SUMMARY
Artemisia afra [Jacq] (Asteraceae) phytotherapy is widely used for its medicinal properties in traditional practices. In this study we investigated whether extracts of A. afra are capable of controlling mycobacterial replication. For Mycobacterium aurum cultured in the presence of aqueous-, methanol- and dichloromethane (DCM) extracts of A. afra we found that bacterial replication was inhibited by the dichloromethane extract only. Activity of the DCM extract was confirmed in dose-dependent studies against both M. aurum and M. tuberculosis with an IC50 = 270 μg/ml and IC50 = 290 μg/ml, respectively. Fractionation of the DCM extract and evaluation of its efficacy in vitro found that most of the antimycobacterial activity was associated with isolate fraction C8 that contained several sesquiterpene lactones, the most prominent of which are Artemin and Arsubin. Evaluation of the bactericidal efficacy in vitro showed that isolate fraction C8 reduced replication of M. aurum and M. tuberculosis in a dose-dependent manner with IC50 = 1.9 μg/ml and IC50 = 2.0 μg/ml, respectively, and an MIC = 10 μg/ml. Further, isolate fraction C8 and the DCM extract was administered to M. tuberculosis-infected mice at a tolerated dose of 1000 mg/kg for up to 26 weeks and mycobacterial burdens compared to untreated-, INH/RIF treated- and aqueous-extract-treated animals to assess its bactericidal activity in vivo. Bacterial replication remained unaffected during treatment with either isolate fraction C8 or the DCM extract resulting in pulmonary and splenic bacilli burdens comparable to that of untreated mice. In contrast, INH/RIF treatment cleared M. tuberculosis infection after only 8 weeks to undetectable levels. Interestingly, treatment of M. tuberculosis-infected mice with aqueous extract of A. afra regulated pulmonary inflammation during early infection notwithstanding its inability to inhibit mycobacterial growth. This study clearly demonstrates that A. afra contains in vitro anti-mycobacterial activity, modulates pulmonary inflammation in early mycobacterial infection, and that the mouse experimental tuberculosis model may serve as a useful assay for evaluating the utility of phytotherapy.
Keywords: Artemisia afra, Mycobacterium aurum, Mycobacterium tuberculosis, Plant extracts
1. Introduction
Tuberculosis remains a global health burden with >9 million new infections annually and a mortality of 1.5 million individuals1.The emergence of multidrug-resistant strains and the presence of HIV in high-incidence populations contribute significantly to sustaining the problem. The standard treatment advocated by the WHO for active tuberculosis remains a therapeutic approach that includes long-term therapy which incorporates the use of isoniazid, rifampicin, pyrazinimide and ethambutol as frontline drugs. However inherent deficiencies in this approach include the potential for the development of multidrug resistance due to non-compliance as a consequence of the long duration of treatment and the lack of similarly efficient therapeutics with few known side effects should treatment be unsuccessful. The escalation of HIV/AIDS and tuberculosis co-infections requires compatibility between tuberculosis and antiretroviral therapies which limits the capacity to treat patients. All current therapeutic strategies target active tuberculosis and do not address latent tuberculosis infection. Thus, there is a need to identify and develop new drugs that will improve on the duration of treatment, enhance safety, improve compatibility with other drug therapies and have the potential to target latent tuberculosis.
Natural products contribute significantly as a source for the derivation of lead compounds and development of drugs that are introduced into the market. Traditional knowledge applications and its use of plant extracts in medicinal practices provide an excellent data base for potential identification of sources that may yield lead compounds with bioactive properties. Although several South African medicinal plants can actively inhibit microbial replication2, recently McGaw et al. (2008) highlighted the applications of these in the treatment of tuberculosis symptoms and concluded that many of these display anti-mycobacterial activity3. Artemisia afra [Jacq] (Asteraceae), also referred to as Africa wormwood through its resemblance to A. vulgaris (English wormwood), is commonly found in most parts of Southern Africa and is used widely in traditional medicine practices against respiratory ailments including fever, colds, coughs, bronchitis, asthma and chest complaints4.
Furthermore, its traditional use as an anti-infectious therapy has found scientific support in studies of its anti-pathogenic qualities and it was shown to have antimicrobial5,6 as well as antifungal7 properties. The essential oil of A. afra which contains 1.8 cineole, thujone, camphor and borneol specifically inhibited growth of Aspergillus ochraceus, A. niger, A. parasiticus, Candida albicans, Alternaria alternata and Geotrichum candidum7.
We have previously investigated the safety of the aqueous extract and reported on its behavioural and pharmaco-toxicological effects after acute and chronic administration in mice and rats, respectively8. In this study we investigated the inhibitory potential of A. afra extracts against M. tuberculosis. We report here on its anti-mycobacterial properties on both M. aurum and M. tuberculosis in culture and evaluation of efficacy in an M. tuberculosis aerosol inhalation murine challenge model that utilizes the active fractions incorporated into solid animal feed.
