Abstract
The total synthesis of the indole alkaloid hirsutine has been achieved, with a key step being the application of our phosphine-catalyzed [4 + 2] annulation of an imine with ethyl α-methylallenoate. From commercially available indole-2-carboxaldehyde, the target was synthesized in 14 steps and 6.7% overall yield.
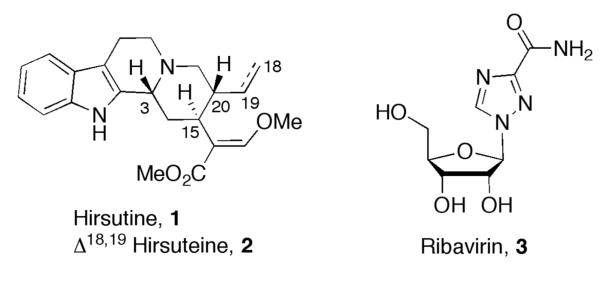
The hooks of Uncaria rhynchophylla, U. sinensis, and U. macrophylla are used in traditional Chinese herbal medicine as spasmolytic, analgesic, and sedative treatments for many symptoms associated with hypertension and cerebrovascular disorders.1 Many compounds have been isolated from these plants, including indole alkaloids, oxyindole alkaloids, and phenylpropanoids.2 Hirsutine (1) and hirsuteine (2), two of the major indole alkaloids isolated from the Uncaria species, have been demonstrated to exert central depressive and vasodilatory effects,3,4 as well as protective effects (through inhibition of Ca2+ influx) against neuronal death in cultured rat cerebellar granule cells.5 In addition, hirsutine displays antihypertensive, negative chronotropic, and antiarrhythmic activity.6 Recently, hirsutine has attracted the attention of the medical community for its ability to inhibit the growth of influenza A virus (subtype H3N2) with an EC50 value of 0.40–0.57 >g/mL; thus, hirsutine is 10–20 times more potent than the clinically used drug ribavirin (3, Figure 1).7 Many research groups have taken the initiative to study and synthesize hirsutine and its various derivatives in the hope that these compounds will find significant medicinal use.8
Figure 1.
Structures of hirsutine, hirsuteine, and ribavirin.
Of all the various approaches toward functionalized 1,2,5,6-tetrahydropyridines, our phosphine-catalyzed [4 + 2] annulation of α-methylallenoates with imines has emerged as one of the premiere methodologies.9 Although many natural products contain a tetrahydropyridine motif, the phosphine-catalyzed [4 + 2] annulation has not been applied previously to the synthesis of fused tetracyclic indole alkaloids.9b,10 Herein, we disclose our total synthesis of hirsutine using our [4 + 2] annulation methodology as a key step. The strategy developed in this study should also be applicable as a new route for the synthesis of other corynantheine indole alkaloids.
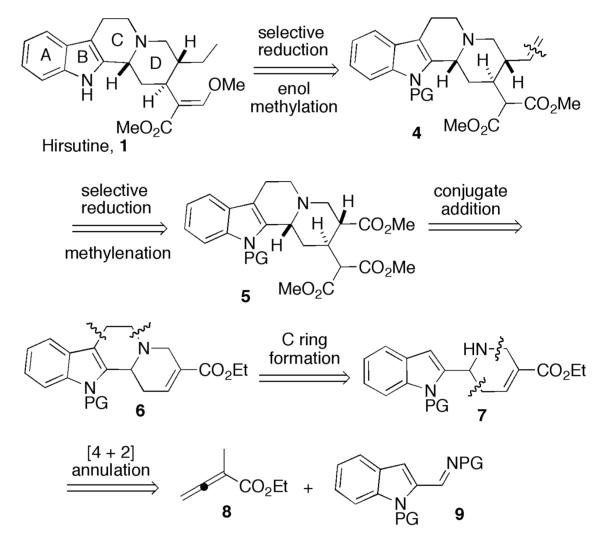
Scheme 1 outlines our retrosynthetic analysis of hirsutine. We envisioned the β-methoxy acrylate motif to arise from methylation of the enol form of the corresponding aldehyde, which would result from partial reduction of the malonate functionality in intermediate 4. We suspected that the vinyl group, which could be hydrogenated to the ethyl group in hirsutine, of 4 could be introduced through selective reduction of the isolated ester group in 5 in the presence of the malonate moiety, followed by Wittig olefination. We expected the malonate group to be installed through a diastereoselective conjugate addition of the malonate anion onto the functionalized enoate tetracycle 6,11 which would be derived through intramolecular N-alkylation of the free amine of the tricycle 7. In the key step, we anticipated the indole tricycle 7 to result directly from our phosphine-catalyzed [4 + 2] annulation between ethyl α-methylallenoate (8) and a suitably protected 2-indolylimine (9).
