Abstract
Ambulatory blood pressure monitoring (ABPM) is the best method of detecting abnormal blood pressure (BP) in patients with chronic kidney disease (CKD), whose hypertension may be missed with office BP measurements. We report ABPM findings in 332 children 1 year after entry in the Chronic Kidney Disease in Children (CKiD) cohort study.
All subjects underwent casual and ambulatory BP measurement. BP was categorized based on casual and ABPM results into normal, white coat, masked, and ambulatory hypertension. Only half of the subjects had a normal ABPM. BP load was elevated (>25%) in 52% (n= 172) while mean BP was elevated in 32% (n= 105). In multivariate analysis, those using an ACE inhibitor (ACEi) were 89% more likely to have a normal ABPM than those who did not report using an ACEi (OR: 1.89, 95%CI: 1.17, 3.04). For every 20% faster decline in annualized GFR change, the odds of an abnormal ABPM increased 26% (OR: 1.26, 95%CI: 0.97, 1.64; p= 0.081). A 2.25 fold increase in urine protein:creatinine ratio annualized change was associated with a 39% higher odds of an abnormal ABPM (OR: 1.39, 95%CI: 1.06, 1.82; p= 0.019).
Abnormalities on ABPM are common in children with CKD, and are strongly associated with known risk factors for end stage renal disease. Individuals on ACEi were less likely to have abnormal ABPM, suggesting a possible therapeutic intervention. ABPM should be used to monitor risk and guide therapy in children with CKD.
Keywords: Ambulatory blood pressure, chronic kidney disease, proteinuria, pediatric, Epidemiology, Blood pressure measurement/monitoring
Introduction
Uncontrolled hypertension (HTN) is a known risk factor for increased cardiovascular morbidity and for progression of chronic kidney disease (CKD) in adults.1, 2 HTN is also common in children with (CKD). We have previously shown that over 25% of children with CKD had elevated casual blood pressure, and nearly one-third of these were not receiving antihypertensive medications, indicating that HTN in pediatric CKD may be frequently undertreated or even untreated entirely.3
Ambulatory blood pressure monitoring (ABPM) has been demonstrated to be a more accurate technique than office blood pressure to stratify cardiovascular risk, especially in patients with CKD 4–6, and is also more predictive of disease progression.7–14 Few studies, however, have characterized the prevalence of ambulatory hypertension in CKD or quantified the association between the degree of ambulatory hypertension and other factors related to the progression of kidney disease in children.
In this report, we summarize the initial ABPM data collected from participants in the Chronic Kidney Disease in Children (CKiD) study, a multicenter observational cohort study being conducted in the United States and Canada. Our aims in this report are to: (1) present our experience with coordinating ambulatory blood pressure monitoring across a North American multicenter study; and (2) describe the distribution of Ambulatory BP, hypertension, and antihypertensive medication use in this large cohort of children with CKD and (3) to investigate the association between markers of kidney disease progression over one year and the presence of abnormal ambulatory BP.
Methods
Study Population and Design
The CKiD study, currently underway at 48 North American pediatric nephrology centers, is designed to evaluate CKD disease progression in children and its putative role on growth and development, cardiovascular, and psychological parameters. Details of the CKiD study design have been previously published.15 The CKiD study protocol was reviewed and approved by the Institutional Review Boards of each participating center.
Eligibility criteria for enrollment was age between 1 to 16 years with an estimated Schwartz formula16 glomerular filtration rate (GFR) 30 to 90 mL/min per 1.73 m2, and signed written informed consent by a parent or guardian plus signed assent according to local requirements. Exclusion criteria included solid organ, bone marrow or stem cell transplant, dialysis within the 3 months before enrollment, cancer/leukemia or HIV treatment within the past year, pregnancy within the past year, inability to complete protocol procedures, enrollment in a randomized clinical trial in which treatment is masked, or an intent move away from the participating center in the near future.
Measurements
Blood Pressure
All casual BPs were measured by auscultation using an aneroid sphygmomanometer (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL). Aneroid calibration and recertification in auscultatory BP measurement technique occurred annually for all equipment and personnel. By protocol, casual blood pressures (BP) were measured at each CKiD visit (annual) and the standardized methods for obtaining casual BP have been previously described.3 Study personnel measured auscultatory BP three times at each study visit and the mean was used as the participant’s casual BP for the present analysis.
Each participant’s casual BP was classified according to the National High Blood Pressure Education Program (NHBPEP) Fourth Report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents17: normotensive (<90th percentile), pre-hypertensive (≥90th and <95th percentiles or >120/80), and hypertensive (≥95th percentile). In this analysis, participants’ casual BP obtained within 30 days of ABPM placement were used to classify their ambulatory blood pressure category (see below).
ABPM
A Spacelabs 90217 monitor (SpaceLabs Healthcare, Issaquah, WA) was used for all ambulatory blood pressure monitorings, which occurred one year after study entry, and repeated every 2 years thereafter. The data described in this report were obtained one year after study enrollment. Monitors were sent from and analyzed at a central site at the University of Texas Health Science Center at Houston. Arm circumference was measured at each local site with appropriate cuffs selected according to the 4th Report recommendations.17 All participating clinical sites received annual training in monitor placement from the ABPM Center.