2. Materials and methods
2.1. Plants
Artemisia afra [Jacq] (Asteraceae) was obtained from Montague Garden (Western Cape, South Africa) and authenticated as correct by the Department of Botany, University of the Western Cape. Leaves were separated from stalks, washed in distilled water and dried at 30°C. Plant material was stored at room temperature. An exemplar was vouchered (specimen number 6639) and deposited in the herbarium at the University of the Western Cape.
2.2. Plant extracts and formulated feed preparation, storage and analysis
Preparation of aqueous extract
Dried leaves (200 g) were suspended in distilled water (4 litres) and the mixture was boiled for 30 minutes. The preparation obtained, was left to cool before it was filtered and the filtrate freeze-dried. The final powder obtained was weighed, put into stoppered brown bottle and then sent to HEPRO Cape Gamma, a facility providing sterilization by gamma irradiation (radiation conditions: mass: 0.5; dose kGy: 18; time: 2 h). The sterilized extract was stored at room temperature in a dark cupboard until required [yield, 39.9 g (20%)].
Preparation of crude DCM extract
Plant material (1 kg) was extracted in 10 L of dichloromethane (DCM) at room temperature. The mixture was stirred vigorously at room temperature for 3 hours using an Overhead stirrer and then allowed to stand overnight. The extract (extract 1) was recovered by filtration, followed by removal of solvent using rotary evaporation. The plant material was re-extracted two more times with 5 L of DCM each time and all three extracts were finally combined [yield, 147 g (15%)].
Fractionation of the DCM extract
The DCM extracts of Artemisia afra were fractionated to isolate the active compounds. The initial fractionation of A. afra DCM extract was done by column chromatography. The active crude DCM extract (96 g) was chromatographed over a silica gel 60–120 mm Merck (Germany) column (80 cm × 6 cm) eluted with a gradient of ethyl acetate in n-hexane v/v (i.e. 0; 100; 100; 100; 500; 500; 750; 1000) followed by methanol in ethyl acetate v/v (0; 100; 500; 500; 1000). Fractions were pooled or separated based on thin-layer chromatography analyses. The first fractionation step yielded 12 isolates designated C1–C12, with the second step yielding 9 fractions (which were evaluated against M. aurum), within which fraction C8 containing various compounds, was the most active. High-resolution impact mass spectrometry of isolate C8 was performed on a VG-70 SEQ mass spectrometer operating at 70 eV, whilst nuclear magnetic resonance spectra of C8 were recorded on a Varian 300 Mhz (VXR 300).
Preparation of animal diet
Phytotherapy was administered to animals as a feed prepared in batches of 1000 g using a formulated food diet, AIN 93G purified rodent diet obtained from Dyets Incorporation, to provide a plant extract dosage of 1 g/kg/day assuming an average weight of 22 g/mouse and a daily food consumption of 2 g. The calculated dose of aqueous extract was weighed and dissolved in 500 ml of boiling distilled water; for the DCM extract, the material was first dissolved in Tween 60 then mixed with boiling water, for a final concentration of 0.8% Tween 60. 500 g of formulated feed was weighed into a beaker and the extract in boiling water mixed with the finely powdered food and stirred to form a homogeneous paste. The paste was pelleted into small pieces of 2×1.5 mm size, and the pellets were dried at 30°C for three days and sent to HEPRO Cape Gamma for sterilisation by gamma irradiation. All extracts and the sterilised diets were stored at −20°C until required for use.
2.3. Assessment of mycobacterial growth in vitro
Approximately 500 ml of preheated buffer was added to 50 ml of mycobacterial culture and heat-killed over 5 minutes in boiling water. 100 ml of the heat-killed cultures were used to assay for ATP using luciferin/luciferase reagent in a firefly bioluminescence assay or via optical densitometry at 600 nm.
2.4. Mice
C57Bl/6 were bred and maintained under specific pathogen-free conditions at the University of Cape Town. Female mice, between the ages of 8–12 weeks, were used in experiments which were approved by the Research Ethics Committee of the University of Cape Town and the University of Missouri-Columbia.
2.5. Mycobacterium tuberculosis infection and phytotherapy of animals
Mycobacterium tuberculosis H37Rv (M. tuberculosis) was grown in 10% OADC enriched Difco Middlebrook 7H9 medium containing 0.5% glycerol and incubated at 37°C and grown until log phase. A frozen mycobacterial aliquot was rapidly thawed at 37°C and passed 30× through a 29.5 G needle prior to use. Aerosol inhalation infection was performed in a Biosafety Level-3 laboratory using a Glas-Col Inhalation Exposure System Model A4224.
The pulmonary infection dose was confirmed in 10 mice 18–24 hours after infection. Infected animals received a feed formulation that delivered approximately 1000 mg/kg of either aqueous, dichloromethane or isolate fraction C8 extract of A. afra for the duration of the experiment.
2.6. CFU determination
Mice were sacrificed at the indicated time points, organs removed and homogenized in PBS/0.04% Tween 80. Homogenized tissue were plated on 10% OADC enriched Difco Middlebrook 7H10 agar plates in 10-fold serial dilutions. Plates were semi-sealed in plastic bags and incubated for 17–21 days at 37°C, after which the number of mycobacterial colonies were counted and the infection dose calculated.
