Abstract
Background
Coffee is one of the most widely consumed beverages, but the association between coffee consumption and the risk of death remains unclear.
Methods
We examined the association of coffee drinking with subsequent total and cause-specific mortality among 229,119 men and 173,141 women in the National Institutes of Health–AARP Diet and Health Study who were 50 to 71 years of age at baseline. Participants with cancer, heart disease, and stroke were excluded. Coffee consumption was assessed once at baseline.
Results
During 5,148,760 person-years of follow-up between 1995 and 2008, a total of 33,731 men and 18,784 women died. In age-adjusted models, the risk of death was increased among coffee drinkers. However, coffee drinkers were also more likely to smoke, and, after adjustment for tobacco-smoking status and other potential confounders, there was a significant inverse association between coffee consumption and mortality. Adjusted hazard ratios for death among men who drank coffee as compared with those who did not were as follows: 0.99 (95% confidence interval [CI], 0.95 to 1.04) for drinking less than 1 cup per day, 0.94 (95% CI, 0.90 to 0.99) for 1 cup, 0.90 (95% CI, 0.86 to 0.93) for 2 or 3 cups, 0.88 (95% CI, 0.84 to 0.93) for 4 or 5 cups, and 0.90 (95% CI, 0.85 to 0.96) for 6 or more cups of coffee per day (P<0.001 for trend); the respective hazard ratios among women were 1.01 (95% CI, 0.96 to 1.07), 0.95 (95% CI, 0.90 to 1.01), 0.87 (95% CI, 0.83 to 0.92), 0.84 (95% CI, 0.79 to 0.90), and 0.85 (95% CI, 0.78 to 0.93) (P<0.001 for trend). Inverse associations were observed for deaths due to heart disease, respiratory disease, stroke, injuries and accidents, diabetes, and infections, but not for deaths due to cancer. Results were similar in subgroups, including persons who had never smoked and persons who reported very good to excellent health at baseline.
Conclusions
In this large prospective study, coffee consumption was inversely associated with total and cause-specific mortality. Whether this was a causal or associational finding cannot be determined from our data. (Funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.)
Coffee is one of the most widely consumed beverages, both in the United States and worldwide. Since coffee contains caffeine, a stimulant, coffee drinking is not generally considered to be part of a healthy lifestyle. However, coffee is a rich source of antioxidants1 and other bioactive compounds, and studies have shown inverse associations between coffee consumption and serum biomarkers of inflammation2 and insulin resistance.3,4
Considerable attention has been focused on the possibility that coffee may increase the risk of heart disease,5,6 particularly since drinking coffee has been associated with increased low-density lipoprotein cholesterol levels7 and short-term increases in blood pressure.8 Results from a number of studies have been inconsistent.9,10 The heterogeneous findings may be due to differences between case–control and prospective study designs and possibly also to inconsistent control for important confounders such as tobacco smoking. In addition, the numbers of deaths have been small in most studies. Cohort studies do not support a positive association between coffee drinking and mortality, however, and some even suggest a modest inverse association.11–15
Previous studies have also investigated the association between coffee consumption and other major causes of death, and they have shown inverse associations with diabetes,4 inflammatory diseases,11 stroke,16–18 and injuries and accidents,19,20 although associations with cancer have generally been null.15,20–22 The results of studies of coffee consumption and total mortality have been mixed,15,20–31 with associations that have been consistent with either the null hypothesis or a modest inverse effect. Data are lacking to clarify the association between coffee drinking and mortality, to determine whether there is a dose–response relationship, and to assess whether associations are consistent across various subgroups.
We used data from a very large study, the National Institutes of Health (NIH)–AARP Diet and Health Study (ClinicalTrials.gov number, NCT00340015), to determine whether coffee consumption is associated with total or cause-specific mortality.32 The current analysis, involving more than 400,000 participants and 52,000 deaths, had ample power to detect even modest associations and allowed for subgroup analyses according to important baseline factors, including the presence or absence of adiposity and diabetes, as well as cigarette-smoking status.
METHODS
STUDY POPULATION
The NIH–AARP Diet and Health Study has been described previously.32 Between 1995 and 1996, a total of 617,119 AARP members, 50 to 71 years of age, returned a comprehensive questionnaire assessing diet and lifestyle. Participants resided in six states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta and Detroit). Of the respondents, 566,401 completed the questionnaire satisfactorily. Completion of the self-administered questionnaire was considered to imply informed consent to participate in the study.
We excluded from these analyses 15,760 persons whose questionnaires were completed by a spouse or other surrogate correspondent, as well as 51,234 persons with cancer, 65,044 with heart disease, 10,459 who had had a previous stroke, 2082 who did not provide information on coffee use, 15,820 who did not provide information on cigarette smoking, 3731 with an extremely low or high caloric consumption (two times as high as the 75th percentile of caloric intake or two times as low as the 25th percentile of caloric intake), and 11 who died before their completed questionnaire was received. The resulting analytic cohort included 229,119 men and 173,141 women. The NIH–AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the National Cancer Institute.
ASSESSMENT OF EXPOSURE
Participants completed a baseline questionnaire that assessed demographic and lifestyle characteristics and 124 dietary items, as previously described.32 Consumption of fruits, vegetables, red meat, white meat, and saturated fat were adjusted for total energy intake with the use of the nutrient-density approach (i.e., measured per 1000 kcal per day for food groups and as a percentage of total energy for saturated fat).
Coffee consumption was assessed according to 10 frequency categories, ranging from 0 to 6 or more cups per day. In addition, 96.5% of coffee drinkers provided information on whether they drank caffeinated or decaffeinated coffee more than half the time, and we used this information to categorize coffee drinkers.
In a subgroup of 1953 study participants who also completed a 24-hour dietary-recall questionnaire on 2 nonconsecutive days,33 the Spearman coefficient for the correlation between coffee consumption as assessed with this questionnaire and coffee consumption as assessed with the baseline food-frequency questionnaire was 0.80. The respective Spearman correlation coefficients for caffeinated and decaffeinated coffee were 0.64 and 0.48, respectively. Among participants who completed the 24-hour dietary-recall questionnaire, 79% drank ground coffee, 19% drank instant coffee, 1% drank espresso coffee, and 1% did not specify the type of coffee they consumed.