Scheme 1.
Retrosynthetic analysis of hirsutine
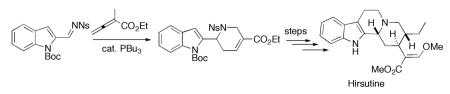
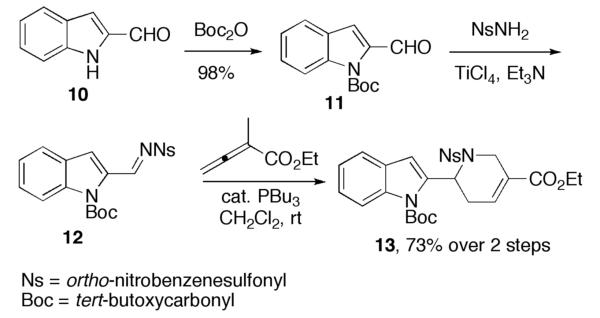
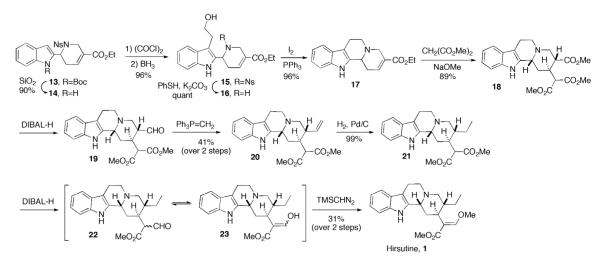
Our synthesis of hirsutine (Scheme 2) commenced with Boc protection of the commercially available indole 2-carboxaldehyde (10).12,13 We then reacted the N-Boc–protected aldehyde 11 with o-nitrobenzenesulfonamide (NsNH2) in the presence of Et3N and catalytic TiCl4 to give the N-(o-nosyl)imine 12.14 Because this imine is hydrolytically labile, we performed the transformations from the aldehyde 11 to the annulation product 13 in one pot. The phosphine-catalyzed annulation of the crude imine 12 with ethyl α-methylallenoate (8) proceeded smoothly under modified conditions to give compound 13. Accordingly, we obtained compound 13 in 73% yield from the aldehyde 11 over two steps.
Scheme 2.
[4 + 2] Annulation in the synthesis of intermediate
We removed the Boc group from compound 13 cleanly, using SiO2 in refluxing toluene, in 90% yield (Scheme 3).15 Acylation at the C3 position of the indole moiety in 14 with oxalyl chloride, followed by reduction of the resulting keto acid chloride with borane, furnished the requisite tryptophol 15. To the best of our knowledge, this transformation is the first example of the reduction of a chlorooxalyl group with borane, instead of its trapping as an alkyl oxalate ester derivative.16
Scheme 3.