2.7. Pulmonary cytokine quantification
Whole lungs were homogenized in 1 ml PBS containing a protease inhibitor (Sigma), centrifuged and the supernatants aliquoted and stored at −80°C until further analysis. Cytokine concentrations were measured by sandwich ELISA using antibodies purchased from R&D Systems Inc, Minneapolis, MN according to the instructions of the manufacturers.
2.8. Statistics
The data are expressed as the mean±SD. Statistical analysis was performed by ANOVA or Student’s t-test. For all tests, a p value of <0.05 was considered significant.
3. Results
3.1. Dichloromethane extract of A. afra inhibits growth of M. aurum and M. tuberculosis H37Rv in vitro
We first asked whether extracts of A. afra displayed inhibitory activity on direct exposure to mycobacteria. Here we used M. aurum as a surrogate for M. tuberculosis to test the efficacy of dichloromethane-, methanol- and water extracts of A. afra at a concentration 200 μg/ml over 48 hours (Table 1). Exposure to isoniazid (20 μg/ml) inhibited growth of M. aurum >95% compared to untreated cultures. We found that both methanol- and water extracts of A. afra displayed no inhibitory activity of mycobacterial growth. Importantly, however, exposure to dichloromethane extract significantly inhibited M. aurum growth with 41.4% of bacteria killed. In view of the antimicrobial activity displayed by the dichloromethane A. afra extract we investigated the dose-dependent killing effect of the extract exposing M. aurum to a concentration range of 62.5–1000 μg/ml over 48 h. We found that culture of M. aurum at increasing concentrations of dichloromethane A. afra extract resulted in increased killing with sterility being achieved at 500 μg/ml (Fig. 1). Further analysis showed that DCM extract had an IC50 = 270 μg/ml against M. aurum (Fig. 2). Considering the inhibitory effects of dichloromethane extracts of A. afra against M. aurum, we next asked whether similar activity can be measured against M. tuberculosis. We therefore cultured M. tuberculosis in the presence of variable concentrations of dichloromethane extract of A. afra and monitored mycobacterial growth over a period of 7 days. We found that dichloromethane extracts of A. afra reduced M. tuberculosis growth in a concentration-dependent manner with an IC50 = 290 μg/ml, similarly to the inhibitory effects noted against M. aurum (Fig. 1).
Table 1.
Inhibition of M. aurum replication
| Treatment | M. aurum growth inhibition |
|---|---|
| Untreated control | 0% |
| Isoniazid (20 μg/ml) | >95% |
| Aqueous extract of A. afra | <25% |
| Methanol extract of A. afra | <25% |
| Dichloromethane extract of A. afra | 41.4% |
Extracts of A. afra (200 μg/ml) were incubated with M. aurum and its growth measured by firefly lucifrase bioluminescence ATP assay after 48 hours. Inhibition is expressed as a percentage of untreated control cultures. Activity was arbitrary assigned for extracts that displayed ≥25% inhibition
Fig. 1.
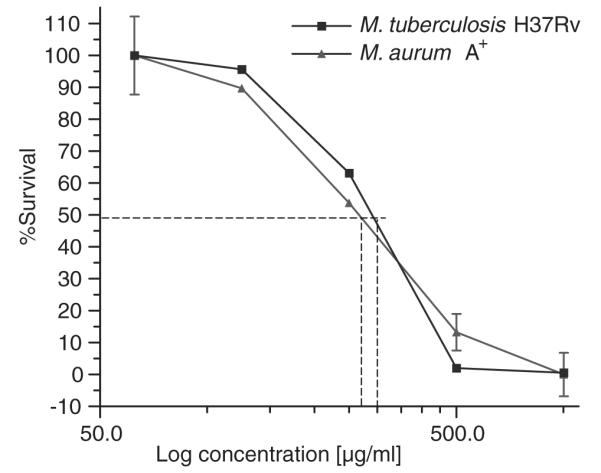
Dose-dependent inhibition of M. aurum and M. tuberculosis by the DCM extract of A. afra in vitro. M. tuberculosis and M. aurum were cultured in the presence of different concentrations of dichloromethane extract of A. afra. Mycobacterial growth was assessed by firefly luciferase bioluminescence ATP assay after 7 days in culture for M. tuberculosis and 48 hours for M. aurum. DCM-extract inhibitory activity had an IC50 = 270 μg/ml for M. aurum and an IC50 = 290 μg/ml for M. tuberculosis. The results represent the mean ±SD of three assays.
Fig. 2.