Monitors were set to measure BP every 20 minutes during the day and night at a bleed step of 8mmHg and participants were instructed to wear the monitor for a continuous 24-hour period. Most monitorings occurred at/near the time of the CKiD study visit. The participant’s family was given a diary to complete, noting times of wake, sleep, and any medication administration while wearing the monitor. After completion, the monitor and diary were returned to the ABPM center for data processing and summarizing. For ABP studies that did not fulfill pre-specified quality parameters (see below), a repeat attempt was made. Summarized ABP data was sent to the CKiD data coordinating center at the Johns Hopkins Bloomberg School of Public Health for centralized data management and analysis.
Definitions
All analyses of ambulatory blood pressure data was performed at the ABPM Coordinating site (PI: JS) using a standardized protocol. Quality of the ABPM studies was defined by the length of time the monitor was actually worn and the number of successful BP recordings. To be acceptable for analysis, we required that the monitor be worn for ≥21 hours, have ≥18 hours with at least one valid BP measured per hour. As additional criteria to ensure adequate representation of both wake and sleep periods, each ABPM had to have at least 1 successful BP recording in ≥75% of wake hours and ≥75% in sleep hours.
The ABP parameters of interest included mean systolic and diastolic BP for parental-reported wake, sleep, and 24-hour periods. From this, systolic and diastolic BP dip status was determined by calculating percent nocturnal drop in mean BP from waking mean values. In addition, wake and sleep BP loads were calculated as the percent of readings at or above the 95th percentile, based on Soergel’s published normative pediatric ABPM data18. This normative dataset has been used as the reference for ambulatory blood pressures. For 24-hour load calculation, a weighted sum of wake and sleep loads was used. Similarly, ABP index was calculated as the mean ambulatory BP divided by the corresponding 95th percentile. Thus, an index of 1 indicates an ambulatory BP equal to the threshold value for a clinical diagnosis of hypertension, and an index of 1.1 is 10% above that threshold.19 Since the 95th percentile is gender and height specific, this measure allows for comparison of BP across a wide range of pediatric normal values.
We also classified studies based upon the recently issued AHA guidelines for pediatric ABPM.20 Using both auscultatory clinical and 24-hour readings, ambulatory blood pressure status was categorized using the following definitions:
Normal BP: casual BP <95th percentile and wake and sleep mean ABP <95th percentile and wake and sleep load < 25%.
Ambulatory HTN: casual BP ≥95th percentile and either (1) wake or sleep mean ABP ≥ 95th percentile, or (2) either wake or sleep load ≥ 25%.
White coat HTN (WCH): casual BP ≥95th percentile and wake and sleep mean ABP < 95th percentile and wake and sleep load < 25%.
Masked HTN: casual BP <95th percentile and either (1) wake or sleep mean ABP ≥ 95th percentile, or (2) either wake or sleep load ≥ 25%.
Given the increased risk of cardiovascular disease in patients with CKD 21, 22 ABP was considered abnormal when either the ambulatory mean or load was elevated. Thus, subjects with AHA classified pre-hypertension (high casual BP, normal mean ambulatory BP and high ambulatory BP load) were considered hypertensive (i.e., abnormal ABPM) in this analysis. Additionally, those with unclassified AHA BP parameters (normal casual, normal mean ABP, high load) were also considered masked hypertensive in this analysis. As noted above, we chose the Soergel 1997 normative ABP data18 since this dataset included shorter children and is thus more applicable to the CKiD population, which exhibits substantial deficits in height23.
Other Variables
Demographic and medical history information included age, gender, self-reported race/ethnicity, underlying CKD diagnosis, duration of CKD, a history of hypertension, use of antihypertensive medications during the past 30 days, history of low birth weight (<2500g) or premature birth (<36 weeks gestation), and family history of hypertension. GFR was measured at visit 1 and visit 2 by plasma disappearance of iohexol.24 Laboratory analyses were performed in the CKiD central laboratory (University of Rochester, Rochester, NY).
Statistical Analysis
Descriptive statistics are presented as medians and interquartile ranges for continuous variables and percentages for categorical variables. ABPM-classified BP groups were defined as normotensive, white coat hypertensive, masked hypertensive, confirmed hypertensive.
Since there was a high prevalence of antihypertensive medication use in this population, we were also interested in the association between the use of any antihypertensive medications and a specific class, ACE inhibitors, and an abnormal ABPM study. We investigated this association between abnormal ABPM and antihypertensive medication use at the time of ABPM monitoring using logistic regression. In two models, the outcome was presence of a normal vs abnormal ABPM and the two exposures of interest were any antihypertensive use and ACE inhibitor use. The models adjusted for age, gender, CKD diagnosis type (glomerular cause vs. non-glomerular cause) iohexol GFR (iGFR) and urine protein:creatinine ratio (uPrCr).
