Abstract
We sought to determine the serological test that could be used for Trypanosoma cruzi seroprevalence studies in Mexico, where lineage I predominates. In a previous study among pregnant women and their newborns in the states of Yucatan and Guanajuato, we reported a 0.8–0.9% of prevalence for T. cruzi–specific antibodies by Stat-Pak and Wiener ELISA. We have expanded this study here by performing an additional non-commercial ELISA and confirming the seropositives with Western blot, using whole antigens of a local parasite strain. We found a seroprevalence of 0.6% (3/500) in Merida and 0.4% in Guanajuato (2/488). The 5 seropositive umbilical cord samples reacted to both non-commercial ELISA and Western blot tests, and only 1 of the maternal samples was not reactive to non-commercial ELISA. A follow-up of the newborns at 10 mo was performed in Yucatan to determine the presence of T. cruzi antibodies in children as evidence of congenital infection. None of the children was seropositive. One newborn from an infected mother died at 2 wk of age of cardiac arrest, but T. cruzi infection was not confirmed. The T. cruzi seroprevalence data obtained with both commercial tests (Stat-Pak and ELISA Wiener) are similar to those from non-commercial tests using a local Mexican strain of T. cruzi.
Chagas’ disease (American trypanosomiasis) is a parasitic infection caused by Trypanosoma cruzi affecting 8–10 million people in Latin America (Schofield et al., 2006) and is a major public health issue in Mexico (Dumonteil, 1999; Cruz-Reyes and Pickering-López, 2006). There is an urgent need to accelerate the development of high-quality diagnostic tests for Chagas’ disease (Médecins Sans Frontières, 2008). In Mexico, most T. cruzi strains belong to lineage I (Bosseno et al., 2002), while most tests have been developed for South America, where lineage II predominates.
The state of Yucatán in the southeast of Mexico is in need of updated seroprevalence data. Population-based seroprevalence studies conducted in the 1970–1980s among different rural communities reported 11.2–18.0% prevalence of infection with T. cruzi (Farfan-Ale et al., 1992; Zavala-Velázquez, 2003). In blood donors, a seroprevalence of up to 5.6% was also reported (Rodriguez-Felix et al., 1995). However, these studies used small sample sizes and were based on a single immunofluorescence assay. In contrast, national serosurveys with much larger sample sizes reported a seroprevalence of 0.0–1.5% for the general population in Yucatan (Velasco-Castrejón et al., 1992) and 1.7% in the blood donors (Guzman Bracho et al., 1998).
In the state of Guanajuato, located in the center of Mexico, a study of the distribution of Chagas’ disease vectors showed that the presence of Triatoma barberi represents a risk for vector transmission in the state, with an estimated 3,500 new cases per year and an overall seroprevalence of 2.6% (Lopez-Cardenas et al., 2005). The national survey reported a seroprevalence of 0.1– 0.4% for the state (Velasco-Castrejón et al., 1992).
Pregnant women with Chagas’ disease can transmit T. cruzi to their fetuses. In Mexico, calculations indicate that almost 155,000 children under 5 yr of age could be infected and that approximately 40,539 women could be at risk of infecting 2,028 fetuses with T. cruzi (Buekens et al., 2008). To our knowledge, there has been just 1 case of congenital Chagas’ disease reported in Mexico (Guzman Bracho et al., 1998). However, a serological study of pregnant women and their babies in 2 hospitals of endemic regions (Chiapas and Veracruz) showed a T. cruzi seroprevalence of 4% among 145 women (Olivera et al., 2006).
In a previous study, we reported a 0.9% prevalence to T. cruzi–specific antibodies by Stat-Pak rapid test (Chembio, New York, New York) in umbilical cord blood (UC) from 2 Mexican hospitals (Merida and Celaya), which was in close agreement with the 0.8% obtained with the Wiener ELISA in maternal (M) venous blood (ELISA Recombinant v. 3.0; Wiener, Rosario, Argentina) (Sosa-Estani et al., 2008). A potential shortcoming of this study was that the commercial tests we used were not based on the Mexican strain. Indeed, it has been suggested that using tests based on local antigens could increase the sensitivity of the diagnosis (Sanchez et al., 2001). Here, we present the results of additional analyses with a non-commercial ELISA test using whole antigens of the H1 strain of T. cruzi, which belongs to lineage I (Bosseno et al., 2000). Additionally, we performed a follow-up at least 10 mo after delivery on infants from Merida, Yucatan, who were positive for at least 2 of 3 diagnostic tests (non-commercial ELISA, Wiener ELISA, or Stat-Pak) in M blood and who had seropositive UC tests to determine the persistence of antibodies against T. cruzi as evidence of congenital infection. We also determined whether antibodies against T. cruzi were present among the infants’ siblings.