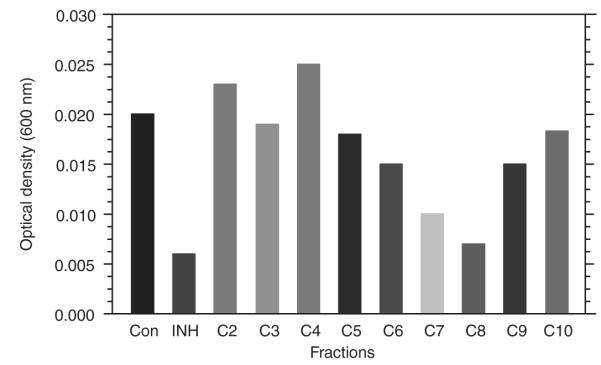
Maximal inhibitory activity is associated with isolate fraction C8. Nine isolate fractions of the DCM extract were generated and tested for activity against M. aurum at a concentration of 5 μg/ml. Mycobacterial growth was measured by optical density at 600 nm. Inhibitory activity was maximum during incubation with isolate fraction C8.
We therefore demonstrated that extracts of A. afra using dichloromethane but not methanol or water yielded compounds with significant antimicrobial activity capable of inhibiting replication of M. tuberculosis and M. aurum.
3.2. Anti-mycobacterial activity is associated with isolate fraction C8 of A. afra
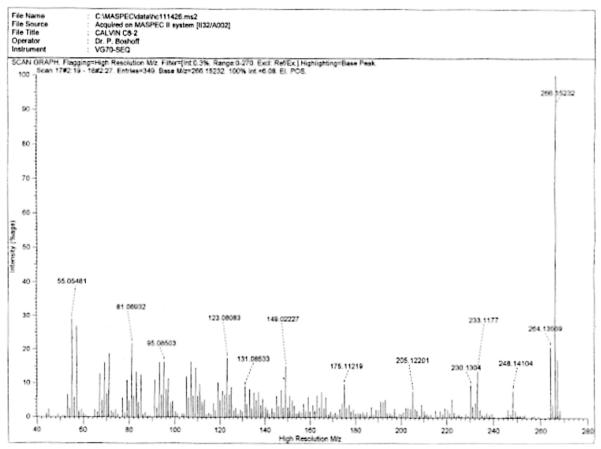
To characterise the anti-mycobacterial properties of A. afra, the dichloromethane extracts were further fractionated yielding 12isolate fractions. Nine of these fractions, labelled C2–C10, were tested for antimicrobial activity against M. aurum at 5 μg/ml in culture assays and bacterial growth determined after 48 h (Fig. 2). Isolates C2, C3, C4, C5 and C10 displayed limited, if any, anti-mycobacterial activity whereas isolate C6 and C9 partially inhibited growth by 20–25%. Most of the anti-mycobacterial effects resided in isolate fractions C7 and C8 where 60–70% inhibition of mycobacterial growth was noted. However, further analysis of isolate fraction C7 found that it was contaminated with material of the C8 isolate fraction (data not shown) which may have contributed to the activity observed in isolate C7. We therefore further investigated the activity of isolate fraction C8 by culturing M. tuberculosis and M. aurum in different concentrations of isolate fraction C8. We observed similar dose-dependent bactericidal activities for both M. tuberculosis and M. aurum with an IC50 = 2.0 μg/ml noted for M. tuberculosis and an IC50 = 1.9 μg/ml for M. aurum. 100% inhibition of mycobacterial replication occurred at 10 μg/ml (Fig. 3). The mass spectrometry and nuclear magnetic resonance analyses of fraction C8 indicated the presence of sesquiterpene lactones, the most prominent of which are Artemin and Arsubin (Fig. 4).
Fig. 3.
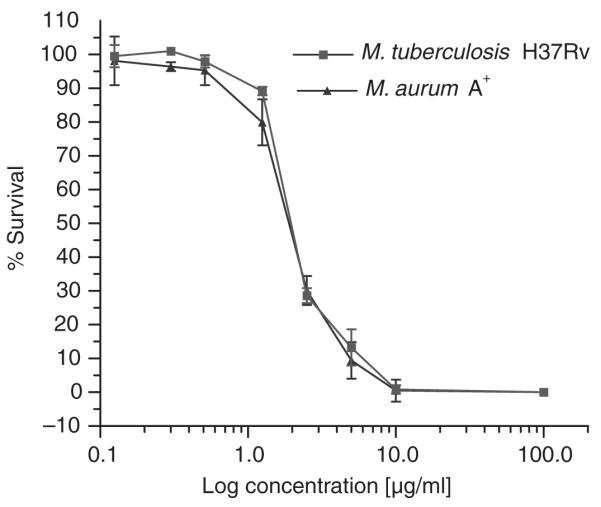
Dose-dependent inhibition of M. aurum and M. tuberculosis by isolate fraction C8 in vitro. M. tuberculosis and M. aurum were cultured in the presence of different concentrations of isolate fraction C8. Mycobacterial growth was assessed by firefly luciferase bioluminescence ATP assay after 7 days in culture for M. tuberculosis and 48 hours for M. aurum. Similar inhibitory activities were found for M. aurum and M. tuberculosis with an IC50 = 1.9 μg/ml and an IC50 = 2.0 μg/ml, respectively. The results represent the mean ±SD of three assays.
Fig. 4.