COHORT FOLLOW-UP
Participants were followed from baseline (1995–1996) until the date of death or December 31, 2008, whichever came first, by means of linkage to the National Change of Address database maintained by the U.S. Postal Service, specific change-of-address requests from participants, and updated addresses returned during other mailings. Vital status was assessed by periodic linkage of the cohort to the Social Security Administration Death Master File, linkage with cancer registries, questionnaire responses, and responses to other mailings.
CAUSES OF DEATH
Specific causes of death were obtained through follow-up linkage to the National Death Index Plus, maintained by the National Center for Health Statistics. We used the International Classification of Diseases, Ninth Revision (ICD-9), and International Classification of Diseases, 10th Revision (ICD-10) codes to classify the underlying cause of death (obtained from death certificates) as follows: cancer (ICD-9, 140–239; ICD-10, C00–C97 and D00–D48), heart disease (ICD-9, 390–398, 401–404, 410–429, and 440–448; ICD-10, I00–I13, I20–I51, and I70–I78), respiratory disease (e.g., pneumonia, influenza, chronic obstructive pulmonary disease, and associated conditions) (ICD-9, 480–487 and 490–496; ICD-10, J10–J18 and J40–J47), stroke (ICD-9, 430–438; ICD-10, I60–I69), injuries and accidents (e.g., accident, suicide, and homicide) (ICD-9, 800–978; ICD-10, V01–X59, Y85–Y86, U03, X60–X84, Y87.0, U01–U02, X85–Y09, Y35, Y87.1, and Y89.0), diabetes (ICD-9, 250; ICD-10, E10–E14), infections (e.g., tuberculosis, septicemia, and other infectious and parasitic diseases) (ICD-9, 001–139; ICD-10, A00–B99), and all other causes.
State data on the incidence of cancer were obtained from the Arizona Cancer Registry, the Georgia Center for Cancer Statistics, the California Cancer Registry, the Michigan Cancer Surveillance Program, the Florida Cancer Data System, the Louisiana Tumor Registry, the New Jersey State Cancer Registry, the North Carolina Central Cancer Registry, the Pennsylvania Cancer Registry, and the Texas Cancer Registry.
STATISTICAL ANALYSIS
Coffee consumption was tabulated according to a number of dietary and lifestyle factors. Hazard ratios and 95% confidence intervals for mortality associated with coffee consumption were estimated with the use of Cox proportional-hazards regression models, with person-years as the underlying time metric; results calculated with age as the underlying time metric were similar. We tested the proportional-hazards assumption by modeling the interaction of follow-up time with coffee consumption and observed no significant deviations. Analyses were conducted with the use of SAS software, version 9.1. Statistical tests were two-sided, and P values of less than 0.05 were considered to indicate statistical significance.
We present risk estimates separately for men and women. Multivariate models were adjusted for the following baseline factors: age; body-mass index (BMI); race or ethnic group; level of education; alcohol consumption; the number of cigarettes smoked per day, use or nonuse of pipes or cigars, and time of smoking cessation (<1 year, 1 to <5 years, 5 to <10 years, or ≥10 years before baseline); health status; presence or absence of diabetes; marital status; level of physical activity; total energy intake; consumption of fruits, vegetables, red meat, white meat, and saturated fat; and use of any vitamin supplement (yes vs. no). In addition, risk estimates for death from cancer were adjusted for history of cancer (other than non-melanoma skin cancer) in a first-degree relative (yes vs. no). For women, status with respect to postmenopausal hormone therapy was also included in multivariate models. Less than 5% of the cohort lacked any single covariate; for each covariate, we included an indicator for missing data in the regression models, if necessary. In a sensitivity analysis, we adjusted for propensity scores34 that reflected associations of coffee consumption with the other variables in the multivariate-adjusted models. Results obtained with the use of propensity-score adjustment were very similar to those from multivariate-adjusted models (Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Hazard ratios for death associated with categories of coffee consumption (<1, 1, 2 or 3, 4 or 5, and ≥6 cups per day), as compared with no coffee consumption, were estimated from a single model. Tests of linear trend across categories of coffee consumption were performed by assigning participants the midpoint of their coffee-consumption category and entering this new variable into a separate Cox proportional-hazards regression model.
In secondary analyses, we determined risk estimates for categories of consumption of caffeinated and decaffeinated coffee and examined associations among prespecified baseline subgroups based on the following: follow-up time; age; cigarette-smoking status; presence or absence of diabetes; BMI; alcohol consumption; self-reported health; high or low consumption of red meat, white meat, fruits, and vegetables; use or nonuse of any vitamin supplement; and, in women, use or nonuse of postmenopausal hormone therapy. For these analyses, we combined the categories of 4 or 5 cups of coffee per day and 6 or more cups per day to preserve numbers in the top category of consumption. P values for interactions were computed by means of likelihood-ratio tests comparing Cox proportional-hazards models with and those without cross-product terms for each level of the baseline stratifying variable, with coffee consumption as an ordinal variable. For total mortality, we performed 12 interaction tests for men and 13 interaction tests for women. We also performed interaction tests for smoking status with eight different outcomes for both men and women. Several differences (P<0.05) would be expected by chance alone.
RESULTS
ASSOCIATION OF COFFEE CONSUMPTION WITH DIETARY AND LIFESTYLE FACTORS
Coffee consumption at baseline was associated with several other dietary and lifestyle factors (Table 1). As compared with persons who did not drink coffee, coffee drinkers were more likely to smoke cigarettes and consume more than three alcoholic drinks per day, and they consumed more red meat. Coffee drinkers also tended to have a lower level of education; were less likely to engage in vigorous physical activity; and reported lower levels of consumption of fruits, vegetables, and white meat. However, coffee drinkers, especially women who drank coffee, were less likely to report having diabetes. About two thirds of coffee drinkers reported drinking predominantly caffeinated coffee.
Table 1.