Synthesis of Hirsutine, 1
The nosyl group of 15 was readily removed in the presence of PhSH and K2CO3 in MeCN at 50 °C.17 Formation of the C-ring through intramolecular N-alkylation proceeded smoothly under the influence of I2 and PPh3 to give the tetracycle 17.18 To install the desired relative stereochemistry at the C3 and C15 positions of hirsutine, we opted for the Michael addition onto the enoate 17, which would favor axial addition. To our delight, Michael addition with dimethyl malonate anion provided the triester 18 in 89% yield as a single diastereoisomer. Compound 18 exhibited the same relative stereochemistry as that found in hirsutine, as confirmed through X-ray diffraction analysis.19
Another key transformation was the selective reduction of the triester 18. The literature appears to be lacking in any precedents for the reduction of an isolated ester in the presence of a malonate ester moiety. After screening many reducing reagents and conditions, we obtained compound 19 in 20% isolated yield after the reaction of 18 with DIBAL-H at –78 °C. We confirmed the structure of 19 through X-ray diffraction analysis.19 Unfortunately, standard olefination conditions, including those of classic Wittig,20 Tebbe,21 Takai,22 and Wilkinson23 reactions, failed to provide the methylenation product. We found, however, that the Wittig reaction proceeded smoothly in the presence of DMSO to give the olefin 20.24 Because the aldehyde 19 was labile toward SiO2, we decided to use the crude product from the selective reduction of 18 directly for the Wittig olefination, obtaining the alkene 20 in 41% yield over two steps from the triester 18.
After reducing the vinyl group through hydrogenation,25,26 we reduced the malonate group of 21 selectively to give the monoaldehyde 22.8c At this point, the classic method for methylation, using HCl and MeOH, failed.8c Nevertheless, TMSCHN2 proved effective in methylating the aldehyde oxygen atom to furnish hirsutine (1) in 31% yield over two steps from 21.27 The spectral data of our synthetic sample matched those reported in the literature.8f,28
Our synthesis exhibits several salient features: (i) phosphine-catalyzed [4 + 2] annulation of ethyl α-methylallenoate with an imine; (ii) reduction of a chlorooxalyl group with borane to install the tryptophol motif, followed by N-alkylation to form ring C; (iii) diastereoselective Michael addition of the malonate anion to establish the correct relative configuration at C15 and C20 of hirsutine; and (iv) selective reduction of an isolated ester in the presence of a malonate moiety. This synthesis also provided, in eight steps and 59% yield, the key tetracyclic intermediate 17, which should be useful in the preparation of other corynantheine indole alkaloids. Further investigations into the syntheses of other corynantheine indole alkaloids and the enantioselective synthesis of hirsutine are underway.
Supplementary Material
Acknowledgment
We thank the NIH (R01GM071779 and P41GM081282) for financial support; Dr. Saeed Khan (UCLA) for the crystallographic analyses; and Prof. Hongchao Guo (China Agricultural University) for providing several allenoates (synthesized in the National Key Technologies R&D Program of China, 2012BAK25B03, CAU) as the substrates. This material is based upon work supported by the NSF under equipment grant no. CHE-1048804.
Footnotes
Supporting Information Available: Representative experimental procedures and spectral data for all new compounds (PDF). Crystallographic data for compounds 18 and 19 (CIF). This material is available free of charge via the internet at http://pubs.acs.org.