High-resolution electron impact mass spectrometry of isolate fraction C8 showed two molecular ions at m/z 266.15232 and m/z 264.13569. The calculated values of the ions were 266.15181 m/z and 264.13616 m/z The data further suggest the molecular formulae of the two compounds to be C15H22O4 and C15H20O4, respectively. 1H-NMR spectra suggests that these compounds are Artemin and Arsubin.
4. In vivo activity of isolate fraction C8, dichloromethane extract and aqueous extract of A. afra against M. tuberculosis
To evaluate the efficacy of isolate fraction C8 against M. tuberculosis in vivo we incorporated the respective A. afra extracts into a formulated feed that allowed an approximate tolerated daily administration dose of 1000 mg/kg of the specific fraction.
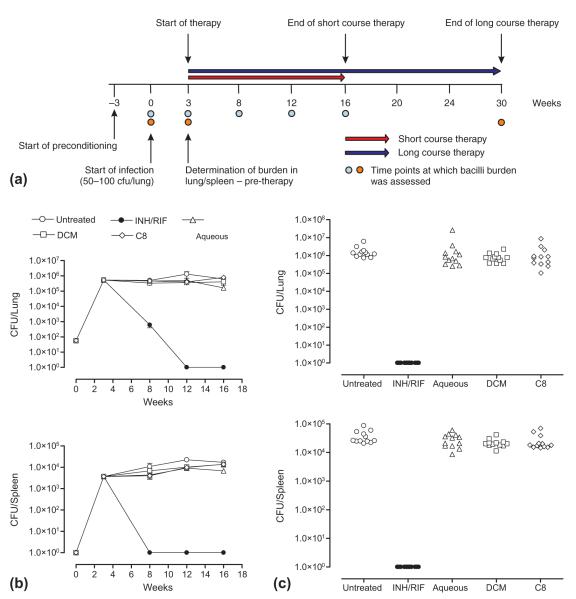
We assessed in vivo efficacy of the dichloromethane extract in parallel to activity of the isolate fraction C8 in view of its antimicrobial activity observed in vitro and included the water extract of A. afra as a control. The scheme for infection and therapy is shown in Fig. 5a. Briefly, mice preconditioned on formulated feed for 3 weeks were infected with M. tuberculosis at a dose of 50–100 cfu/lung. After 3 weeks, when the pulmonary bacilli burden reached approximately 1×106 cfu/lung, therapy was initiated and continued for either 12 weeks as a short therapeutic course or 20 weeks as a long therapeutic course. We found that INH/RIF treatment applied for 5 weeks reduced pulmonary bacilli burdens by 3 log10 and further treatment for 4 weeks rendered bacilli-undetectable by culture (Fig. 5b). In the spleen, bacilli growth decreased by >3 log10 to undetectable levels after only 5 weeks treatment. In contrast, pulmonary and splenic bacilli burdens of untreated mice remained unchanged for the duration of the experiment.
Fig. 5.
Isolate fraction C8 and DCM extract of A. afra are inactive against M. tuberculosis in vivo. (a) Infection and therapy design for efficacy evaluation of A. afra extracts against M. tuberculosis in vivo. (b) Pulmonary and splenic bacilli burdens of M. tuberculosis-infected mice measured after 13 weeks treatment with isolate fraction C8 (1000 mg/kg), DCM extract (1000 mg/kg), aqueous extract (1000 mg/kg) and INH/RIF (25 mg/kg). (c) Pulmonary and splenic bacilli burdens of M. tuberculosis-infected mice measured after 26 weeks treatment with isolate fraction C8 (1000 mg/kg), DCM extract (1000 mg/kg), aqueous extract (1000 mg/kg) and INH/RIF (25 mg/kg).
Assessment of pulmonary and splenic bacilli levels after application of the respective A. afra extracts for 13 weeks showed no significant differences between the untreated animals or any of the animal groups that received the isolate fraction C8, the dichloromethane extract or the water extract of A. afra. We next asked whether increasing the duration will improve the outcome of phytotherapy. In this study, M. tuberculosis-infected animals were subjected to treatment for 26 weeks after which pulmonary and splenic bacilli burdens were assessed. INH/RIF treatment rendered infected animals sterile in contrast to untreated animals that had an approximate pulmonary bacilli burden of 1 106 cfu/lung and a splenic burden in excess of 1×104 cfu/lung (Fig. 5c). However, we found that increasing the duration of treatment with either the isolate fraction C8 or the dichloromethane extract of A. afra did not improve the outcome of disease. We found no significant difference in pulmonary or splenic bacilli burdens between untreated infected animals or animals treated with either the isolate fraction C8 or the dichloromethane extract of A. afra.