MATERIALS AND METHODS
Study design and study populations: Hospital study
We conducted a cross-sectional descriptive study in 2 endemic areas of T. cruzi infection in Mexico, i.e., Hospital Materno-Infantil in Merida, Yucatán, which attends 5,097 childbirths per year (15% of the childbirths statewide), and Hospital General in Celaya-Guanajuato, which attends 5,000 childbirths per year (45% of the childbirths countywide). The inclusion criteria were women ≥18 yr old at the time of delivery, having single live births, and who consented to participate. M and UC samples were obtained from each woman who agreed to participate and her newborn, respectively. At the time of delivery, 5 ml UC blood were obtained with a syringe in vacutainer tubes with ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. During the first 24 hr postpartum, 5 ml of M venous blood were also collected in EDTA vacutainer tubes.
Follow-up of seropositive cases
Infants and their mothers from Merida, Yucatan, who were positive for at least 2 of 3 diagnostic tests (non-commercial ELISA, Wiener ELISA, or Stat-Pak) were followed at least 10 mo after delivery. We used the same tests as with seropositive newborns to determine the presence of antibodies against T. cruzi as evidence of congenital infection (Chagas’ Stat-Pak and Wiener ELISA tests). We also determined whether antibodies against T. cruzi were present among the infants’ siblings (newborns and children less than18 yr old). The follow-up of seropositive infants and mothers from Guanajuato was conducted by local authorities, and the results were not available for publication.
Serologic tests to detect antibodies against T. cruzi
The Chagas’ Stat-Pak (Chembio Diagnostic Systems, Medford, New York) is a rapid immunochromatographic screening test for the detection of anti–T. cruzi antibodies in whole blood (Luquetti et al., 2003; Ponce et al., 2005). It uses a unique combination of T. cruzi recombinant antigens (B13, 1F8, and H49/JL7), which are bound to the membrane, and a specific antibody-binding protein, which is conjugated to dye particles (Umezawa et al., 2003). We also used a commercially available ELISA kit based on recombinant T. cruzi antigens (Chagatest ELISA Recombinant v. 3.0; Wiener Laboratories, Rosario, Argentina) (Caballero et al., 2007), according to the manufacturer's instructions.
We prepared a non-commercial indirect ELISA based on a whole parasite lysate from a local strain H1 of T. cruzi (Cruz-Chan et al., 2009). Briefly, 96-well microplates were coated with 2 μg/ml of T. cruzi epimastigote lysate in carbonate buffer (100 μl/well) for 12 hr at 4 C. The plates were blocked to prevent false positive results by adding 200 μl of PBS buffer with 0.05% Tween 20 (PBST) and 1% of bovine serum albumin (BSA) and incubated for 1 hr at 37 C; they were then washed 3 times with PBST. Plasma samples were diluted 1:100 in PBST with 0.3% BSA, and 100-μl aliquots of diluted plasma were placed in each well. Positive and negative controls samples were diluted 1:20, and 100-μl aliquots of the controls were deposited in each well. The microplates were then incubated 1 hr at 37 C and washed 5 times with PBST. Anti-human IgG conjugated to peroxidase (goat anti-human IgG-HRP conjugate) and diluted 1:4,000 in PBS was added to the wells and incubated for 30 min at 37 C. Plates were washed 5 times with PBST and once with PBS. O-phenyldiamine substrate (0.5 mg/ml) dissolved in citrate buffer pH 5.0 was added, and plates were incubated for 40 min at 37 C; the reaction was stopped by adding 2 N sulfuric acid. The optical density readings were performed in an ELISA reader at an excitation wavelength of 490 nm.
All cases with at least 1 seroreactive test were tested by Western blot (WB). Parasites from T. cruzi isolate H1 were harvested by centrifugation (800 g for 5 min at room temperature), washed in PBS buffer, and lysed with Laemmli sample buffer with a protease inhibitor cocktail. The samples were then boiled 5 min prior to loading on the gel; 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and proteins were blotted to nitrocellulose membranes. The membranes were blocked with TBST (Tris buffered saline Tween 20; 10 mM Tris HCl, 150 mM NaCl, 5 mM Tween 20, pH 8.0, and 1% nonfat milk) for 30 min at room temperature, incubated overnight at 4 C with serum samples, and diluted 1:100 in milk–TBST–nonfat milk, which was followed by 3 washes with TBST. Afterward, membranes were incubated with anti-human IgG, conjugated to alkaline phosphatase (AP), washed 3 times with TBST, and developed with nitro-blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3′-indolyl phosphate p-toluidine (BCIP).
In addition to positive and negative controls, a conjugated anti-IgG human control was used to discard unspecific bands. Interpretation of the WB results was based on established criteria (Jimenez-Coello et al., 2008). A serum sample was considered positive when it recognized at least 5 antigenic bands from a group of 10 with the highest frequency of recognition; the result was considered indeterminate when the sample recognized 1 to 4 antigenic bands and negative when the serum sample showed no reactivity. Bands considered for diagnosis interpretation were different than those observed in the conjugate control.