Additionally, we investigated the association between markers of CKD disease progression and the presence of an abnormal ABPM (as the dependent variable) using available longitudinal data (Visits 1 and 2). CKD disease progression was determined for two renal markers: iGFR and uPrCr (putative exposures), which were measured at Visit 1 (at study entry 1 year prior to the ABPM protocol) and at Visit 2 (at the same time as the ABPM study). Using a logistic regression model, the outcome was the presence of abnormal ABPM and the independent variables of interest were the annualized ratio of Visit 2 level to Visit 1 level in each renal marker and the geometric mean of the two levels. Specific confounders were identified a priori as important to control for: age, sex and CKD diagnosis. The interpretation of the coefficient is expressed as the odds ratio associated with a k-fold difference in the ratio (i.e., comparing a subject whose ratio of current to previous levels over a year is k-fold the ratio of an otherwise similar individual used as a reference). For each variable, k was calculated as the standard deviation of the annual percent change: 0.80-fold difference in the ratio of current iGFR to previous iGFR (i.e., a 20% lower decline over one year), and 2.25-fold increase in uPrCr.
Statistical significance was assessed at the 0.05 level. All analyses were performed using SAS 9.1 statistical software (SAS Institute, Cary, NC).
Results
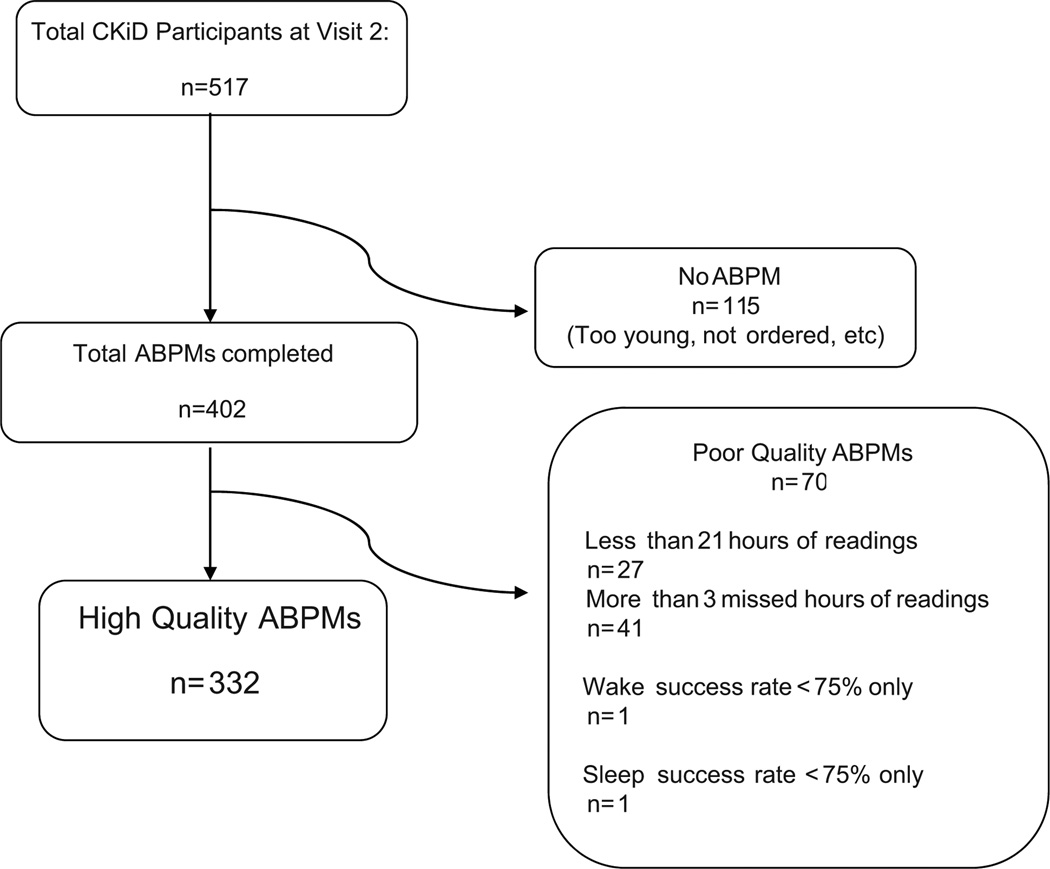
The first CKiD ABPM was performed on January 25, 2006. At the time of the current analysis, there were 515 completed Visit 2 records. Twenty one subjects never had an ABPM ordered (see Figure 1). Of 626 shipments of ABP monitors from the coordinating center, 97% were delivered in time for subject use. A total of 414 ABPM studies were completed in 476 total subjects (87%). The most common factor leading to an unsuccessful ABPM attempt was postponement of study visit. Monitor malfunction (n=3) or non-return (n=5) were not major difficulties considering the geographic scope of the study. An ABPM suitable for analysis was successfully completed on the initial attempt in 276 subjects. The remaining participants who attempted an ABPM study were unable to meet the quality criteria described above. These 113 participants had the baseline ABPM procedure repeated following their unsuccessful attempt, with half returning quality data on the second attempt (58/113). Despite repeat attempts, 68 participants had fewer than 21 hours of successful monitoring or missed more than 3 hours and thus, were excluded. The other 2 excluded subjects had either poor wake success rate (i.e., <75% of wake hours with a valid BP reading; n= 1) or poor sleep success rate (n= 1). Overall, there were 332 high quality ABPM studies included in the present analysis (See Figure 1).