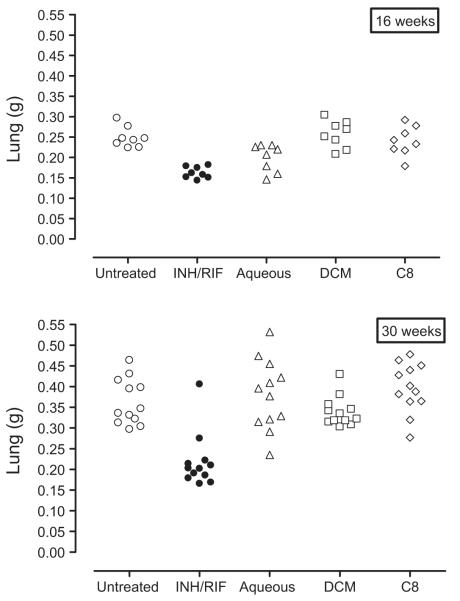
Further, we measured lung and spleen weight as a surrogate of inflammation during infection. It is well established that a controlled, inflammatory immune response leading to the establishment of properly defined bactericidal granulomas is critical for effective control of bacilli replication. However, an inability of the host to eliminate mycobacteria may lead to persistent infection and a chronic, uncontrolled inflammatory response resulting in detrimental pathology. We found lung weights increased significantly in M. tuberculosis-infected animals that did not receive therapy. In contrast, lung weights of M. tuberculosis-infected animals treated with INH/RIF for 16 weeks were similar to that of naive mice and were significantly less compared to untreated M. tuberculosis-infected animals. Furthermore, we found that the lung weights of M. tuberculosis-infected animals treated with either the isolate fraction C8 or the dichloromethane extract of A. afra were similar to that of untreated M. tuberculosis-infected animals but significantly higher than M. tuberculosis-infected animals receiving INH/RIF therapy. Surprisingly however, lung weights of M. tuberculosis-infected animals treated with the aqueous extract of A. afra for 16 weeks were similar to those of M. tuberculosis-infected animals which received INH/RIF treatment, and significantly lower compared to untreated animals or groups treated with isolate fraction C8 and the dichloromethane extract of A. afra (Fig. 6). This suggested that treatment with the aqueous extract of A. afra regulated inflammation during host immune responses.
Fig. 6.
Aqueous extract of A. afra is anti-inflammatory during pulmonary M. tuberculosis infection. Lung weights of mice treated with isolate fraction C8 (1000 mg/kg), DCM extract (1000 mg/kg), aqueous extract (1000 mg/kg) and INH/RIF (25 mg/kg) were measured at 16 weeks and 30 weeks post infection. Significant reduction of lung weights in infected mice treated with aqueous extract was noted at 16 weeks post infection. p < 0.05.
However, the regulatory effect appeared transient as prolonged therapy of 26 weeks showed no significant differences in the lung weights of animals treated with the aqueous extract of A. afra or the untreated infected animals, animals treated with isolate fraction C8 or animals which received the dichloromethane extract of A. afra.
Our findings therefore demonstrate that isolate fraction C8 and dichloromethane extracts of A. afra are inactive against M. tuberculosis in vivo and fail to reduce the bacilli burden. We found however that treatment with the aqueous extract of A. afra may regulate pulmonary inflammation during early M. tuberculosis infection but that the effect may be transient.
4.1. Long-term therapy of M. tuberculosis-infected mice with isolate fraction C8 inhibits IL-12p40 synthesis
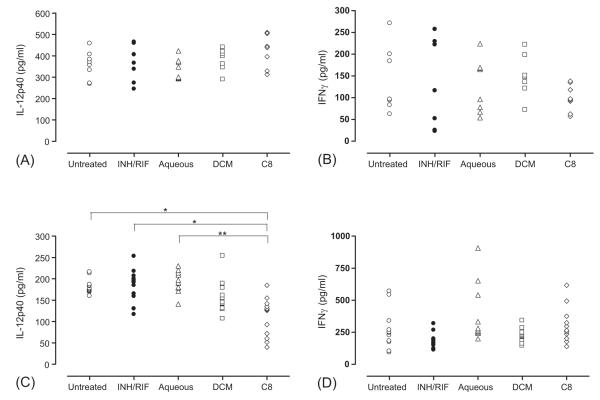
The absence of bactericidal activity against M. tuberculosis in vivo excluded the potential of the fractions to have direct inhibitory effects on bacterial replication but we postulated that they still may have had an impact on host immune regulation. We therefore measured levels of IL-12p40 and IFNg in lung homogenates (Fig. 7) during short-term therapy (2 months post infection) and long-term therapy (6 months post infection). Comparative levels of IL-12p40 or IFNg were equivalent at 2 months post infection in treated or untreated animals. Surprisingly however, treatment of animals with the DCM fraction showed reduced levels of IL-12p40 compared to either untreated, INH/RIF-treated or aqueous-fraction-treated animals and significantly so (p < 0.05) when treated with isolate fraction C8. This suggested that chronic treatment with isolate fraction C8 can potentially modulate IL-12 synthesis in M. tuberculosis-infected animals without affecting outcome of disease. Analysis of IFNg synthesis in long-term treated and untreated M. tuberculosis-infected animals showed no significant differences between any of the groups.
Fig. 7.
Pulmonary IL-12p40 and IFNg synthesis during short- and long-term phytotherapy of M. tuberculosis-infected mice. Lung homogenates of M. tuberculosis-infected treated and untreated mice were analysed for IL-12p40 (A, C) or IFNg (B, D) after either 2 months (A, B) or 6 months (C, D). The data represent 7–12 mice per group. *p < 0.01; **p < 0.001.