Collection and processing of samples for the follow-up study
At the time we visited the families in their households, we drew 5 ml of venous blood from the mother. We immediately performed the Stat-Pak test on site using 10 μl of whole blood and stored the remaining blood in a vacutainer EDTA tube. After transporting the blood samples to the laboratory, we separated the plasma in order to perform the Wiener ELISA and stored the rest of the plasma for future studies. We also obtained blood samples from the children by finger prick and used 10 μl of blood to perform Stat-Pak tests on site. Another 100-μl sample of blood was collected in a capillary tube with heparin for the ELISA tests to be performed in the laboratory.
Clinical and sociodemographic questionnaires
Clinical and sociodemographic data were obtained from clinical records or by interviewing the mothers during their hospital stays. We also interviewed the seropositive mothers we followed up in Yucatan and collected the following information during household visits: type of housing; number of inhabitants; presence of pets or domestic animals; knowledge about the vector (pictures of Triatoma dimidiata were shown); and whether anyone in the home had been bitten by triatomine or had presented fever, tiredness, or chagoma (the bite wound).
Ethical aspects
The study protocol, with written informed consent, was approved by the Institutional Review Board of the Tulane University Health Sciences Center and the ethics committees of the respective collaborating institutions at the Autonomous University of Yucatan and the University of Guanajuato. We asked mothers to give informed consent in writing, and we asked for assent to participate from their children between 7 and 18 yr of age.
Statistical analysis
We used Epidat v. 3.1 (Xunta de Galicia y Organizacion Panamericana de la Salud, 2006) to perform McNemar tests comparing 2 serological tests performed on the same patient.
RESULTS
We studied a total of 988 women, 488 in Celaya and 500 in Merida, and their newborns between September 2006 and February 2007. Table I shows that age was most frequently between 20 and 29 yr, and education was 7 or more years in both Celaya and Merida. More women were from urban areas in Celaya as well as in Merida. Parity (data were not recorded from 2 women in Celaya), mode of delivery, and birth outcomes were also similar in the 2 study sites.
Table I.
Sociodemographic characteristics of mothers.
| Celaya |
Merida |
Total |
||||
|---|---|---|---|---|---|---|
| Variable | n | (%) | n | (%) | n | (%) |
| Age group | ||||||
| 18–19 | 90 | 18.4 | 85 | 17.0 | 175 | 17.7 |
| 20–29 | 263 | 53.9 | 326 | 65.2 | 589 | 59.6 |
| 30–39 | 122 | 25.0 | 81 | 16.0 | 203 | 20.5 |
| 40–90 | 13 | 2.7 | 8 | 1.6 | 21 | 2.1 |
| Total | 488 | 100 | 500 | 100 | 988 | 100 |
| Education | ||||||
| <7 yr | 217 | 44.5 | 217 | 43.4 | 434 | 43.9 |
| ≥7 yr | 271 | 55.5 | 283 | 56.6 | 554 | 56.1 |
| Total | 488 | 100 | 500 | 100 | 988 | 100 |
| Residence area | ||||||
| Rural | 188 | 38.5 | 113 | 23.0 | 300 | 30.4 |
| Urban | 300 | 61.5 | 387 | 77.0 | 688 | 67.7 |
| Total | 488 | 100 | 500 | 100 | 988 | 100 |
| Parity | ||||||
| Primiparous | 139 | 28.5 | 179 | 35.8 | 318 | 32.3 |
| Multiparous | 347 | 71.4 | 321 | 64.2 | 670 | 67.7 |
| Total | 486 | 100 | 500 | 100 | 986 | 100 |
| Gestational age (wk) | ||||||
| 18–31 | 3 | 0.6 | 2 | 0.4 | 5 | 0.5 |
| 32–36 | 35 | 7.2 | 33 | 6.6 | 68 | 6.9 |
| 37–50 | 450 | 92.2 | 463 | 93.0 | 913 | 92.6 |
| Total | 488 | 100 | 498 | 100 | 986 | 100 |
| Type of delivery | ||||||