Baseline Characteristics of the Study Participants, According to Daily Coffee Consumption.*
| Characteristic | Men (N = 229,119) | Women (N = 173,141) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Coffee (N = 21,080) | 1 Cup (N = 33,961) | 2 or 3 Cups (N = 97,144) | 4 or 5 Cups (N = 32,084) | ≥6 Cups (N = 10,139) | No Coffee (N = 20,865) | 1 Cup (N = 31,355) | 2 or 3 Cups (N = 68,250) | 4 or 5 Cups (N=17,434) | ≥6 Cups (N = 5,152) | |
| Age (yr) | ||||||||||
|
| ||||||||||
| Median | 61.1 | 63.5 | 62.5 | 61.0 | 60.3 | 60.9 | 62.9 | 62.2 | 61.6 | 61.0 |
|
| ||||||||||
| Interquartile range | 56.4–65.7 | 58.6–67.2 | 57.7–66.5 | 56.6–65.5 | 55.9–64.8 | 56.1–65.6 | 58.1–66.7 | 57.5–66.3 | 57.0–65.8 | 56.4–65.3 |
|
| ||||||||||
| Non-Hispanic white (%)† | 91.1 | 91.1 | 95.1 | 96.9 | 97.3 | 89.8 | 87.2 | 93.9 | 96.5 | 97.2 |
|
| ||||||||||
| Family history of cancer (%)‡ | 46.3 | 46.3 | 48.2 | 48.6 | 48.5 | 51.3 | 51.1 | 52.2 | 52.7 | 52.6 |
|
| ||||||||||
| Currently married (%) | 83.0 | 85.5 | 86.4 | 85.2 | 83.4 | 45.4 | 46.6 | 46.1 | 43.5 | 37.8 |
|
| ||||||||||
| College graduate (%) | 53.0 | 46.7 | 46.6 | 44.9 | 37.2 | 33.9 | 31.3 | 31.0 | 28.7 | 24.3 |
|
| ||||||||||
| Median body-mass index§ | 26.4 | 26.5 | 26.6 | 26.7 | 26.6 | 25.9 | 25.8 | 25.5 | 25.1 | 25.0 |
|
| ||||||||||
| Diabetes (%) | 8.4 | 8.6 | 8.0 | 8.1 | 8.0 | 7.4 | 7.0 | 5.2 | 4.5 | 4.4 |
|
| ||||||||||
| Current smoker (%) | 4.8 | 6.5 | 10.9 | 20.3 | 34.7 | 8.1 | 8.8 | 15.9 | 31.1 | 48.1 |
|
| ||||||||||
| >3 Alcoholic drinks/day (%) | 6.3 | 10.4 | 12.7 | 13.1 | 11.6 | 1.8 | 2.3 | 3.1 | 3.3 | 3.0 |
|
| ||||||||||
| Vigorous physical activity ≥5 times/wk (%) | 24.8 | 21.3 | 20.5 | 19.7 | 20.6 | 18.2 | 16.6 | 16.1 | 16.0 | 15.4 |
|
| ||||||||||
| Poor or fair self-reported health (%) | 7.5 | 7.5 | 6.6 | 7.0 | 9.3 | 12.2 | 10.2 | 8.2 | 8.2 | 10.0 |
|
| ||||||||||
| Median total energy intake (kcal/day) | 1869 | 1830 | 1882 | 2007 | 2175 | 1444 | 1432 | 1463 | 1540 | 1642 |
|
| ||||||||||
| Median servings of food | ||||||||||
|
| ||||||||||
| Fruits (servings/day) | 1.5 | 1.4 | 1.2 | 1.1 | 0.8 | 1.8 | 1.8 | 1.6 | 1.4 | 1.2 |
|
| ||||||||||
| Vegetables (servings/day) | 1.9 | 1.9 | 1.9 | 1.8 | 1.7 | 2.3 | 2.3 | 2.2 | 2.2 | 2.0 |
|
| ||||||||||
| Red meat (g/day) | 33.1 | 34.6 | 36.5 | 38.3 | 40.5 | 25.6 | 25.6 | 27.1 | 28.4 | 29.6 |
|
| ||||||||||
| White meat (g/day) | 25.8 | 26.1 | 26.0 | 24.4 | 21.5 | 28.3 | 30.1 | 29.5 | 27.3 | 23.9 |
|
| ||||||||||
| Use of any vitamin supplement (%) | 59.6 | 58.2 | 58.1 | 57.6 | 55.5 | 68.4 | 67.0 | 67.6 | 66.1 | 62.4 |
|
| ||||||||||
| Previous or current use of postmenopausal hormone therapy (%) | 52.4 | 54.8 | 55.3 | 51.2 | 45.1 | |||||
All exposures were associated with coffee drinking, with P<0.001 for trends across categories, except for diabetes in men (P = 0.002) and self-reported health status in men (P = 0.33).
Race or ethnic group was self-reported.
Nonmelanoma skin cancer in a first-degree relative was excluded from this category.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
COFFEE CONSUMPTION AND TOTAL MORTALITY
During 14 years of follow-up (median, 13.6 years; total person-years, 5,148,760), 33,731 men and 18,784 women died. In age-adjusted analyses, coffee consumption was associated with increased mortality among both men (Table 2) and women (Table 3). However, after multivariate adjustment for potential confounders, particularly smoking (Table 1 in the Supplementary Appendix), a modest inverse association between coffee drinking and total mortality was observed for both sexes. Hazard ratios for death among men who drank coffee, as compared with men who did not drink coffee, were as follows: 0.99 (95% confidence interval [CI], 0.95 to 1.04) for less than 1 cup of coffee per day, 0.94 (95% CI, 0.90 to 0.99) for 1 cup, 0.90 (95% CI, 0.86 to 0.93) for 2 or 3 cups, 0.88 (95% CI, 0.84 to 0.93) for 4 or 5 cups, and 0.90 (95% CI, 0.85 to 0.96) for 6 or more cups (P<0.001 for trend across categories) (Table 2). Hazard ratios among women who drank coffee, as compared with those who did not, were as follows: 1.01 (95% CI, 0.96 to 1.07) for less than 1 cup of coffee per day, 0.95 (95% CI, 0.90 to 1.01) for 1 cup, 0.87 (95% CI, 0.83 to 0.92) for 2 or 3 cups, 0.84 (95% CI, 0.79 to 0.90) for 4 or 5 cups, and 0.85 (95% CI, 0.78 to 0.93) for 6 or more cups (P<0.001 for trend across categories) (Table 3).
Table 2.