Figure 1.
Flow diagram of included ambulatory blood pressure monitorings in the study
The median age of participants undergoing ABPM was 12 years [interquartile range: 9, 16 years], with a gender and ethnic mix similar to the larger CKiD cohort. Among these participants, 19% (n= 63) had a glomerular cause of CKD. Nearly half the subjects (n= 152), had a previous diagnosis of hypertension and 69% (n= 224) were treated with anti-hypertensive medication at the time of the ABPM. Of those treated, 77% (n= 173) were using an ACE-inhibitor, and 14% (n=31) were using an ARB medication. (Table 1)
Table 1.
Baseline Demographics comparing attainable (n= 332), unattainable (n= 70), and not attempted (n=115) ABPMs
| Characteristic | Median [IQR] or % (n) | |||
|---|---|---|---|---|
| Attainable n= 332 |
Unattainable n= 70 |
p- value* |
No ABPM study conducted n= 115 |
|
| Age | 12 [9, 15] | 9 [6, 15] | 0.01 | 10 [5,15] |
| Male | 59% (195) | 70% (49) | 0.08 | 61% (70) |
| Race | ||||
| Caucasian | 73% (242) | 57% (40) | 0.01 | 55% (63) |
| African American | 18% (59) | 30% (21) | 0.03 | 30% (35) |
| Other | 9% (31) | 13% (9) | 0.38 | 15% (17) |
| Hispanic ethnicity | 15% (50) | 11% (8) | 0.57 | 14% (16) |
| Height Percentile | 24 [8, 51] | 26 [7, 48] | 0.99 | 32 [11, 60] |
| Weight Percentile | 44 [19, 78] | 45 [18, 76] | 0.93 | 53 [23, 81] |
| Weight (Kg) | 42 [29, 58] | 35 [21, 57] | 0.02 | 36 [18, 57] |
| BMI percentile | 61 [29, 89] | 56 [37, 82] | 0.69 | 66 [38, 89] |
| Percent obese, BMI > 95th % | 17% (55) | 14% (10) | 0.72 | 15% (17) |
| Iohexol GFR, mL/min per 1.73m2 | 44 [32, 55] | 48 [34, 64] | 0.10 | 46 [36, 60] |
| Urine protein:creatinine† | 0.44 [0.18, 1.07] | 0.44 [0.18, 1.24] | 0.56 | 0.41 [0.14, 1.26] |
| Low birthweight | 17% | 19% | 0.60 | 22% (24) |
| Premature birth | 10% | 20% | 0.03 | 20% (21) |
| Primary Disease | ||||
| Glomerular | 19% (63) | 30% (21) | 0.05 | 19% (22) |
| Duration of CKD, years | 9 [4, 12] | 6 [5, 10] | 0.18 | 7 [3, 11] |
| History of HTN | 46% (152) | 50% (34) | 0.69 | 38% (44) |
| Current HTN by Casual Readings | 19% (61) | 18% (12) | 0.87 | 14% (14) |
| Use of Anti-hypertensive | 68% (226) | 64% (45) | 0.49 | 52% (60) |
| ACE-inhibitor | 52% (173) | 46% (32) | 0.36 | 37% (43) |
| Calcium Channel Blocker | 14% (46) | 16% (11) | 0.71 | 11% (13) |
| ARB | 9% (31) | 11% (8) | 0.66 | 10% (11) |
For successful ABPMs, the youngest age was 2 years and the oldest was 18 years.
p-values based on Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables comparing attainable to unattainable ABPM studies
Iohexol GFR and urine protein: creatinine were measured at Visit 2, at the time of the ABPM data collection.
We compared subjects who successfully completed a study with those who were unable to do so and those who never had an ABPM study at all in order to understand potential barriers to collecting valid ABPM data. (Table 1) Successful subjects (n= 332) tended to be older, of white race, heavier and not born prematurely compared to those who did not complete a successful study (n= 70). There were no significant differences in gender, BMI, GFR, hypertensive history or antihypertensive use between these two groups. Those who were did not complete an ABPM study tended to be similar to those who had an ABPM study that was not successful.
Descriptive results of ambulatory blood pressure
Among those with a quality ABPM study, 151 subjects had a completely normal ABPM (45.7%), with the remaining subjects having an abnormal ABPM. Overall, 30% were defined as hypertensive based on mean ambulatory blood pressures alone (either wake or sleep). A higher proportion of subjects had elevated blood pressure loads above 25% during the sleep state for both systolic (33%) and diastolic (39%) measurements than during the wake state for systolic (28%) and diastolic (22%) measurements. We also investigated ambulatory hypertension by wake or sleep state, where hypertension was defined as a systolic or diastolic mean greater than the 95th Soergel limit or systolic or diastolic load greater than 25%. Using this definition, sleep HTN (65%) was more prevalent than wake HTN (33%).
