpretation is in addition supported by the finding of our study that increased tonic power is associated with a decrease in PLI, as both types of oscillatory responses are associated with decreased cognitive performance. Therefore, it can be hypothesized that phasic EEG decrements in the theta and delta range during sleep deprivation represent capacity limits of the human information processing system. Further, this means that throughout sleep deprivation it becomes gradually harder for the brain to exactly adjust itself and to respond properly (i.e. accurate timing or small EEG phase variability) to perceptual stimuli.
It would be interesting to relate the observed phasic EEG changes in conditions of elevated homeostatic sleep pressure with specific brain regions using neuroimaging. Further research is also needed to investigate the influence of circadian modulations on these phasic EEG measures. Classically, a constant routine protocol could be used to focus on endogenous circadian rhythms normally embedded within the sleep–wake cycle (Cajochen et al., 1999). Thereby it would be possible to measure the influence of the circadian system and the sleep–wake homeostat on event-related cognitive performance. Further studies of that kind would also allow controlling more rigorously unspecific factors like metabolic influences that might have affected our EEG results over the course of sleep deprivation. Additionally, it is unknown how task difficulty would modulate the described association of phasic EEG activity with homeostatic sleep pressure (e.g. Sternberg task with different memory loads). One could speculate that a more demanding task than the PVT would even show clearer associations between performance decrements and, for example, PLI estimates over the course of sleep deprivation. Future research has to reveal whether ‘approaching’ sleepiness – not yet evident in performance decrements – could be anticipated using these phasic EEG measures, which is a finding with potential impact on any profession where human errors can be fatal: such as transportation, medical settings (e.g. surgery) or nuclear power plants.
Taken together it is suggested that phasic EEG measures as those presented are a serious complement and extension of conventional EEG analysis when studying impaired attention and alertness during prolonged wakefulness. To date there is (besides intracranial research) probably no more direct way to tackle changes in neuronal timing inevitably emerging together with sleepiness.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Christina Schmidt (Centre for Chronobiology, Psychiatric University Clinics Basel, Switzerland) and Stefan Hawelka (Department of Psychology, University of Salzburg, Austria) for their assistance in implementing the psychomotor vigilance task. This research was supported by the Austrian Science Fund (P-16849-B02; P-T397-B02).
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am. J. Physiol. 1999;277:R1771–R1779. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am. J. Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Knoblauch V, Kräuchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–2281. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Prazinko B, Caldwell JL. Body posture affects electroencephalographic activity and psychomotor vigilance task performance in sleep-deprived subjects. Clin. Neurophysiol. 2003;114:23–31. doi: 10.1016/s1388-2457(02)00283-3. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Arce C, Ramos J, Lorenzo I, Guevara MA. Time course of reaction time and EEG while performing a vigilance task during total sleep deprivation. Sleep. 1996;19:563–569. doi: 10.1093/sleep/19.7.563. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Arce C, Del Río-Portilla IY, Pérez-Garci E, Guevara MA. Amplitude reduction in visual event-related potentials as a function of sleep deprivation. Sleep. 1999;22:181–189. [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Meth. Instr. Com. 1985;16:652–655. [Google Scholar]
- Drapeau C, Carrier J. Fluctuation of waking electroencephalogram and subjective alertness during a 25-hour sleep-deprivation episode in young and middle-aged subjects. Sleep. 2004;27:55–60. doi: 10.1093/sleep/27.1.55. [DOI] [PubMed] [Google Scholar]
- Freunberger R, Klimesch W, Doppelmayr M, Höller Y. Visual P2 component is related to theta phase-locking. Neurosci. Lett. 2007;426:181–186. doi: 10.1016/j.neulet.2007.08.062. [DOI] [PubMed] [Google Scholar]
- Fründ I, Busch NA, Schadow J, Koerner U, Herrmann CS. From perception to action: phase-locked gamma oscillations correlate with reaction times in a speeded response task. BMC Neurosci. 2007;8:27. doi: 10.1186/1471-2202-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliaud E, Taillard J, Sagaspe P, Valtat C, Bioulac B, Philip P. Sharp and sleepy: evidence for dissociation between sleep pressure and nocturnal performance. J. Sleep Res. 2008;17:11–15. doi: 10.1111/j.1365-2869.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- Gruber WR, Klimesch W, Sauseng P, Doppelmayr M. Alpha phase synchronization predicts P1 end N1 latency and amplitude size. Cereb. Cortex. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- Hanslmayer S, Klimesch W, Sauseng P, et al. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005;375:64–68. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc. Natl Acad. Sci. USA. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Baulk SD. Awareness of sleepiness when driving. Psychophysiology. 2004;41:161–165. doi: 10.1046/j.1469-8986.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Res. Cogn. Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, et al. Oscillatory EEG correlates of episodic trace decay. Cereb. Cortex. 2006;16:280–290. doi: 10.1093/cercor/bhi107. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci. Biobehav. Rev. 2007;3:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J. Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P, Akerstedt T. Transport and industrial safety, how are they affected by sleepiness and sleep restriction? Sleep Med. Rev. 2006;10:347–356. doi: 10.1016/j.smrv.2006.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.