Association of Daily Coffee Consumption with Total and Cause-Specific Mortality among 229,119 Men.*
| Cause of Death | All Participants | No Coffee (N = 21,080) | <1 Cup (N = 34,710) | 1 Cup (N = 33,961) | 2 or 3 Cups (N = 97,144) | 4 or 5 Cups (N= 32,084) | ≥6 Cups (N= 10,139) | P Value for Trend |
|---|---|---|---|---|---|---|---|---|
| All causes | ||||||||
| No. of deaths (%) | 33,731 | 2766 (13.1) | 4931 (14.2) | 5049 (14.9) | 14,115 (14.5) | 4966 (15.5) | 1904 (18.8) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.02 (0.98–1.07) | 0.99 (0.94–1.03) | 1.03 (0.99–1.07) | 1.21 (1.15–1.27) | 1.60 (1.51–1.69) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.95–1.04) | 0.94 (0.90–0.99) | 0.90 (0.86–0.93) | 0.88 (0.84–0.93) | 0.90 (0.85–0.96) | <0.001 | |
| Cancer | ||||||||
| No. of deaths (%) | 13,402 | 946 (4.5) | 1729 (5.0) | 1824 (5.4) | 5804 (6.0) | 2219 (6.9) | 880 (8.7) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.06 (0.98–1.14) | 1.06 (0.98–1.15) | 1.25 (1.17–1.34) | 1.57 (1.46–1.70) | 2.13 (1.95–2.34) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.01 (0.93–1.09) | 0.96 (0.89–1.04) | 1.00 (0.93–1.07) | 1.04 (0.96–1.12) | 1.08 (0.98–1.19) | 0.02 | |
| Heart disease | ||||||||
| No. of deaths (%) | 8,127 | 712 (3.4) | 1193 (3.4) | 1243 (3.7) | 3353 (3.5) | 1184 (3.7) | 442 (4.4) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.96 (0.87–1.05) | 0.94 (0.86–1.03) | 0.95 (0.87–1.03) | 1.12 (1.02–1.23) | 1.44 (1.28–1.62) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.93 (0.85–1.02) | 0.92 (0.84–1.01) | 0.86 (0.79–0.94) | 0.87 (0.79–0.96) | 0.88 (0.78–1.00) | 0.03 | |
| Respiratory disease | ||||||||
| No. of deaths (%) | 2,512 | 169 (0.8) | 351 (1.0) | 352 (1.0) | 1046 (1.1) | 409 (1.3) | 185 (1.8) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.17 (0.97–1.40) | 1.07 (0.89–1.28) | 1.21 (1.03–1.43) | 1.64 (1.37–1.96) | 2.63 (2.13–3.24) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.05 (0.87–1.27) | 0.93 (0.77–1.11) | 0.83 (0.70–0.98) | 0.83 (0.69–1.00) | 0.81 (0.65–1.00) | 0.004 | |
| Stroke | ||||||||
| No. of deaths (%) | 1,327 | 125 (0.6) | 221 (0.6) | 222 (0.7) | 555 (0.6) | 141 (0.4) | 63 (0.6) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.80–1.24) | 0.91 (0.73–1.14) | 0.87 (0.72–1.06) | 0.76 (0.60–0.97) | 1.21 (0.89–1.64) | 0.57 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.79–1.24) | 0.92 (0.73–1.15) | 0.84 (0.68–1.02) | 0.65 (0.51–0.84) | 0.83 (0.61–1.14) | 0.003 | |
| Injuries and accidents | ||||||||
| No. of deaths (%) | 1,211 | 113 (0.5) | 186 (0.5) | 202 (0.6) | 492 (0.5) | 168 (0.5) | 50 (0.5) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.96 (0.76–1.21) | 1.01 (0.80–1.27) | 0.90 (0.73–1.10) | 1.00 (0.78–1.26) | 1.00 (0.72–1.40) | 0.93 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.98 (0.77–1.24) | 1.02 (0.80–1.29) | 0.88 (0.71–1.09) | 0.87 (0.68–1.12) | 0.72 (0.51–1.02) | 0.02 | |
| Diabetes | ||||||||
| No. of deaths (%) | 850 | 87 (0.4) | 165 (0.5) | 154 (0.5) | 310 (0.3) | 107 (0.3) | 27 (0.3) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.10 (0.85–1.42) | 0.98 (0.75–1.27) | 0.73 (0.57–0.92) | 0.83 (0.62–1.10) | 0.71 (0.46–1.10) | 0.001 | |
| Multivariate-adjusted hazard ratio (95% CI)‡ | 1.00 | 1.07 (0.82–1.39) | 1.00 (0.76–1.31) | 0.75 (0.59–0.96) | 0.80 (0.60–1.08) | 0.60 (0.39–0.94) | <0.001 | |
| Infections | ||||||||
| No. of deaths (%) | 685 | 68 (0.3) | 112 (0.3) | 124 (0.4) | 276 (0.3) | 80 (0.2) | 25 (0.2) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.95 (0.70–1.28) | 0.99 (0.74–1.34) | 0.82 (0.63–1.07) | 0.79 (0.57–1.09) | 0.85 (0.54–1.35) | 0.08 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.95 (0.70–1.29) | 1.03 (0.76–1.40) | 0.83 (0.63–1.10) | 0.70 (0.50–0.98) | 0.59 (0.37–0.95) | 0.001 | |
| Other causes | ||||||||
| No. of deaths (%) | 5,617 | 546 (2.6) | 974 (2.8) | 928 (2.7) | 2279 (2.3) | 658 (2.1) | 232 (2.3) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.02 (0.92–1.13) | 0.91 (0.81–1.01) | 0.84 (0.76–0.92) | 0.81 (0.73–0.91) | 1.00 (0.86–1.16) | 0.002 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.02 (0.92–1.13) | 0.92 (0.82–1.02) | 0.81 (0.74–0.89) | 0.71 (0.63–0.80) | 0.72 (0.61–0.84) | <0.001 | |
CI denotes confidence interval. The numbers of deaths are for participants who died during follow-up. Multivariate analyses were adjusted for the following factors at baseline: age; body-mass index; race or ethnic group; level of education; alcohol consumption; the number of cigarettes smoked per day, use or nonuse of pipes or cigars, and time of smoking cessation (<1 year, 1 to <5 years, 5 to <10 years, or ≥10 years before baseline); health status; diabetes (yes vs. no); marital status; physical activity; total energy intake; consumption of fruits, vegetables, red meat, white meat, and saturated fat; and use or nonuse of vitamin supplements. In addition, risk estimates for death from cancer were adjusted for history of cancer (other than nonmelanoma skin cancer) in a first-degree relative (yes vs. no).
Table 3.