References
- (1)(a).Yano SJ. Traditional Sino Japanese Medicine. 1987;8:47–52. [Google Scholar]; (b) Hsieh CL, Chen MF, Li TC, Li SC, Tang NY, Hsieh CT, Pon CZ, Lin JG. Am. J. Chin. Med. 1999;27:257–264. doi: 10.1142/S0192415X9900029X. [DOI] [PubMed] [Google Scholar]
- (2).Shimada Y, Goto H, Kogure T, Shibahara N, Sakakibara I, Sasaki H, Terasawa K. Am. J. Chin. Med. 2001;29:173–180. doi: 10.1142/S0192415X01000198. [DOI] [PubMed] [Google Scholar]
- (3).Ozaki Y. Nippon Yakurigaku Zasshi. 1989;94:17–26. doi: 10.1254/fpj.94.17. [DOI] [PubMed] [Google Scholar]
- (4).Ozaki Y. Nippon Yakurigaku Zasshi. 1990;95:47–54. doi: 10.1254/fpj.95.2_47. [DOI] [PubMed] [Google Scholar]
- (5).Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H, Terasawa K. J. Pharm. Pharmacol. 1999;51:715–722. doi: 10.1211/0022357991772853. [DOI] [PubMed] [Google Scholar]
- (6).Masumiya H, Saitoh T, Tanaka Y, Horie S, Aimi N, Takayama H, Shigenobu K. Life Sci. 1999;65:2333–2341. doi: 10.1016/s0024-3205(99)00500-7. [DOI] [PubMed] [Google Scholar]
- (7).Takayama H, Iimura Y, Kitajima M, Aimi N, Konno K, Inoue H, Fujiwara M, Mizuta T, Yokota T, Shigeta S, Tokuhisa K, Hanasaki Y, Katsuura K. Bioorg. Med. Chem. Lett. 1997;7:3145–3148. [Google Scholar]
- (8).Syntheses of hirsutine: Aimi N, Yamanaka E, Endo J, Sakai S, Haginiwa J. Tetrahedron. 1973;29:2015–2021. Brown RT, Chapple CL, Charalambides AA. J. Chem. Soc., Chem. Commun. 1974:756–757. Brown RT, Chapple CL. J. Chem. Soc., Chem. Commun. 1974:740–742. Wenkert E, Vankar YD, Yadav JS. J. Am. Chem. Soc. 1980;102:7971–7972. Brown RT, Ford MJ. Tetrahedron Lett. 1990;31:2033–2036. Gomez-Pardo D, Desmaële D, d’Angelo J. Tetrahedron Lett. 1992;33:6633–6636. Lounasmaa M, Jokela R, Laine C, Hanhinen P. Heterocycles. 1998;49:445–450. Tietze LF, Zhou Y. Angew. Chem., Int. Ed. 1999;38:2045–2047. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<2045::AID-ANIE2045>3.0.CO;2-U. Deiters A, Pettersson M, Martin SF. J. Org. Chem. 2006;71:6547–6561. doi: 10.1021/jo061032t. Synthesis of hirsuteine: Naito T, Miyata O, Ninomiya I. Heterocycles. 1987;26:1739–1742.
- (9)(a).Zhu X-F, Lan J, Kwon O. J. Am. Chem. Soc. 2003;125:4716–4717. doi: 10.1021/ja0344009. [DOI] [PubMed] [Google Scholar]; (b) Wurz RP, Fu GC. J. Am. Chem. Soc. 2005;127:12234–12235. doi: 10.1021/ja053277d. [DOI] [PubMed] [Google Scholar]; (c) Lu K, Kwon O. Org. Synth. 2009;86:212–224. doi: 10.1002/0471264229.os086.21. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xiao H, Chai Z, Wang H-F, Wang X-W, Cao D-D, Liu W, Lu Y-P, Yang Y-Q, Zhao G. Chem. Eur. J. 2011;17:10562–10565. doi: 10.1002/chem.201100850. [DOI] [PubMed] [Google Scholar]; (e) Fan YC, Kwon O. Phosphine Catalysis. In: List B, editor. Science of Synthesis. Asymmetric Organocatalysis, Vol. 1, Lewis Base and Acid Catalysts. Georg Thieme; Stuttgart: 2012. pp. 723–782. [Google Scholar]
- (10).For the application of Kwon’s [4 + 2] annulation to the synthesis of bridged tetracyclic indole alkaloids, see: Tran YS, Kwon O. Org. Lett. 2005;7:4289–4291. doi: 10.1021/ol051799s.