Blood pressure classification using combined casual and ambulatory values are summarized in Table 3. Of these, 138 (42%) subjects had both normal BP by casual and ambulatory measurements. White coat HTN was diagnosed in only 13 (4%) subjects, while 116 (35%) were classified as having masked HTN by ABPM criteria. There were 48 (14%) subjects with confirmed hypertension of whom 21 had “severe” ambulatory HTN (loads > 50%). Although similar in gender across all groups, those with white coat HTN were younger than the overall group. Ambulatory HTN was more common in African Americans and slightly more common among those with glomerular disease as a primary cause of CKD. As expected from the definitions, those with masked HTN had lower casual BP than those with either white coat HTN or ambulatory HTN. Similarly, systolic loads were much higher in subjects with masked (26%) and ambulatory HTN (47%) than those with white coat HTN (7%). In general, those with masked HTN had more abnormal BP loads than abnormal mean BP. Additionally, elevated sleep blood pressures were more common than elevated wake pressures.(Table 2)
Table 3.
Characteristics by ABPM classifications, percents or median (interquartile range).
| Characteristic | Normal (n= 138) |
WCH (n= 13) |
Masked HTN (n= 116) |
Confirmed HTN (n= 48) |
|---|---|---|---|---|
| Age | 13 [10, 15] | 7 [5, 12] | 12 [9, 16] | 10 [9, 15] |
| % Male | 60% (83) | 54% (7) | 59% (69) | 55% (27) |
| Race | ||||
| Caucasian | 79% (109) | 85% (11) | 68% (79) | 64% (31) |
| AA | 12% (16) | 8% (1) | 21% (24) | 27% (13) |
| Other | 9% (13) | 8% (1) | 11% (13) | 8% (4) |
| Hispanic ethnicity | 17% (23) | 8% (1) | 13% (15) | 21% (10) |
| Height %ile | 27 [9, 61] | 19 [8, 48] | 24 [7, 50] | 19 [6, 41] |
| Weight %ile | 41 [21, 80] | 30 [6, 71] | 52 [22, 80] | 37 [14, 67] |
| Weight (Kg) | 46 [34, 57] | 25 [18, 30] | 43 [30, 58] | 34 [27, 53] |
| BMI %ile | 57 [24, 89] | 52 [14, 71] | 68 [37, 92] | 57 [30, 78] |
| Percent obese, BMI > 95th% | 17% (23) | 8% (1) | 18% (21) | 15% (7) |
| Iohexol GFR | 43 [31, 55] | 50 [36, 71] | 46 [36, 59] | 35 [23, 51] |
| 1o Disease Glomerular | 20% (27) | 8% (1) | 20% (23) | 23% (11) |
| Years of CKD | 9 [5, 13] | 6 [5, 8] | 7 [4, 11] | 8 [3, 10] |
| Hx of HTN | 44% (60) | 46% (6) | 44% (50) | 57% (26) |
| Anti-HTN | 73% (101) | 62% (8) | 68% (79) | 65% (31) |
| ACE-i | 62% (86) | 54% (7) | 49% (57) | 33% (16) |
| Ca Blocker | 5% (7) | 23% (3) | 16% (19) | 31% (15) |
| ARB | 11% (15) | 0% (0) | 9% (10) | 8% (4) |
| LVMI | 30 [26, 35] | 35 [27, 40] | 33 [27, 39] | 36 [29, 44] |
| % LVH | 9% (11) | 11% (1) | 16% (17) | 31% (12) |
| Urine protein:creatinine | 0.41 [0.15, 0.90] | 0.22 [0.14, 0.63] | 0.47 [0.20, 1.02] | 0.99 [0.30, 2.22] |
| Proteinuria uP/C > 2 | 7% (9) | 8% (1) | 11% (12) | 32% (15) |
| Blood Pressure parameters | ||||
| Casual SBP | 104 [97, 110] | 115 [106, 121] | 107 [101, 115] | 122 [117, 131] |
| Casual SBP %ile | 36 [19, 63] | 92 [82, 97] | 65 [ 44, 77] | 97 [92, 99] |
| Casual DBP | 61 [55, 67] | 71 [63, 75] | 65 [59, 73] | 80 [75, 86] |
| Clinic DBP %ile | 47 [24, 65] | 0.96 [0.64, 0.97] | 62 [41, 85] | 96 [92, 98] |
| 24o SBP Index | 0.85 [0.81, 0.89] | 0.89 [0.87, 0.90] | 0.96 [0.92, 0.99] | 1.01 [0.96, 1.06] |
| 24o SBP Mean | 104 [99, 111] | 102 [99, 111] | 115 [110, 122] | 121 [114, 128] |
| SBP Load | 3% [0%, 7%] | 6% [4%, 7%] | 28% [14%, 42%] | 48% [25%, 70%] |
| % Dip (S) | 13 [9, 16] | 11 [11, 16] | 10 [7, 13] | 11 [8, 14] |
| % Dip (D) | 19 [15, 23] | 20 [16, 23] | 15 [11, 19] | 16 [12, 20] |
| Other ABPM measures | ||||
| Wake SBP Index | 0.86 [0.81, 0.90] | 0.90 [0.86, 0.92] | 0.94 [0.91, 0.98] | 1.00 [0.94, 1.04] |
| Sleep SBP Index | 0.86 [0.80, 0.89] | 0.89 [0.85, 0.91] | 0.97 [0.93, 1.01] | 1.01 [0.96, 1.06] |
| Wake DBP Index | 0.79 [0.74, 0.83] | 0.81 [0.78, 0.85] | 0.89 [0.82, 0.94] | 0.97 [0.91, 1.02] |
| Sleep DBP Index | 0.81 [0.75, 0.86] | 0.87 [0.80, 0.90] | 0.97 [0.92, 1.02] | 1.02 [0.96, 1.14] |
| Wake SBP Load | 3% [0%, 7%] | 6% [3%, 12%] | 24% [10%, 38%] | 48% [22%, 64%] |
| Sleep SBP Load | 0% [0%, 8%] | 3% [0%, 6%] | 32% [14%, 47%] | 51% [24%, 81%] |
| Wake DBP Load | 3% [0%, 6%] | 3% [0%, 10%] | 15% [6%, 28%] | 38% [18%, 64%] |
| Sleep DBP Load | 5% [0%, 11%] | 7% [5%, 15%] | 36% [23%, 50%] | 56% [35%, 80%] |
Note: 17 KIDs were not included due to missing clinic BP measurements.