Association of Daily Coffee Consumption with Total and Cause-Specific Mortality among 173,141 Women.*
| Cause of Death | All Participants | No Coffee (N =20,865) | <1 Cup (N = 30,085) | 1 Cup (N = 31,355) | 2 or 3 Cups (N = 68,250) | 4 or 5 Cups (N = 17,434) | ≥6 Cups (N = 5152) | P Value for Trend |
|---|---|---|---|---|---|---|---|---|
| All causes | ||||||||
| No. of deaths (%) | 18,784 | 2161 (10.4) | 3221 (10.7) | 3388 (10.8) | 7140 (10.5) | 2099 (12.0) | 775 (15.0) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.94–1.05) | 0.93 (0.88–0.98) | 0.93 (0.89–0.98) | 1.13 (1.07–1.20) | 1.51 (1.39–1.64) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.01 (0.96–1.07) | 0.95 (0.90–1.01) | 0.87 (0.83–0.92) | 0.84 (0.79–0.90) | 0.85 (0.78–0.93) | <0.001 | |
| Cancer | ||||||||
| No. of deaths (%) | 7,750 | 783 (3.8) | 1153 (3.8) | 1313 (4.2) | 3110 (4.6) | 1016 (5.8) | 375 (7.3) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.90–1.08) | 1.02 (0.93–1.11) | 1.14 (1.05–1.23) | 1.52 (1.38–1.67) | 2.00 (1.77–2.27) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.90–1.08) | 1.01 (0.92–1.10) | 0.98 (0.90–1.06) | 1.01 (0.92–1.12) | 1.03 (0.90–1.16) | 0.66 | |
| Heart disease | ||||||||
| No. of deaths (%) | 3,701 | 461 (2.2) | 673 (2.2) | 683 (2.2) | 1379 (2.0) | 378 (2.2) | 127 (2.5) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.96 (0.86–1.08) | 0.86 (0.76–0.96) | 0.83 (0.75–0.93) | 0.95 (0.83–1.09) | 1.16 (0.95–1.41) | 0.67 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.00 (0.89–1.13) | 0.91 (0.81–1.03) | 0.85 (0.76–0.95) | 0.78 (0.68–0.90) | 0.72 (0.59–0.88) | <0.001 | |
| Respiratory disease | ||||||||
| No. of deaths (%) | 1,791 | 187 (0.9) | 315 (1.0) | 279 (0.9) | 698 (1.0) | 208 (1.2) | 104 (2.0) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.11 (0.92–1.33) | 0.85 (0.71–1.03) | 1.03 (0.88–1.22) | 1.29 (1.06–1.57) | 2.35 (1.85–2.99) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.09 (0.91–1.31) | 0.84 (0.69–1.01) | 0.79 (0.67–0.93) | 0.65 (0.53–0.79) | 0.77 (0.61–0.99) | <0.001 | |
| Stroke | ||||||||
| No. of deaths (%) | 966 | 115 (0.6) | 191 (0.6) | 168 (0.5) | 369 (0.5) | 91 (0.5) | 32 (0.6) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.09 (0.87–1.38) | 0.84 (0.66–1.06) | 0.89 (0.72–1.10) | 0.92 (0.70–1.21) | 1.18 (0.79–1.74) | 0.82 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.15 (0.91–1.45) | 0.89 (0.70–1.13) | 0.93 (0.75–1.15) | 0.82 (0.62–1.09) | 0.84 (0.56–1.25) | 0.05 | |
| Injuries and accidents | ||||||||
| No. of deaths (%) | 462 | 57 (0.3) | 91 (0.3) | 114 (0.4) | 153 (0.2) | 36 (0.2) | 11 (0.2) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 1.07 (0.77–1.49) | 1.22 (0.88–1.67) | 0.77 (0.57–1.05) | 0.74 (0.49–1.12) | 0.81 (0.42–1.54) | 0.004 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.11 (0.80–1.55) | 1.27 (0.92–1.76) | 0.77 (0.56–1.06) | 0.64 (0.42–0.98) | 0.57 (0.29–1.10) | <0.001 | |
| Diabetes | ||||||||
| No. of deaths (%) | 446 | 71 (0.3) | 95 (0.3) | 93 (0.3) | 140 (0.2) | 38 (0.2) | 9 (0.2) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.90 (0.66–1.22) | 0.79 (0.58–1.08) | 0.56 (0.42–0.75) | 0.63 (0.42–0.93) | 0.53 (0.27–1.07) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.00 (0.73–1.36) | 0.91 (0.67–1.25) | 0.77 (0.57–1.03) | 0.82 (0.54–1.23) | 0.57 (0.28–1.16) | 0.03 | |
| Infections | ||||||||
| No. of deaths (%) | 448 | 65 (0.3) | 77 (0.3) | 96 (0.3) | 152 (0.2) | 37 (0.2) | 21 (0.4) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.79 (0.57–1.10) | 0.88 (0.64–1.20) | 0.66 (0.50–0.89) | 0.66 (0.44–0.99) | 1.36 (0.83–2.22) | 0.63 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 0.82 (0.59–1.15) | 0.93 (0.68–1.29) | 0.69 (0.51–0.94) | 0.60 (0.40–0.91) | 0.97 (0.58–1.61) | 0.13 | |
| Other causes | ||||||||
| No. of deaths (%) | 3,220 | 422 (2.0) | 626 (2.1) | 642 (2.0) | 1139 (1.7) | 295 (1.7) | 96 (1.9) | |
| Age-adjusted hazard ratio (95% CI) | 1.00 | 0.98 (0.87–1.11) | 0.89 (0.79–1.01) | 0.76 (0.68–0.85) | 0.81 (0.70–0.94) | 0.96 (0.77–1.20) | <0.001 | |
| Multivariate-adjusted hazard ratio (95% CI) | 1.00 | 1.02 (0.90–1.16) | 0.94 (0.83–1.07) | 0.79 (0.71–0.89) | 0.74 (0.64–0.87) | 0.72 (0.57–0.90) | <0.001 | |
CI denotes confidence interval. The numbers of deaths are for participants who died during follow-up. Multivariate analyses were adjusted for the following factors at baseline: age; body-mass index; race or ethnic group; level of education; alcohol consumption; the number of cigarettes smoked per day, use or nonuse of pipes or cigars, and time of smoking cessation (<1 year, 1 to <5 years, 5 to <10 years, or ≥10 years before baseline); health status; diabetes (yes vs. no); marital status; physical activity; total energy intake; consumption of fruits, vegetables, red meat, white meat, and saturated fat; use or nonuse of vitamin supplements; and use or nonuse of postmenopausal hormone therapy. In addition, risk estimates for death from cancer were adjusted for history of cancer (other than nonmelanoma skin cancer) in a first-degree relative (yes vs. no).