5. Discussion
The genus Artemisia is globally exploited for its use in medicinal applications. A. afra [JacQ] (Asteraceae) is a species indigenous to Southern- and East Africa, and widely used for traditional purposes including treatment for respiratory ailments4. In this study we investigated the anti-mycobacterial properties of A. afra [JacQ] (Asteraceae) against M. tuberculosis in culture and assessed its therapeutic potential in a murine model during aerosol inhalation challenge.
Various species within the genus Artemisia display antimicrobial activity, capable of inhibiting the growth of a large range of bacterial species9–11. Compound derivatives of natural products which display inhibitory activity specifically against M. tuberculosis or related organisms have extensively been reviewed12. Such compounds include alkenes, alkynes, peptides, alkaloids, terrenes and steroids. Clearly evident is the lack of compounds derived from Artemisia indicating that the genus remains unexploited as a source of natural product derivatives targeting different mycobacterial species. Here we initially examined the anti-mycobacterial activities of aqueous-, methanol- and dichloromethane extracts of the species A. afra against M. aurum. We found that bioactivity resided in the dichloromethane extract, and that the methanol- and aqueous extract were non-inhibitory. Interestingly, aqueous extracts of 22 plants used in Mexican traditional medicine for respiratory ailments similarly did not show any bioactivity in mycobacterial growth assays, whereas hexane and methanol extracts inhibited M. tuberculosis and M. avium13. Further, from 21 plant extracts used for traditional medicines in South Africa, most of the antibacterial activity was associated with methanol- and not aqueous extracts14. This would suggest that such active ingredients would not be extracted in traditional practices where application is usually associated with boiling of plant material in water.
In our studies, activity of the dichloromethane extract against M. aurum was confirmed in dose-dependent studies and, in addition, showed that the extract had similar dose-dependent activity against M. tuberculosis. Our findings contrast with that of a recent study by Mativandlela et al. (2008) which found that A. afra extract inhibited M. smegmatis but failed to inhibit M. tuberculosis15. Our findings are highly significant in view of other studies which reported that antimicrobial activity of natural products are largely targeted towards Gram-positive rather than Gram-negative bacteria due to impermeability associated with the cell wall. M. tuberculosis, although weakly Gram-negative, has a highly complex cell-wall structure that excludes most hydrophobic compounds and is uniquely adapted for survival of the pathogen. Further fractionation of the dichloromethane extract yielded isolate fraction C8 which had an improved IC50 concentration of approximately 100× against both M. aurum and M. tuberculosis.
According to our knowledge, in vivo antimicrobial efficacy studies of A. afra reported here are novel for any species of A. afra. Previously we found that oral administration of the aqueous extract of A. afra was non-toxic to rodents when administered acutely and had low chronic toxicity potential8. We similarly established that administration of the dichloromethane- and isolate fraction C8 extract was non-toxic and was tolerated at an oral dose of 1000 mg/kg over a 3-month period (unpublished). Here we found that isolate fraction C8 and the dichloromethane extract administered in feed at a dose of 1000 mg/kg to infected mice was inactive against M. tuberculosis unlike INH/RIF treatment which effectively cleared bacilli burden. The failure to observe in vivo efficacy may have been attributable to several factors including serum concentration, modification post absorption or failure to reach the target organs. Alternative mechanisms of delivery of these compounds may enhance in vivo efficacy such as delivery via an aerosol route to animals post-exposure and will be addressed in future studies.
An unexpected finding was the anti-inflammatory potential associated with the aqueous extract of A. afra. Here we found that pulmonary inflammation was significantly reduced but that it was associated with high pulmonary bacilli burdens. Resolution of pathology and reduced inflammation is usually associated with clearance of infection as evident in mice treated with INH/RIF. In this study inflammation treatment with aqueous extract was as efficient in regulating inflammation as treatment with INH/RIF. Anti-inflammatory activity associated with different species of Artemisia has previously been described. Interestingly, Hong et al. (2004) showed that aqueous extracts from A. capillaris Thunb. reduced LPS-mediated inflammatory responses in rat liver and human hepatocarcinoma cells16. Thus, here we provide the first evidence of in vivo anti-inflammatory effects of an Artemisia species associated with M. tuberculosis infection. Further, the immunomodulatory potential of isolate fraction C8 on IL-12p40 synthesis during long-term therapy was surprising and unexpected. The reduction in IL-12p40 levels in this group, however, did not correlate with a higher bacilli burden in view of evidence which showed that IL-12 is essential for protection against acute M. tuberculosis infection and is a requirement to maintain latency during persistent infection17–19. Although reduced it appears that IL-12 levels present in animals treated with isolate C8 during chronic infection were sufficient to inhibit reactivation.