Table 2.
Ambulatory Blood Pressure Parameters for attainable ABPM studies (n= 332).
| ABPM Parameter |
Index variables Median [IQR] |
Load Variables Median [IQR] |
% with index above 1 % (n) |
% with Load above 25% % (n) |
|---|---|---|---|---|
| Wake Systolic | 0.91 [0.85, 0.96] | 10% [3%, 28%] | 13% (43) | 28% (93) |
| Wake Diastolic | 0.83 [0.79, 0.92] | 7% [2%, 21%] | 7% (24) | 22% (72) |
| Sleep Systolic | 0.91 [0.86, 0.98] | 12% [0%, 38%] | 18% (61) | 33% (110) |
| Sleep Diastolic | 0.89 [0.81, 0.98] | 17% [4%, 40%] | 20% (66) | 39% (129) |
Almost 13% of the cohort (n=38) had a circadian BP pattern that is not included in the 2008 AHA guidelines. These subjects had normal casual and 24-hour mean BP readings but BP loads higher than 25%. Given that a substantial portion of ABPM readings were abnormal, and this population was already defined as a high risk group (i.e., a clinical diagnosis of CKD), we classified these subjects as having an abnormal ABPM study25. Thus, they were included as having masked hypertension (Table 3).
Use of antihypertensive and ACE inhibitor medications
A majority of this study sample reported any antihypertensive therapy use (68%, Table 1) and about half (52%) of the study sample were being treated with ACE inhibitors. There were similar proportions of subjects treated with any antihypertensive medications (ACE inhibitors, ARBs, diuretics and/or calcium-channel blockers) among those with a normal ABPM (68%) and an abnormal ABPM (71%). In a logistic regression model with adjustment for age, gender, CKD diagnosis, iohexol GFR level and uPrCr, those on any antihypertensive medications had a 13% higher odds of a normal ABPM compared to those not on any antihypertensive medications (OR: 1.13, 95%CI: 0.69, 1.95), but this association was not significant. When we investigated the association with ACE inhibitor use compared to no ACE inhibitor use among all subjects (regardless of other antihypertensive medications), there was a stronger and significant association with this specific class of antihypertensives and the odds of having a normal ABPM: those using an ACE inhibitor were 89% more likely to have a normal ABPM than those who did not report using an ACE inhibitor (OR: 1.89, 95%CI: 1.17, 3.04).
Association of CKD disease progression and abnormal ABPM
Table 4 describes the effect of iGFR and uPrCr annual change and average level on the odds of having an abnormal ABPM. The average level of iGFR between visits 1 and 2 was 42.5 ml/min/1.73m2 and the average level of uPrCr was 0.43. The average annual decline in iGFR was 0.96 (or a decrease of about 4% per year) while uPrCr increased by about 8% per year. The standard deviation of this annual change was 0.80 for iGFR and 2.25 for uPrCr. For the one-year longitudinal change in iGFR, a 20% lower difference in the ratio of current level (visit 2) to previous level at visit 1 (i.e., a 0.80-fold difference in decline which corresponds to one standard deviation of ratios of the sample) was associated with a 26% higher odds of having an abnormal ABPM independent of the average level of uPrCr and change in uPrCR and adjusting for average level of GFR at the two visits, age, sex and CKD diagnosis (OR: 1.26, 95%CI: 0.97, 1.64; p= 0.081). This effect was borderline significant and showed that a faster decline was associated with an increased odds of having an abnormal ABPM. The effect of average iGFR level was not associated with abnormal ABPM (p= 0.435)
Table 4.
