Abstract
Spectacular images of neutrophils ejecting nuclear chromatin and bactericidal proteins, in response to microbes, were first reported in 2004. As externalized chromatin could entangle bacteria, these structures were named neutrophil extracellular traps (NETs). Subsequent studies identified microorganisms and sterile conditions that stimulate NETs, and additional cell types that release extracellular chromatin. NETs’ release is the most dramatic stage in a cell death process called NETosis. Experimental evidence suggests that NETs participate in pathogenesis of autoimmune and inflammatory disorders, with proposed involvement in glomerulonephritis, chronic lung disease, sepsis and vascular disorders. Exaggerated NETosis or diminished NET clearance likely increases risk of autoreactivity to NET components. The biological significance of NETs is just beginning to be explored. A more complete integration of NETosis within immunology and pathophysiology will require better understanding of NET properties associated with specific disease states and microbial infections. This may lead to the identification of important therapeutic targets.
Keywords: Neutrophil extracellular traps, autoimmunity, neutrophils, infections
Neutrophils and NETs
Neutrophils are the most abundant leukocytes in mammals and, as a first line of defense against microbes, they play crucial roles in innate immune responses. The homeostasis of neutrophils is maintained by balancing their short lifespan in the circulation with their regulated release from the bone marrow. Neutrophils have various types of granules, containing hundreds of proteins. These include bactericidal proteins, some of them with important effects on innate and adaptive immune responses, such as α-defensins, the cathelicidin human cationic antimicrobial protein 18 (hCAP18) and lactoferrin, among others. LL-37, a proteolytic product of hCAP18, has important chemotactic and immunostimulatory effects, as discussed below(1).
The mechanisms used by neutrophils to eliminate microbes include phagocytosis, reactive oxygen species’ (ROS) generation and the release of microbicidal molecules from granules (degranulation). In 2004, another distinct antimicrobial activity was described: neutrophils extrude a meshwork of chromatin fibers that are decorated with granule-derived antimicrobial peptides and enzymes such as neutrophil elastase (Figure 1), cathepsin G, and myeloperoxidase (MPO)(2). These structures, called neutrophil extracellular traps (NETs), represent an important strategy to immobilize and kill invading microorganisms. The NET scaffold consists of chromatin fibers with a diameter of 15–17 nm; DNA and histones represent the major NET constituents (2). Mass spectrometry has identified various additional proteins associated with NETs, including components from various types of granules. While conserved proteins are found in extracellular trap fractions, it is still unclear if the peptide composition of these structures may vary depending on the specific stimulus that promotes NET formation(3, 4).
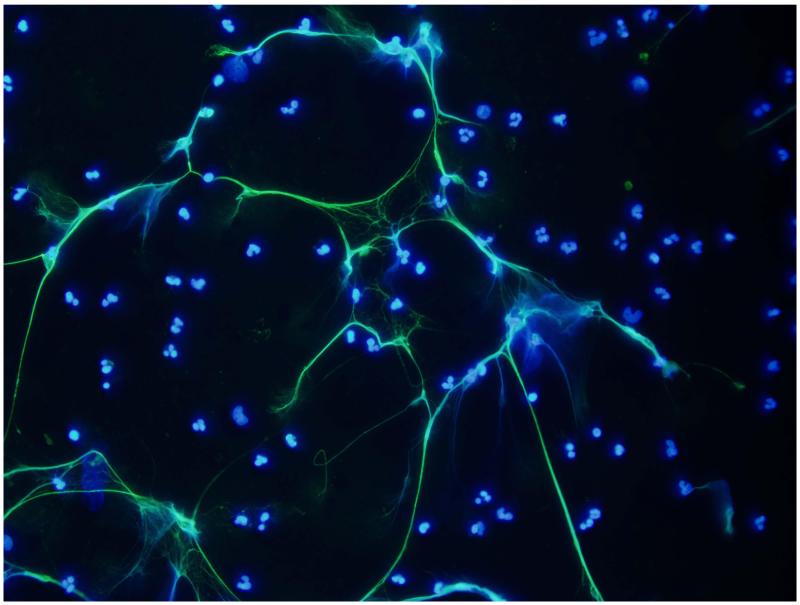
Figure 1.