The model presented here is novel in its application of phytotherapy to M. tuberculosis-infected animals. Here we administered phytotherapeutics as an integral component of a formulated feed, providing several advantages and bypassing several difficulties generally associated with phytotherapy applications. It is clear that a formulated feed incorporating isoniazid/rifampicin applied for 7 weeks reduces pulmonary bacilli burden by 6 log10 to undetectable levels. Although phytotherapies administered in drinking water of mice can successfully be used, the application is limited by the water solubility of the plant extracts rendering many phytotherapies, known to have significant water-insoluble active components, unable to be administered in this manner.
The alternative approach of dosing animals by oral gavage is widely used and proven to be effective with the advantage of applying a known dose to each animal. However the procedure becomes labour intensive and cumbersome; requiring daily administration for larger animal studies with significant cost implications. Further, the procedure is invasive and daily handling results in animal stress. The model presented here eliminates all of these disadvantages and can be applied successfully for most hydrophobic phytotherapies.
In summary, we have successfully demonstrated that a dichloromethane extract of A. afra contains anti-mycobacterial activity which can inhibit both rapid growing M. aurum and virulent M. tuberculosis replication. This activity however was not evident during extended preclinical evaluation. Nonetheless this study has shown that A. afra is a viable source for identifying antimicrobial compounds and contains anti-inflammatory substances that may potentially be useful for clinical application. Further, we describe a new application model where water-insoluble phytochemicals can be applied successfully in therapy.
Acknowledgements
This project was supported by Grant U19AT003264 from the National Center For Complementary & Alternative Medicine and the Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Complementary & Alternative Medicine, the National Institutes of Health or the Fogarty International Center. We gratefully acknowledge the support of many in the International Center for Indigenous Phytotherapy Studies, at the University of Cape Town, the University of the Western Cape and the University of Missouri-Columbia.
Footnotes
Competing interests: The authors declare no financial conflict of interest.
References
- 1.WHO Report 2008: Global tuberculosis control – surveillance, planning, financing.
- 2.van Vuuren SF. Antimicrobial activity of South African medicinal plants. J Ethnopharmacol. 2008;119:462–72. doi: 10.1016/j.jep.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 3.McGaw LJ, Lall N, Meyer JJ, Eloff JN. The potential of South African plants against Mycobacterium infections. J Ethnopharmacol. 2008;119:482–500. doi: 10.1016/j.jep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 4.van Wyk BE. A broad review of commercially important Southern African medicinal plants. J Ethnopharmacol. 2008;119:342–55. doi: 10.1016/j.jep.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Graven EDS, Mavi S, Gundidza MG, Svoboda KP. Antimicrobial and antioxidative properties of the volatile (essential) oil of Artemisia afra Jacq. Flavour Fragrance J. 1992;7:121–3. [Google Scholar]
- 6.Mangena T, Muyima NY. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected bacteria and yeast strains. Lett Appl Microbiol. 1999;28:291–6. doi: 10.1046/j.1365-2672.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 7.Gundidza M. Antifungal activity of essential oil from Artemisia afra Jacq. Cent Afr J Med. 1993;39:140–2. [PubMed] [Google Scholar]
- 8.Mukinda JT, Syce JA. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J Ethnopharmacol. 2007;112:138–44. doi: 10.1016/j.jep.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Benli M, Kaya I, Yigit N. Screening antimicrobial activity of various extracts of Artemisia dracunculus L. Cell Biochem Funct. 2007;25:681–6. doi: 10.1002/cbf.1373. [DOI] [PubMed] [Google Scholar]
- 10.Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732–8. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Tan RX, Zheng WF, Tang HQ. Biologically active substances from the genus Artemisia. Planta Med. 1998;64:295–302. doi: 10.1055/s-2006-957438. [DOI] [PubMed] [Google Scholar]
- 12.Copp BR, Pearce AN. Natural product growth inhibitors of Mycobacterium tuberculosis. Nat Prod Rep. 2007;24:278–97. doi: 10.1039/b513520f. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Arellanes A, Meckes M, Ramirez R, Torres J, Luna-Herrera J. Activity against multidrug-resistant Mycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytother Res. 2003;17:903–8. doi: 10.1002/ptr.1377. [DOI] [PubMed] [Google Scholar]
- 14.Rabe T, van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol. 1997;56:81–7. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 15.Mativandlela SP, Meyer JJ, Hussein AA, Houghton PJ, Hamilton CJ, Lall N. Activity against Mycobacterium smegmatis and M. tuberculosis by extract of South African medicinal plants. Phytother Res. 2008;22:841–5. doi: 10.1002/ptr.2378. [DOI] [PubMed] [Google Scholar]
- 16.Hong SH, Seo SH, Lee JH, Choi BT. The aqueous extract from Artemisia capillaris Thunb. inhibits lipopolysaccharide-induced inflammatory response through preventing NF-kappaB activation in human hepatoma cell line and rat liver. Int J Mol Med. 2004;13:717–20. [PubMed] [Google Scholar]
- 17.Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174:4185–92. doi: 10.4049/jimmunol.174.7.4185. [DOI] [PubMed] [Google Scholar]
- 18.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S. Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–24. [PubMed] [Google Scholar]