COFFEE CONSUMPTION AND CAUSE-SPECIFIC MORTALITY
Specific causes of death were also examined. After multivariate adjustment, coffee appeared to be inversely associated with most major causes of death in both men and women, including heart disease, respiratory disease, stroke, injuries and accidents, diabetes, and infections. In contrast, there was no significant association between coffee consumption and deaths from cancer in women. There was a borderline positive association in men: among 13,402 deaths from cancer, 880 deaths were reported among men who drank 6 or more cups of coffee per day (hazard ratio for the comparison with men who did not drink coffee, 1.08; 95% CI, 0.98 to 1.19; P = 0.02 for trend).
SUBGROUP ANALYSES
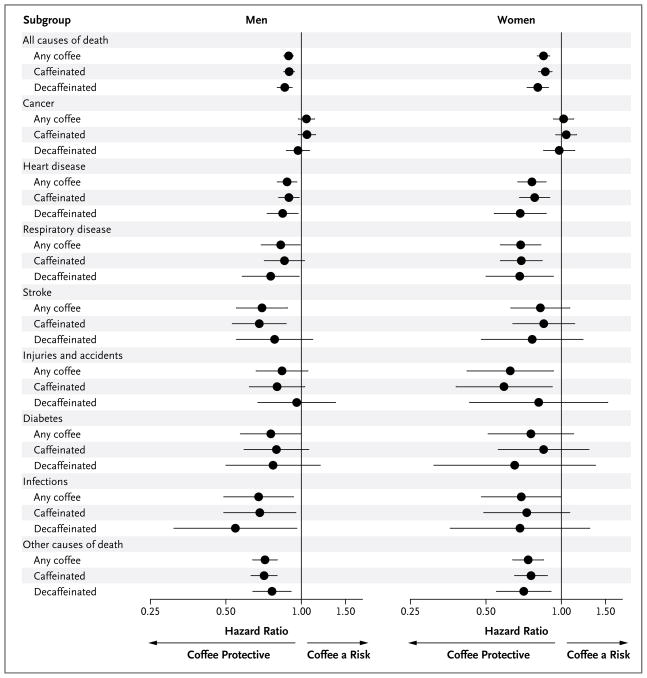
In analyses stratified according to the predominant type of coffee consumed (caffeinated or decaffeinated), the association of coffee drinking with total mortality and individual causes of death appeared to be similar for the two types of coffee (Fig. 1, and Tables 2 and 3 in the Supplementary Appendix).
Figure 1. Subgroup Analysis of Associations between the Consumption of 4 or More Cups of Coffee per Day and Total and Cause-Specific Mortality.
Hazard ratios for death from all causes and from specific causes are for the comparison of men and women who drank 4 or more cups of coffee per day with those who did not drink coffee. Participants were classified as drinking caffeinated or decaffeinated coffee according to whether they reported drinking caffeinated or decaffeinated coffee more than half the time. Risk estimates for other categories of coffee consumption are shown in Tables 2 and 3 in the Supplementary Appendix. Risk estimates were adjusted for the following factors at baseline: age; body-mass index; race or ethnic group; level of education; alcohol consumption; the number of cigarettes smoked per day, use or non-use of pipes or cigars, and time of smoking cessation (<1 year, 1 to <5 years, 5 to <10 years, or ≥10 years before baseline); health status; diabetes (yes vs. no); marital status; physical activity; total energy intake; consumption of fruits, vegetables, red meat, white meat, and saturated fat; and use or nonuse of vitamin supplements. In addition, risk estimates for death from cancer were adjusted for history of cancer (other than nonmelanoma skin cancer) in a first-degree relative (yes vs. no). In women, risk estimates were also adjusted for use or nonuse of postmenopausal hormone therapy. Horizontal lines represent 95% confidence intervals.
Coffee consumption was associated with a number of risk factors for death. Results of subgroup analyses are shown in Figure 2, with more detailed results shown in Tables 4 and 5 in the Supplementary Appendix. Associations between coffee consumption and mortality were generally similar across subgroups stratified according to duration of follow-up and the following baseline factors: age; diabetes (yes vs. no); BMI; alcohol consumption; high or low consumption of red meat, white meat, fruits, and vegetables; use or nonuse of any vitamin supplement; and, in women, use or nonuse of postmenopausal hormone therapy. The largest differences across strata were observed for cigarette-smoking status, with stronger inverse associations between coffee drinking and mortality among men and women who never smoked or were former smokers than among those who were current smokers (P<0.001 for interaction in men and P = 0.002 for interaction in women), and for self-reported health at baseline, with stronger associations among participants reporting good or very good to excellent health than among those reporting poor to fair health (P<0.001 for interaction in both men and women).
Figure 2. Subgroup Analysis of Associations between the Consumption of 4 or More Cups of Coffee per Day and Total Mortality.
Hazard ratios for death from any cause are for the comparison of men and women who drank 4 or more cups of coffee per day with those who did not drink coffee. The multivariate model was adjusted for the following factors at baseline: age; body-mass index (BMI; the weight in kilograms divided by the square of the height in meters); race or ethnic group; level of education; alcohol consumption; the number of cigarettes smoked per day, use or nonuse of pipes or cigars, and time of smoking cessation (<1 year, 1 to <5 years, 5 to <10 years, or ≥10 years before baseline); health status; diabetes (yes vs. no); marital status; physical activity; total energy intake; consumption of fruits, vegetables, red meat, white meat, and saturated fat; use or nonuse of vitamin supplements; and, in women, use or non-use of postmenopausal hormone therapy. Risk estimates for other categories of coffee consumption are shown in Tables 4 and 5 in the Supplementary Appendix. High and low dietary-intake categories are split at the median. Horizontal lines represent 95% confidence intervals. P values for interactions were computed with the use of likelihood-ratio tests comparing Cox proportional-hazards models with and without cross-product terms for each level of baseline stratifying variables, with coffee consumption as an ordinal variable. P values for the years of follow-up were derived from testing the addition of a cross-product term for follow-up with coffee consumption.