| Vaginal | 296 | 60.7 | 286 | 57.2 | 582 | 58.9 |
| Cesarean section | 192 | 39.3 | 214 | 42.8 | 406 | 41.1 |
| Total | 488 | 100 | 500 | 100 | 988 | 100 |
| Birthweight (g) | ||||||
| <2,500 | 27 | 5.5 | 33 | 6.6 | 58 | 5.9 |
| 2,500–2,999 | 102 | 20.9 | 130 | 26.0 | 231 | 23.4 |
| 3,000–3,499 | 229 | 46.9 | 231 | 46.2 | 462 | 46.8 |
| 3,500 or more | 130 | 26.6 | 106 | 21.2 | 237 | 24.0 |
| Total | 488 | 100 | 500 | 100 | 988 | 100 |
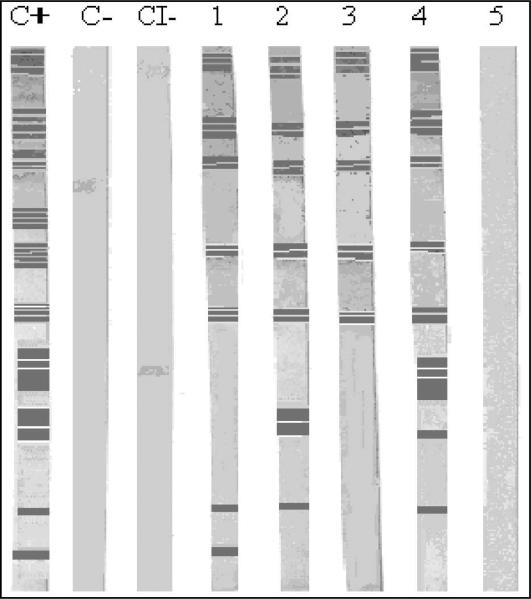
We analyzed 988 maternal (M) samples and 988 umbilical cord (UC) samples. We found a seroprevalence of 0.6% (3/500) in Merida and 0.4% in Guanajuato (2/488), which was determined by 2 commercial serologic tests (Wiener ELISA and Chagas’ Stat-Pak). Table II shows the results we reported previously using commercial tests and the additional results from the noncommercial ELISA. The seroprevalence for M samples was 0.6% with the Stat-Pak, 0.8% with the Wiener ELISA, and 1.1% with the non-commercial ELISA. For the UC samples, we found a seroprevalence of 0.9% with the Stat-Pak, 0.6% with the Wiener ELISA, and 0.5% with the non-commercial ELISA. There was no statistically significant differences (McNemar tests P > 0.05) between the results from Stat-Paks and Wiener ELISA versus non-commercial ELISA. Tables III and IV show the correspondence between the 3 tests for M and UC samples, respectively, together with the confirmation test by Western blot. Figure 1 shows a WB gel of reactive samples (with lines) and 1 non-reactive sample (without lines).
Table II.
Prevalence of reactive samples in maternal and umbilical cord samples.
| Celaya n = 488 |
Merida n = 500 |
Total n = 988 |
||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Maternal samples | ||||||
| Stat-Pak | 2 | 0.4 | 4 | 0.8 | 6 | 0.6 |
| Wiener ELISA | 4 | 0.8 | 4 | 0.8 | 8 | 0.8 |
| Non-C ELISA* | 4 | 1.0 | 6 | 1.2 | 10 | 1.1 |
| Umbilical cord samples | ||||||
| Stat-Pak | 5 | 1.0 | 4 | 0.8 | 9 | 0.9 |
| Wiener ELISA | 2 | 0.4 | 4 | 0.8 | 6 | 0.6 |
| Non-C ELISA | 2 | 0.4 | 3 | 0.6 | 5 | 0.5 |
Non-C ELISA = non-commercial ELISA.
Table III.
Reactivity of IV mother samples at the time of delivery.
| Mother ID | S-Pak* | W ELISA* | Non-C ELISA* | WB* |
|---|---|---|---|---|
| 2430 | + | + | – | + |
| 2473 | – | + | – | + |
| 2478 | – | – | + | – |
| 2505 | – | – | + | – |
| 2511 | + | + | + | + |
| 3129 | – | + | – | + |
| 3145 | – | – | + | – |
| 4002 | + | + | + | + |
| 4124 | – | + | + | + |
| 4458 | + | + | + | + |
| 4509 | + | + | + | + |
| 4030 | – | – | + | – |
| 4106 | – | – | + | + |
| 4455 | + | – | – | – |
S-Pak = Stat-Pak; W ELISA = Wiener ELISA; Non-C ELISA = non-commercial ELISA; WB = Western blot; + = positive test; – = negative test.
Table IV.
Reactivity of umbilical cord samples from newborns at the time of delivery.
| Newborn ID | S-Pak* | W ELISA* | Non-C ELISA* | WB* |
|---|---|---|---|---|
| 2031 | + | – | – | NR‡ |
| 2272 | + | – | – | NR |
| 2322 | + | – | – | NR |
| 2430 | + | + | + | + |
| 2473 | – | – | – | NR |
| 2478 | – | – | – | NR |
| 2505 | – | – | – | NR |
| 2511 | + | + | + | + |
| 3129 | – | – | – | NR |
| 3145 | – | – | – | NR |
| 4002† | + | + | + | + |
| 4030 | – | – | – | NR |
| 4056 | + | – | – | – |
| 4106 | – | + | – | + |
| 4124 | – | – | – | NR |
| 4458 | + | + | + | + |
| 4509 | + | + | + | + |
| 4455 | – | – | – | NR |
S-Pak = Stat-Pak; W ELISA = Wiener ELISA; Non-C ELISA = non-commercial ELISA; WB = Western blot; + = positive test; – = negative test.
the baby died of cardiac arrest in the second week after birth.