Descriptive statistics and results from logistic regression model with abnormal ABPM as the dependent variable and average 2 year level and annual change in iGFR and urine protein:creatinine as the independent variables.*
| Variable | Average | Standard deviation |
Odds ratio of abnormal ABPM for a one standard deviation difference |
|---|---|---|---|
| Geometric mean level of GFR of visits 1 and 2 |
42.5 ml/min | 1.73m2 | 1.46 | 0.90 (0.80, 1.10) p= 0.435 |
| Annual change in iGFR (Visit 2 level / Visit 1 level) |
0.96 | 0.80 | 1.26 (0.97, 1.64) p= 0.081 |
| Geometric mean level of uPrCr of visits 1 and 2 |
0.43 mg/mg creatinine | 3.16 | 1.16 (0.90, 1.51) p= 0.246 |
| Annual change in uPrCr (Visit 2 level / Visit 1 level) |
1.08 | 2.25 |
1.39 (1.06, 1.82) p= 0.019 |
Model adjusted for age, sex and CKD diagnosis (glomerular vs. non-glomerular underlying cause of CKD).
A larger effect was observed for changes in uPrCr. Specifically, a one standard deviation increase in uPrCr change for this population (e.g., 2.25-fold increase) was associated with a 39% higher odds of having an abnormal ABPM (OR: 1.39, 95%CI: 1.06, 1.82; p= 0.019) when controlling for confounders, including the average uPrCr level of the two visits. (Table 4) Similar to iGFR, the average level of uPrCr was not related to increased odds of having an ABPM (p= 0.246). The interactions with the average level with average change were not significant for iGFR (p= 0.126) and uPrCr (p= 0.944) and were therefore not included in this final model.
Discussion
Ambulatory BP monitoring is a powerful tool for the investigation of cardiovascular risk and has been endorsed for use in children in selected clinical settings17, 20 A major limitation to more widespread use of ABPM in children is lack of expertise in the technique: it can be challenging to ensure successful data collection in this young population. Accordingly, few multicenter studies have included ABPM. In this report, we demonstrate that ABPM can be successfully implemented in a large multicenter pediatric study if standardized procedures and central oversight are provided. We further show the high prevalence of abnormally high ambulatory BP in this cohort, which was importantly detected during sleep, leading to a large proportion classified as having masked hypertension. Perhaps most significantly, we also demonstrate a positive association between the presence of an abnormal ABPM and faster rates of increasing uPrCr, which is an important marker for CKD progression.
Given that a diagnosis of CKD was an inclusion criterion in this cohort, our definition of ambulatory HTN is more conservative than most published reports. While the recent American Heart Association guidelines 20 contain classifications of abnormal ABPM studies which are not considered hypertensive (ambulatory pre-hypertension), any abnormality in either wake or sleep SBP or DBP means (i.e., mean BP > 95th%ile) or load (i.e., load > 25%) was considered diagnostic of HTN in this analysis. Compared to otherwise healthy patients with elevated BP, the high prevalence of cardiovascular complications in individuals with CKD supports this more inclusive definition. This inclusive definition of HTN also helps solve a problematic dilemma faced by clinicians: a significant number of subjects (13%) in the current analysis were found to have an ambulatory BP pattern that is not described by the guidelines. Namely, the current AHA guidelines do not address classification of ABPM studies with elevated loads, but normal mean BP. In the population described in this report, many of these subjects have been previously diagnosed as hypertensive and even more are on antihypertensive mediations; thus supporting our inclusion of them in the hypertensive group.
One potential weakness of the current analysis is the heterogeneity of subjects classified as having masked HTN. We chose to categorize subjects as having masked hypertension based on inadequately controlled BP, rather than excluding those with self-reported antihypertensive medication use. While this classification may not be directly applicable to the general population, the universal diagnosis of CKD in this sample is an essential consideration. Indeed, any patient with CKD and abnormal circadian variation in BP is likely at increased risk of adverse health outcomes, regardless of previous diagnosis or current treatment. Further, another asset to using ambulatory BP monitoring is in determining the effectiveness of therapy, and this is an accepted and important indication for the technology.
Self-reported ACE inhibitor use was associated with a higher odds of normal ABPM compared to those not on an ACE inhibitors. Since a significant portion of those with controlled ABP were also on therapy, this suggests that perhaps those who are not on ACE inhibitors may benefit from this therapy in terms of better BP management, but we are unable to infer causality in this analysis. The relationship is complex and this finding may also be explained by the extensive use of ACE inhibitors for their anti-proteinuric effect in subjects without a history of hypertension. Indeed, there may be significant confounding in this relationship (that is, those who have good BP control may be taking ACE inhibitors for CKD management) and this is important to note in interpreting the association.
The present analysis also describes the association between renal function decline and the presence of abnormal ABPM. A more dramatic one-year decline in function (i.e., a decrease in GFR and an increase in uPrCr) was associated with higher odds of abnormal ABPM. Interestingly, a one standard deviation increase in uPrCr level ratio (i.e., level at Visit 2 / level at Visit 1) was more strongly associated with odds of an abnormal ABPM than a one standard deviation decrease in GFR level ratio (OR: 1.39 vs. 1.26). Indeed, in this model which adjusted for important covariates, the effect for uPrCr level ratio was significant (p= 0.019) while the effect for GFR level ratio was borderline significant (p= 0.081) with the same trend. Of note, the average level of GFR and uPrCr was not significantly associated with having an abnormal ABPM, in contrast to the change in levels.