We further examined associations between coffee consumption and deaths from cancer and other causes according to smoking status (Tables 6 and 7 in the Supplementary Appendix). The results were similar for most outcomes across categories of smoking status, with the exception of death from heart disease, with associations that appeared to be null in current smokers (P = 0.002 for interaction in men and P = 0.05 for interaction in women). We also noted significant interactions between smoking and coffee consumption with respect to the overall risk of death from cancer; associations appeared to be modestly inverse for men and women who had never smoked, but not for those who were former or current smokers. However, associations between coffee consumption and death from cancer were not significant for any single category of coffee consumption.
DISCUSSION
In this large, prospective U.S. cohort study, we observed a dose-dependent inverse association between coffee drinking and total mortality, after adjusting for potential confounders (smoking status in particular). As compared with men who did not drink coffee, men who drank 6 or more cups of coffee per day had a 10% lower risk of death, whereas women in this category of consumption had a 15% lower risk. Similar associations were observed whether participants drank predominantly caffeinated or decaffeinated coffee. Inverse associations persisted among many subgroups, including participants who had never smoked and those who were former smokers and participants with a normal BMI and those with a high BMI. Associations were also similar for deaths that occurred in the categories of follow-up time examined (0 to <4 years, 4 to <9 years, and 9 to 14 years).
Our study was larger than prior studies, and the number of deaths (>52,000) was more than twice that in the largest previous study.22 Whereas the results of previous small studies have been inconsistent, our results are similar to those of several larger, more recent studies, including the Health Professionals Follow-up Study and the Nurses’ Health Study.21 In the Health Professionals Follow-up Study, the hazard ratio for death among men who drank 6 or more cups of coffee per day, as compared with men who drank less than 1 cup per month, was 0.80 (95% CI, 0.62 to 1.04). In the Nurses’ Health Study, the corresponding hazard ratio for women was 0.83 (95% CI, 0.73 to 0.95). In the Japan Collaborative Cohort Study for Evaluation of Cancer Risk,22 the hazard ratio for death among men who drank 4 or more cups of coffee per day, as compared with men who drank less than 1 cup per day, was 0.80 (95% CI, 0.68 to 0.95); the corresponding hazard ratio for women was 0.89 (95% CI, 0.66 to 1.20). In the Miyagi Cohort Study,15 the hazard ratio for death among men who drank 3 or more cups of coffee per day, as compared with men who never drank coffee, was 0.89 (95% CI, 0.74 to 1.08); the corresponding hazard ratio for women was 0.75 (95% CI, 0.53 to 1.05).
We noted inverse associations between coffee drinking and most major causes of death, with the exception of cancer. Associations between coffee drinking and the risk of death from heart disease have been particularly controversial, and several studies have suggested an increased risk among coffee drinkers.9,10 Nevertheless, the inverse associations observed in our study are consistent with those in a recent meta-analysis of this association. In that analysis, the relative risk of death among men in the highest category of coffee consumption, as compared with men in the lowest category of coffee consumption, was 0.89 (95% CI, 0.78 to 1.03).10 Although some previous studies showed differences in risk according to the interval between baseline and the date of death,9 we observed similar associations for deaths occurring early or late in follow-up.
Our results are concordant with previous studies showing inverse associations between coffee consumption and diabetes,4 stroke,16–18 and death due to inflammatory diseases.11 In addition, we observed an inverse association of coffee consumption with deaths from injuries and accidents. The mechanism of this association is unclear and could reflect chance or residual confounding, although similar results were reported in the Nurses’ Health Study and the Kaiser Permanente Multiphasic Health Checkup cohorts.19,20 In contrast to other outcomes, a modest borderline positive association was observed in men for coffee consumption and mortality from cancer, with a null association observed in women. Findings from previous studies were typically null.15,20–22
Several explanations for our findings are possible. As in all observational studies, associations could reflect confounding by unmeasured or poorly measured confounders. Although coffee consumption was inversely associated with diabetes, it was also positively associated with a number of behaviors that are considered unhealthy and are associated with an increased risk of death, such as tobacco smoking,35 consumption of red meat,36 and heavy alcohol use.37 Tobacco smoking was the strongest confounder in the multivariate analysis, and the inverse association between coffee consumption and mortality tended to be stronger among persons who had never smoked or were former smokers than among those who were current smokers, suggesting that residual confounding by smoking status, if present, attenuated the inverse associations between coffee drinking and mortality in our study.
Reverse causality is another possible explanation, since persons with chronic disease and poor health might abstain from coffee drinking. However, we excluded persons who had cancer or cardiovascular disease at baseline. Moreover, the results were similar when data from the first 4 or 9 years of follow-up were excluded, and associations were stronger among persons reporting good or very good to excellent health at baseline than among those reporting poor to fair health, arguing against this possibility.
Several limitations of our study should be noted. Coffee consumption was assessed by self-report at a single time point and may not reflect long-term patterns of consumption. The distinction between persons who drank caffeinated coffee and those who drank decaffeinated coffee was subject to misclassification, since these categories were defined on the basis of consumption of either beverage more than half the time. We lacked data on how coffee was prepared (espresso, boiled, or filtered), and the constituents of coffee may differ according to the method of preparation.
Given the observational nature of our study, it is not possible to conclude that the inverse relationship between coffee consumption and mortality reflects cause and effect. However, we can speculate about plausible mechanisms by which coffee consumption might have health benefits. Coffee contains more than 1000 compounds that might affect the risk of death. The most well-studied compound is caffeine, although similar associations for caffeinated and decaffeinated coffee in the current study and a previous study21 suggest that, if the relationship between coffee consumption and mortality were causal, other compounds in coffee (e.g., antioxidants, including polyphenols) might be important.1,38
In summary, this large prospective cohort study showed significant inverse associations of coffee consumption with deaths from all causes and specifically with deaths due to heart disease, respiratory disease, stroke, injuries and accidents, diabetes, and infections. Our results provide reassurance with respect to the concern that coffee drinking might adversely affect health.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
We thank the participants in this study for their outstanding cooperation.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect those of the cancer registries.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
This article is dedicated to the memory of Arthur Schatzkin, the visionary investigator who founded the NIH–AARP Diet and Health Study.