NR = not reported.
Figure 1.
Western blot analysis of epimastigote forms of the Trypanosoma cruzi H1 strain recognized by mother samples from Merida (lanes 1–5). Positive controls serum from Chagasic patient (C+), negative control (C–), and a conjugated anti-IgG human control (CI–). Serum with positive reactivity (lanes 1–4): 4002, 4106, 4124, 4458, 4509. Sera with negative reactivity (lane 5): 4030.
According to the Wiener ELISA and Stat-Pak tests, 3/4 mothers who could be followed were still seropositive 10 mo after delivery (numbers 4002, 4458, and 4509), and their seropositivity was confirmed by WB, while 1 was seronegative at this time (4124). None of their children was seropositive at 10 mo, nor were any of the siblings (n = 7). One newborn from a seropositive mother (4002) died of cardiac arrest in the second week after birth, apparently without presenting symptoms of Chagas’ disease. Two of the 3 seropositive mothers had spent their childhood in other states of Mexico (Chiapas and Tabasco) that are highly endemic for Chagas’ disease, and another mother lived in a house infested with triatomines. The ages of the seropositive mothers were 31, 26, and 27 yr, and they were all multiparous. The birth weights of their newborns were normal, between 3,000 and 3,499 g, and they each reached about 38–40 wk of gestational age. Two seropositive mothers had cesarean deliveries, and 1 had a vaginal delivery. The 3 mothers had 7 or more years of education.
Table III shows the M results of the WB performed in samples that were positive from at least 1 of the tests (Stat-Pak, Wiener ELISA, or non-commercial ELISA). Four samples were positive from all 4 tests performed (4002, 4458, 4509, and 2511). Two samples that were positive with the Wiener ELISA test showed additional positive results only by WB (2473 and 3129). The sample that only reacted with the Stat-Pak did not react by WB or any other tests (4455). Most (4/5) of the samples that reacted only with the non-commercial ELISA did not react with WB (4030, 2478, 2505, 3145), and only 1 sample (4106) that was seroreactive with the non-commercial ELISA also reacted with WB. All samples that reacted with both the Stat-Pak and Wiener ELISA reacted with WB (4002, 4458, 4509, 2430, and 2511), and similar results were obtained with the follow-up samples. In UC (Table IV), 1 sample that was positive by Wiener ELISA was additionally seroreactive only by WB (4106) (Table IV); however, in the follow-up study, this sample was seronegative by Stat-Pak and Wiener ELISA at 10 mo after birth. All UC samples that were positive with commercial Wiener ELISA and Stat-Pak tests were positive with the non-commercial ELISA test (4002, 4458, 4509, 2430, 2511) and were confirmed by WB (Table IV).
DISCUSSION
We investigated the seroprevalence of T. cruzi infection in mothers and infants from 2 Mexican populations using serological tests based on local antigens, since such tests may have a higher sensitivity compared to commercial serological tests. Overall, we found similar seroprevalence results with commercial and non-commercial tests and a reasonably good agreement between them. Importantly, no additional positive cases were identified using the non-commercial ELISA with WB confirmation, compared to the use of the Stat-Pak rapid test and Wiener recombinant ELISA. The agreement between tests was greater for UC samples compared to IV samples, which showed more discrepancies.
In this project, the Chagas’ Stat-Pak test was selected for screening purposes because it is easy to use, provides rapid results, and can be processed on-site. We acknowledge that a few studies have reported a somewhat limited or even inadequate performance of the Stat-Pak rapid test for evaluation of serum samples from certain regions, such as in Bolivia (Roddy et al., 2008), Peru (Verani et al., 2009), and southern Mexico (Dhiman et al., 2009). This poor performance has often been attributed to antigenic differences of the T. cruzi strains circulating in these regions. Nonetheless, most other studies in diverse regions/countries including Bolivia, Honduras, Venezuela, and Argentina have found this test to perform very well (Luquetti et al., 2003; Ponce et al., 2005; Chippaux et al., 2008; Ji et al., 2009). Additionally, a comparative evaluation of T. cruzi diagnostic tests using a panel of serum samples from 10 Latin American countries and covering most of the geographic range of endemic countries further confirmed the high sensitivity and specificity of the Chagas’ Stat-Pak test (Otani et al., 2009).