Perspectives
In conclusion, ABPM has emerged as an important diagnostic tool to aid clinicians in detecting increased cardiovascular risk in patients with CKD. This analysis demonstrates the feasibility of measuring ABPM in a multicenter trial, highlights the significant burden of HTN in patients with CKD, and reveals an important association between CKD disease progression and the presence of ambulatory HTN. Given these important findings, ABPM should be used to monitor risk and trigger therapeutic interventions in children with CKD.
Novelty and Significance.
What is New?
This manuscript describes the baseline ambulatory blood pressure data from the NIH Funded CKiD cohort study. Blood pressure data from more than 300 children with CKD are analyzed and presented.
What is Relavent?
This study demonstrates the feasibility of measuring ABPM in a multicenter trial, highlights the significant burden of HTN in pediatric patients with CKD, and reveals an important association between CKD disease progression and the presence of ambulatory HTN.
Summary
Ambulatory blood pressure monitoring is a useful tool in assessing disease risk in children with chronic kidney disease. The current study reports baseline ABP data in 322 children enrolled in the CKiD cohort study.
Acknowledgements
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, PhD) and data coordinating center (Principal Investigator) at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, PhD). The CKiD web site is located at www.statepi.jhsph.edu/c kid.
Sources of Funding
The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, and UO1-DK-66116).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosures
None.
References
- 1.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in african-american and white men. 16-year mrfit findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 2.Oldrizzi L, Rugiu C, De Biase V, Maschio G. The place of hypertension among the risk factors for renal function in chronic renal failure. Am J Kidney Dis. 1993;21:119–123. doi: 10.1016/s0272-6386(12)70115-4. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA. Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension. 2008;52:631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R. Ambulatory blood pressure and cardiovascular events in chronic kidney disease. Semin Nephrol. 2007;27:538–543. doi: 10.1016/j.semnephrol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
- 6.Wuhl E, Mehls O, Schaefer F. Antihypertensive and antiproteinuric efficacy of ramiprilin children with chronic renal failure. Kidney Int. 2004;66:768–776. doi: 10.1111/j.1523-1755.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JT. Impact of ambulatory blood pressure monitoring on the management of hypertension in children. Blood Press Monit. 2000;5:211–216. doi: 10.1097/00126097-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Mitsnefes MM, Kimball TR, Daniels SR. Office and ambulatory blood pressure elevation in children with chronic renal failure. Pediatr Nephrol. 2003;18:145–149. doi: 10.1007/s00467-002-1030-z. [DOI] [PubMed] [Google Scholar]
- 9.Soergel M, Schaefer F. Effect of hypertension on the progression of chronic renal failure in children. Am J Hypertens. 2002;15:53S–56S. doi: 10.1016/s0895-7061(01)02296-8. [DOI] [PubMed] [Google Scholar]
- 10.Wuhl E, Hadtstein C, Mehls O, Schaefer F. Home, clinic, and ambulatory blood pressure monitoring in children with chronic renal failure. Pediatr Res. 2004;55:492–497. doi: 10.1203/01.PDR.0000106863.90996.76. [DOI] [PubMed] [Google Scholar]
- 11.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–450. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70:1000–1007. doi: 10.1038/sj.ki.5001695. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Zanchetti A, Agabiti-Rosei E, Benemio G, De Cesaris R, Fogari R, Pessina A, Porcellati C, Rappelli A, Salvetti A, Trimarco B. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. Sample study group. Study on ambulatory monitoring of blood pressure and lisinopril evaluation. Circulation. 1997;95:1464–1470. doi: 10.1161/01.cir.95.6.1464. [DOI] [PubMed] [Google Scholar]
- 14.Gimpel C, Wuhl E, Arbeiter K, Drozdz D, Trivelli A, Charbit M, Gellermann J, Dusek J, Jankauskiene A, Emre S, Schaefer F Group fET. Superior consistency of ambulatory blood pressure monitoring in children: Implications for clinical trials. Journal of hypertension. 2009;27:1568–1574. doi: 10.1097/HJH.0b013e32832cb2a8. 1510.1097/HJH.1560b1013e32832cb32832a32838. [DOI] [PubMed] [Google Scholar]
- 15.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA. Design and methods of the chronic kidney disease in children (ckid) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 17.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 18.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: A multicenter trial including 1141 subjects. Journal of Pediatrics. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 19.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–908. doi: 10.1161/01.hyp.0000013266.40320.3b. [DOI] [PubMed] [Google Scholar]
- 20.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S. Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the american heart association atherosclerosis, hypertension, and obesity in youth committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 21.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191–197. doi: 10.1067/mpd.2002.125910. [DOI] [PubMed] [Google Scholar]
- 22.Lilien MR, Groothoff JW. Cardiovascular disease in children with ckd or esrd. Nat Rev Nephrol. 2009;5:229–235. doi: 10.1038/nrneph.2009.10. [DOI] [PubMed] [Google Scholar]
- 23.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA. Metabolic abnormalities, cardiovascular disease risk factors, and gfr decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 6:2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–1848. doi: 10.1007/s00467-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 25.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Sleep-time blood pressure as a therapeutic target for cardiovascular risk reduction in type 2 diabetes. Am J Hypertens. 2012;25:325–334. doi: 10.1038/ajh.2011.231. [DOI] [PubMed] [Google Scholar]