References
- 1.Gómez-Ruiz JA, Leake DS, Ames JM. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem. 2007;55:6962–9. doi: 10.1021/jf0710985. [Erratum, J Agric Food Chem 2007;55:8284.] [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–93. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 3.Arion WJ, Canfield WK, Ramos FC, et al. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997;339:315–22. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 4.Huxley R, Lee CM, Barzi F, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–63. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 5.Coffee drinking and acute myocardial infarction: report from the Boston Collaborative Drug Surveillance Program. Lancet. 1972;2:1278–81. [PubMed] [Google Scholar]
- 6.Jick H, Miettinen OS, Neff RK, Shapiro S, Heinonen OP, Slone D. Coffee and myocardial infarction. N Engl J Med. 1973;289:63–7. doi: 10.1056/NEJM197307122890203. [DOI] [PubMed] [Google Scholar]
- 7.Jee SH, He J, Appel LJ, et al. Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2001;153:353–62. doi: 10.1093/aje/153.4.353. [DOI] [PubMed] [Google Scholar]
- 8.Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23:921–8. doi: 10.1097/01.hjh.0000166828.94699.1d. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S. A meta-analysis of coffee, myocardial infarction, and coronary death. Epidemiology. 1993;4:366–74. doi: 10.1097/00001648-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Wu JN, Ho SC, Zhou C, et al. Coffee consumption and risk of coronary heart diseases: a meta-analysis of 21 prospective cohort studies. Int J Cardiol. 2009;137:216–25. doi: 10.1016/j.ijcard.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 11.Andersen LF, Jacobs DR, Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am J Clin Nutr. 2006;83:1039–46. doi: 10.1093/ajcn/83.5.1039. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg JA, Chow G, Ziegelstein RC. Caffeinated coffee consumption, cardiovascular disease, and heart valve disease in the elderly (from the Framingham Study) Am J Cardiol. 2008;102:1502–8. doi: 10.1016/j.amjcard.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Garcia E, van Dam RM, Willett WC, et al. Coffee consumption and coronary heart disease in men and women: a prospective cohort study. Circulation. 2006;113:2045–53. doi: 10.1161/CIRCULATIONAHA.105.598664. [DOI] [PubMed] [Google Scholar]
- 14.Mineharu Y, Koizumi A, Wada Y, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65:230–40. doi: 10.1136/jech.2009.097311. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama K, Kuriyama S, Akhter M, et al. Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in Japanese women. J Nutr. 2010;140:1007–13. doi: 10.3945/jn.109.109314. [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Männistö S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Coffee and tea consumption and risk of stroke subtypes in male smokers. Stroke. 2008;39:1681–7. doi: 10.1161/STROKEAHA.107.504183. [DOI] [PubMed] [Google Scholar]
- 17.Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke. 2011;42:908–12. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, Logroscino G, Hu FB, van Dam RM. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116–23. doi: 10.1161/CIRCULATIONAHA.108.826164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawachi I, Willett WC, Colditz GA, Stampfer MJ, Speizer FE. A prospective study of coffee drinking and suicide in women. Arch Intern Med. 1996;156:521–5. [PubMed] [Google Scholar]
- 20.Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993;3:375–81. doi: 10.1016/1047-2797(93)90064-b. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–14. doi: 10.7326/0003-4819-148-12-200806170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamakoshi A, Lin Y, Kawado M, Yagyu K, Kikuchi S, Iso H. Effect of coffee consumption on all-cause and total cancer mortality: findings from the JACC study. Eur J Epidemiol. 2011;26:285–93. doi: 10.1007/s10654-011-9548-7. [DOI] [PubMed] [Google Scholar]
- 23.Happonen P, Läärä E, Hiltunen L, Luukinen H. Coffee consumption and mortality in a 14-year follow-up of an elderly northern Finnish population. Br J Nutr. 2008;99:1354–61. doi: 10.1017/S0007114507871650. [DOI] [PubMed] [Google Scholar]
- 24.Heyden S, Tyroler HA, Heiss G, Hames CG, Bartel A. Coffee consumption and mortality: total mortality, stroke mortality, and coronary heart disease mortality. Arch Intern Med. 1978;138:1472–5. [PubMed] [Google Scholar]
- 25.Iwai N, Ohshiro H, Kurozawa Y, et al. Relationship between coffee and green tea consumption and all-cause mortality in a cohort of a rural Japanese population. J Epidemiol. 2002;12:191–8. doi: 10.2188/jea.12.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen BK, Bjelke E, Kvåle G, Heuch I. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst. 1986;76:823–31. [PubMed] [Google Scholar]
- 27.Kleemola P, Jousilahti P, Pietinen P, Vartiainen E, Tuomilehto J. Coffee consumption and the risk of coronary heart disease and death. Arch Intern Med. 2000;160:3393–400. doi: 10.1001/archinte.160.22.3393. [DOI] [PubMed] [Google Scholar]
- 28.LeGrady D, Dyer AR, Shekelle RB, et al. Coffee consumption and mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 1987;126:803–12. doi: 10.1093/oxfordjournals.aje.a114717. [DOI] [PubMed] [Google Scholar]
- 29.Lindsted KD, Kuzma JW, Anderson JL. Coffee consumption and cause-specific mortality: association with age at death and compression of mortality. J Clin Epidemiol. 1992;45:733–42. doi: 10.1016/0895-4356(92)90051-n. [DOI] [PubMed] [Google Scholar]
- 30.Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med. 2007;44:305–10. doi: 10.1016/j.ypmed.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward M, Tunstall-Pedoe H. Coffee and tea consumption in the Scottish Heart Health Study follow up: conflicting relations with coronary risk factors, coronary disease, and all cause mortality. J Epidemiol Community Health. 1999;53:481–7. doi: 10.1136/jech.53.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 33.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH–AARP (National Institutes of Health–American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11:183–95. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Oza S, Thun MJ, Henley SJ, Lopez AD, Ezzati M. How many deaths are attributable to smoking in the United States? Comparison of methods for estimating smoking-attributable mortality when smoking prevalence changes. Prev Med. 2011;52:428–33. doi: 10.1016/j.ypmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169:562–71. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaridze D, Brennan P, Boreham J, et al. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet. 2009;373:2201–14. doi: 10.1016/S0140-6736(09)61034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagimoto K, Lee KG, Ochi H, Shibamoto T. Antioxidative activity of heterocyclic compounds found in coffee volatiles produced by Maillard reaction. J Agric Food Chem. 2002;50:5480–4. doi: 10.1021/jf025616h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.