In a recently published paper (Roddy et al., 2008), the Chagas’ Stat-Pak tested in Bolivia yielded high specificity (99%, 95% confidence interval [CI] = 98.4–99.4%), with 18/1,792 false positives results, but a low sensitivity (93.4%, 95% CI = 87.4– 97.1%). Another study of the prevalence of Chagas’ disease in southern Bolivia (Chippaux et al., 2008), using the Stat-Pak, underestimated by 3.4% the overall prevalence in a sample of 995 people. Although the divergences between the Chagas’ Stat-Pak and the ELISA were not significant, the authors highlight the importance of performing a confirmation serological test. In a Spanish study of immigrants from Latin America, 25/224 (11.2%) samples were found to be reactive using the Stat-Pak test, but only 3 of the samples were positive using both ELISA serological tests that were concordant in all cases (Soriano et al., 2009).
The sensitivity and specificity of serological tests that require a reading with the naked eye can change with each reader's criteria; this may be the cause of the different values obtained in different studies for the Chagas’ Stat-Pak. Also, a recent multicenter study using a panel of serum samples from Mexico confirmed that the geographical origin of the serum samples and the use of T. cruzi I or II as a source of antigens did not affect the performance of serological assays, which included the Stat-Pak rapid test, as well as the Wiener crude extract and recombinant ELISAs (Luquetti et al., 2009). However, as discussed by Otani et al. (2009), serum panels are often biased to include “consensus” samples, which may falsely increase the sensitivity of the tests; as such, it remains important to further evaluate the tests in population studies.
While several mothers were found to have been infected by T. cruzi, we did not find confirmed congenital cases in the infants at 10 mo of age, or in their siblings. However, it must be noted that our sample size was small. Congenital transmission rates are still poorly known, but maternal-fetal transmission of T. cruzi generally occurs in 2–12% of infected pregnant mothers (Olivera et al., 2006) and large-scale studies are thus needed to identify cases in Mexico. Nonetheless, the identification of T. cruzi–infected mothers in these regions of Mexico suggests that surveillance programs should be designed for the prevention and treatment of congenital transmission cases (Buekens et al., 2008).
Our study indicated a seroprevalence of T. cruzi infection in mothers of 0.8–1.2% in Merida, Yucatan, and 0.4–1.0% in Celaya, Guanajuato (Table II). For Yucatan, these data are very consistent with previous national serosurveys, which reported a seroprevalence of up to 1.5% for the general population in the 1980s (Velasco-Castrejón et al., 1992) and of 1.7% in blood donors (Guzman Bracho et al., 1998). Importantly, this suggests that Chagas’ disease epidemiology and transmission have not changed much in this region in the past 20–30 yr. Also, because most of the studied population lived in urban areas (80.6%), our data further confirm the importance of Chagas’ disease testing in such urban areas, as suggested previously (Guzmán-Tapia et al., 2007). In the case of Guanajuato, our seroprevalence data are somewhat higher than that of the previous national survey, which reported a seroprevalence of 0.1–0.4% (Velasco-Castrejon et al., 1992), but lower than some other estimates (Lopez-Cardenas et al., 2005). In any case, these results clearly confirm the existence of a significant risk of transmission of T. cruzi in this state, in addition to others in Mexico, such as Chiapas, and warrant further epidemiological studies of Chagas’ disease.
In conclusion, our study confirmed the presence of T. cruzi infection in pregnant women from both urban and rural areas from Yucatan and Guanajuato States, Mexico. We also validated the use of Stat-Pak rapid tests and Wiener ELISA tests as the tools of choice for T. cruzi diagnosis in Mexico, because tests based on the Mexican strain with local antigens did not facilitate the identification of additional positive patients. Finally, our results strengthen the need for the design and implementation of preventive programs for the detection and treatment of cases of vertical transmission in these states, and most likely in the larger part of Mexico.
ACKNOWLEDGMENTS
We thank colleagues from the Hospital Materno-Infantil of Yucatan, Mexico, especially Dr. Luis Roberto Cardenas Hernandez (Director of Maternity), and Dr. Benito Argaez Tuz, Chief of Maternity Training. We also acknowledge the help and support of Dr. Angel Ramos-Ligonio and technicians in the laboratory of molecular biology (Centro de Investigaciones Regionales, Dr. Hideyo Noguchi, Universidad Autónoma de Yucatán) for their assistance in processing Western blot assays. We thank Dr. Gregorio Martín del Campo, Director of the Hospital General Celaya, Guanajuato, QFB Omar Villanueva Rodriguez, Chief of the Clinical Laboratory of the same hospital, Isabel Gutierrez, and Adriana Palscencia for sample collection and processing. We thank Dr. Miguel Rosado-Vallado of Laboratory of Parasitology (Centro de Investigaciones Regionales, Dr. Hideyo Noguchi, Universidad Autónoma de Yucatán) for critical readings. This project was supported by the Tulane University School of Public Health and Tropical Medicine, and was funded in part by the National Institutes of Health Fogarty International Center grant D43 TW007784.