- (11).Rosenmund P, Casutt M, Wittich M. Liebigs Ann. Chem. 1990:233–238. [Google Scholar]
- (12).Although the starting material 10 is commercially available, it can be synthesized from indole-2-carboxylic acid through sequential reduction (LiAlH4) and oxidation (activated MnO2); see: Jeught SV, Vos ND, Masschelein K, Ghiviriga I, Stevens CV. Eur. J. Org. Chem. 2010:5444–5453.
- (13).Biswas S, Singh V, Batra S. Tetrahedron. 2010;66:7781–7786. [Google Scholar]
- (14).Kattuboina A, Li G. Tetrahedron Lett. 2008;49:1573–1577. [Google Scholar]
- (15).Zhang M, Yuan X, Ma L, Zhao J, Gao L. Chem. J. Chin. Univ. 2007;28:2330–2332. [Google Scholar]
- (16)(a).Elks J, Elliott DF, Hems BA. J. Chem. Soc. 1944:629–632. [Google Scholar]; (b) Nogrady T, Doyle TW. Can. J. Chem. 1964;42:485–486. [Google Scholar]; (c) Fuchs JR, Funk RL. J. Am. Chem. Soc. 2004;126:5068–5069. doi: 10.1021/ja049569g. [DOI] [PubMed] [Google Scholar]
- (17).Fukuyama T, Jow C-K, Cheung M. Tetrahedron Lett. 1995;36:6373–6374. [Google Scholar]
- (18).Huang H, Spande TF, Panek JS. J. Am. Chem. Soc. 2003;125:626–627. doi: 10.1021/ja028937i. [DOI] [PubMed] [Google Scholar]
- (19).CCDC 874946 for compound 18 and CCDC 874752 for compound 19 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/data_request/cif.
- (20).Bondarenko L, Hentschel S, Greiving H, Grunenberg J, Hopf H, Dix I, Jones PG, Ernst L. Chem. Eur. J. 2007;13:3950–3963. doi: 10.1002/chem.200601629. [DOI] [PubMed] [Google Scholar]
- (21)(a).Pine SH, Zahler R, Evans DA, Grubbs RH. J. Am. Chem. Soc. 1980;102:3270. [Google Scholar]; (b) Chambers DJ, Evans GR, Fairbanks AJ. Tetrahedron: Asymmetry. 2005;16:45–55. [Google Scholar]
- (22).Okazoe T, Takai K, Oshima K, Utimoto K. J. Org. Chem. 1987;52:4410–4412. [Google Scholar]
- (23).Lebel H, Paquet V. J. Am. Chem. Soc. 2004;126:320–328. doi: 10.1021/ja038112o. [DOI] [PubMed] [Google Scholar]
- (24)(a).Greenwald R, Chaykovsky M, Corey EJ. J. Org. Chem. 1963;28:1128–1129. [Google Scholar]; (b) Aiba Y, Hasegawa D, Marunouchi T, Nagasawa K, Uchiro H, Kobayashi S. Bioorg. Med. Chem. Lett. 2001;11:2783–2786. doi: 10.1016/s0960-894x(01)00561-3. [DOI] [PubMed] [Google Scholar]
- (25).With the vinyl substituent at C20, attempts at partial reduction of the malonate to the corresponding monoaldehyde provided complex mixtures of products.
- (26).Suzuki H, Unemoto M, Hagiwara M, Ohyama T, Yokoyama Y, Murakami Y. J. Chem. Soc., Perkin Trans. 1. 1999:1717–1723. [Google Scholar]
- (27).Aoyama T, Terasawa S, Sudo K, Shioiri T. Chem. Pharm. Bull. 1984;32:3759–3760. [Google Scholar]
- (28)(a).Xin W, Gui X, Wang Z. Chin. Traditional and Herbal Drugs. 2009;40:204–207. [Google Scholar]; (b) Kohda H, Namera A, Koyama A, Yamasaki K, Tani T. Chem. Pharm. Bull. 1996;44:352–357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.