LITERATURE CITED
- Bosseno MF, Barnabé C, Magallón-Gastélum E, Lozanokasten F, Ramsey J, Espinoza B, Brenière SF. Predominance of Trypanosoma cruzi lineage I in Mexico. Journal of Clinical Microbiology. 2002;40:627–632. doi: 10.1128/JCM.40.2.627-632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseno MF, Barnabé C, Magallón-Gastélum E, Lozanokasten F, Ramsey J, Espinoza B, Sanchez B, Breniere SF. Mexican Trypanosoma cruzi stocks: Analysis of minicircle kDNA homologies by cross-hybridization. Memórias do Instituto Oswaldo Cruz. 2000;95:473–476. doi: 10.1590/s0074-02762000000400005. [DOI] [PubMed] [Google Scholar]
- Buekens P, Almendares O, Carlier Y, Dumonteil E, Eberhard M, Gamboa-Leon R, James M, Padilla N, Wesson D, Xiong X. Mother-to-child transmission of Chagas’ disease in North America: Why don't we do more? Maternal and Child Health Journal. 2008;12:283–286. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clinical and Vaccine Immunology. 2007;14:1045–1049. doi: 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP, Postigo JR, Santalla JA, Schneider D, Brutus L. Epidemiological evaluation of Chagas disease in a rural area of southern Bolivia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:578–584. doi: 10.1016/j.trstmh.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Cruz-Chan JV, Bolio-Gonzalez M, Colin-Flores R, Ramirez-Sierra MJ, Quijano-Hernandez I, Dumonteil E. Immuno-pathology of natural infection with Trypanosoma cruzi in dogs. Veterinary Parasitology. 2009;162:151–155. doi: 10.1016/j.vetpar.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Cruz-Reyes A, Pickering-López JM. Chagas disease in Mexico: An analysis of geographical distribution during the past 76 years—A review. Memórias do Instituto Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, Perez-Molina G, Gallegos-Sandoval R, Moreno R, Garg NJ. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clinical and Vaccine Immunology. 2009;16:660–666. doi: 10.1128/CVI.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonteil E. Update on Chagas’ disease in Mexico. Salud Publica de Mexico. 1999;41:322–327. doi: 10.1590/s0036-36341999000400010. [DOI] [PubMed] [Google Scholar]
- Farfan-Ale J, Loroño-Pino MA, Flores-Flores LF, Rosadoparedes EE, Arjona A. Prevalencia de anticuerpos Toxoplasma gondii y Tripanosoma cruzi en el Estado de Yucatán. Revista Biomedica. 1992;3:8–12. [Google Scholar]
- Guzman Bracho C, Lahuerta S, Velasco-Castrejon O. Chagas disease. First congenital case report. Archives of Medical Research. 1998;29:195–196. [PubMed] [Google Scholar]
- Guzmán-Tapia Y, Ramirez-Sierra MJ, Dumonteil E. Urban infestation by Triatoma dimidiata in the city of Mérida, Yucatán, México. Vector Borne and Zoonotic Diseases. 2007;7:597–606. doi: 10.1089/vbz.2007.0133. [DOI] [PubMed] [Google Scholar]
- Ji MJ, Noh JS, Cho BK, Cho YS, Kim SJ, Yoon BS. Evaluation of SD BIOLINE Chagas Ab Rapid kit. The Korean Journal of Laboratory Medicine. 2009;29:48–52. doi: 10.3343/kjlm.2009.29.1.48. [DOI] [PubMed] [Google Scholar]
- Jimenez-Coello M, Poot-Cob M, Ortega-Pacheco A, Guzman-Marin E, Ramos-Ligonio A, Sauri-Arceo C, Acosta-Viana K. American trypanosomiasis in dogs from an urban and rural area of Yucatan, Mexico. Vector Borne and Zoonotic Diseases. 2008;8:755–761. doi: 10.1089/vbz.2007.0224. [DOI] [PubMed] [Google Scholar]
- Lopez-Cardenas J, Gonzalez Bravo FE, Salazar PM, Gallaga JC, Ramírez E, Martinez J, Sánchez-Cordero V, Peterson AT, Ramsey JM. Fine-scale predictions of distributions of Chagas disease vectors in the state of Guanajuato, Mexico. Journal of Medical Entomology. 2005;42:1068–1081. doi: 10.1093/jmedent/42.6.1068. [DOI] [PubMed] [Google Scholar]
- Luquetti AO, Espinoza B, Martínez I, Hernández-Becerril N, Ponce C, Ponce E, Reyes PA, Hernández O, López R, Monteón V. Performance levels of four Latin American laboratories for the serodiagnosis of Chagas disease in Mexican sera samples. Memórias do Instituto Oswaldo Cruz. 2009;104:797–800. doi: 10.1590/s0074-02762009000500023. [DOI] [PubMed] [Google Scholar]
- Luquetti AO, Espinoza B, Martínez I, Hernández-Becerril N, Ponce C, Ponce E, Esfandiari J, Schijman A, Revollo S, Anez N, Zingales B, Ramgel-Aldao R, Gonzalez A, Levin MJ, Umezawa ES, Franco da Silveira J. Chagas’ disease diagnosis: A multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagnostic Microbiology and Infectious Disease. 2003;46:265–271. doi: 10.1016/s0732-8893(03)00051-8. [DOI] [PubMed] [Google Scholar]
- Médecins Sans Frontières. Campaign for Access to Essential Medicines International meeting: New diagnostic tests are urgently needed to treat patients with Chagas disease. Revista da Sociedade Brasileira de Medicina Tropical. 2008;41:315–319. doi: 10.1590/s0037-86822008000300020. [DOI] [PubMed] [Google Scholar]
- Olivera A, Guillen F, Cruz S, Hernandez-Becerril N, Perez E, Cordova G, Reyes PA, Monteon VM. Serological and parasitological screening of Trypanosoma cruzi infection in mothers and newborns living in two Chagasic areas of Mexico. Revista da Sociedade Brasileira de Medicina Tropical. 2006;37:774–777. doi: 10.1016/j.arcmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Otani MM, Vinelli E, Kirchhoff LV, del Pozo A, Sands A, Vercauteren G, Sabino EC. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion. 2009;49:1076–1082. doi: 10.1111/j.1537-2995.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- Ponce C, Ponce E, Vinelli E, Montoya A, de Aguilar V, Gonzalez A, Zingales B, Rangel-Aldao R, Levin MJ, Esfandiari J, et al. Validation of a rapid and reliable test for diagnosis of Chagas’ disease by detection of Trypanosoma cruzi–specific antibodies in blood of donors and patients in Central America. Journal of Clinical Microbiology. 2005;43:5065–5068. doi: 10.1128/JCM.43.10.5065-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy P, Goiri J, Flevaud L, Palma PP, Morote S, Lima N, Villa L, Torrico F, Albajar-Vinas P. Field evaluation of a rapid immunochromatographic assay for detection of Trypanosoma cruzi infection by use of whole blood. Journal of Clinical Microbiology. 2008;46:2022–2027. doi: 10.1128/JCM.02303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Felix ME, Zavala-Velázquez J, Barrera-Pérez MA, Guzmán-Marín E, Ramírez-Sierra MJ, Alvarez-Moguel R. Riesgo de trasmision de la enfermedad de Chagas por donantes de sangre. Revista Biomedica. 1995;6:70–75. [Google Scholar]
- Sanchez B, Monteon V, Reyes PA, Espinoza B. Standardization of micro-enzyme-linked immunosorbent assay (ELISA) and Western blot for detection of Trypanosoma cruzi antibodies using extracts from Mexican strains as antigens. Archives of Medical Research. 2001;32:382–388. doi: 10.1016/s0188-4409(01)00303-4. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends in Parasitology. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Soriano A, Munoz J, Vergés M, Castells C, Portús M, gascon J. Prevalence of Chagas disease in the Latin American immigrant population in a primary health centre in Barcelona (Spain). Acta Tropica. 2009;112:228–230. doi: 10.1016/j.actatropica.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Sosa-Estani S, Gamboa-León R, del Cid-Lemus J, Athabe F, Alger J, Almendares O, Cafferata ML, Chippaux JP, Dumonteil E, Gibbons L, et al. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and México. The American Journal of Tropical Medicine and Hygiene. 2008;79:755–759. [PubMed] [Google Scholar]
- Umezawa ES, Bastos SF, Coura JR, Levin MJ, Gonzalez A, Rangel-Aldao R, Zingales B, Luquetti AO, da Silveira JF. An improved serodiagnostic test for Chagas’ disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion. 2003;43:91–97. doi: 10.1046/j.1537-2995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- Velasco-Castrejón O, Valdespino JL, Tapia-Conyer R, Salvatierra B, Guzmán-Bracho C, Clementina MC, Selpúlveda J. Seroepidemiología de la enfermedad de Chagas en México. Salud Pública México. 1992;34:186–196. [PubMed] [Google Scholar]
- Verani JR, Seitz A, Gilman RH, Lafuente C, Galdoscardenas G, Kawai V, de Lafuente E, Ferrufino L, Bowman NM, Pinedo-Cancino V, et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. American Journal of Tropical Medicine and Hygiene. 2009;80:410–415. [PubMed] [Google Scholar]
- Xunta de Galicia y Organizacion Panamericana de la Salud [14 September 2010];Epidat, v. 3.1. 2006 Available at: http://www.sergas.es/MostrarContidos_N3_T02.aspx?IdPaxina550100&uri5http://dxsp.sergas.es/ApliEdatos/Epidat/default.asp&hifr5900&seccion50.
- Zavala-Velázquez E. La enfermedad de Chagas en el Estado de Yucatán, México (1940–2002). Revista Biomedica. 2003;14:35–43. [Google Scholar]