Representative images of NETs induced in vitro by LPS in human neutrophils. NETs are visualized by costaining of neutrophil elastase (green) and nuclear material (DAPI, blue). Magnification is 40X.
The putative role of extracellular traps in host defense is exemplified by their conserved nature in various vertebrates(5-8), insects(9) and even plants (10). While the initial observation of NET formation placed the process within the context of innate immune responses to infections, recent evidence suggests that these structures also figure prominently at the center of various pathologic states. As such, NETs may function as double-edged swords, serving as effective antimicrobial defenses, but also as putative sources of molecules with immune effector and proinflammatory roles that, in susceptible individuals, may promote tissue damage and autoimmunity. Further, other granulocytes (mast cells and eosinophils) can form extracellular traps upon stimulation, a process thus renamed as ETosis(11, 12). In the case of eosinophils, NET-like structures containing mitochondrial DNA and granular proteins have been proposed to contribute to host defense and to allergic responses(12, 13).
Two models for NET release have been proposed: a) NETosis, a distinct form of active cell death, is characterized by release of decondensed chromatin and granular contents to the extracellular space. During NETosis, nuclear and granular membranes dissolve, and nuclear contents decondense into the cytoplasm. This is followed by plasma membrane rupture and release of chromatin decorated with granular proteins into the extracellular space(2, 14, 15). In contrast to apoptotic cells, netting neutrophils do not appear to display “eat-me” signals, and this may prevent their clearance by phagocytes. Indeed, NETs are disassembled primarily by nucleases(15); b) a DNA/serine protease extrusion mechanism from intact neutrophils, where mitochondrial DNA release is apparently not associated to cell death(16). Further, autophagy may contribute to NETosis (17).
Intact chromatin lattices appear to be required for the antimicrobial function of NETs (2). In addition to microorganisms, NETosis is triggered by other various stimuli, including proinflammatory cytokines (TNF-α, IL-8), platelets, activated endothelial cells (ECs), nitric oxide, monosodium urate crystals, and various autoantibodies(18-26). NETs are identified by various techniques. Fluorescence microscopy and immunohistochemical analysis of transmission electron microscopy data may be preferred approaches to characterize NETosis. Importantly, fibrin may mimic NETs in scanning electron microscopy(27).
Kinetic studies in both human and mouse neutrophils have been published. While phagocytosis and degranulation usually take minutes to occur after microbe exposure, NETosis is a more protracted event(5, 14). It is unclear which factors determine the selection between these alternative antimicrobial activities and whether these processes can coexist in the same cell. About 20-60% of isolated human neutrophils typically release NETs 2-4 hours after stimulation with microbes or PKC activators; however, they respond within minutes when activated by LPS-stimulated platelets under conditions of flow(22). The differences in predisposition to form NETs and the characteristics of these structures made by immature and mature neutrophils remain to be determined. Priming with type I or type II Interferons (IFNs) and subsequent stimulation with C5a leads to NETosis in mature, but not immature neutrophils(28).
Bone marrow-derived neutrophils from common murine strains used in immunology research appear equally capable of forming NETs upon stimulation. When comparing mouse and human neutrophils, murine cells make NETs more slowly and less efficiently and these structures have more compact appearance than human cells(5). Most NETs’ studies in mice use bone marrow neutrophils, whereas the preferred source in humans is peripheral blood. This may account for some differences identified between these species.
Microbes and NETs
An astounding variety of microbes induce NET formation(2, 29-35). NET-inducing stimuli include whole bacteria as well as cell surface components of Gram-positive and Gram-negative bacteria lipoteichoic acid and LPS, and breakdown products of prokaryotic proteins, such as fMLP(18). Notable examples of bacteria that potently induce NETs include S. aureus (36, 37), Streptococcus sp.(38),(29), H. influenzae(30), K. pneumoniae(39), L. monocytogenes(40), M. tuberculosis(31), and S. flexneri (2). Other examples include pathogens such as Yersinia(41) and members of the oral microbiome, including P. gingivalis(33).
Neutrophils from neonates, compared to adult neutrophils, are less capable of forming NETs in response to various microbial stimuli, a phenomenon that may contribute to the enhanced susceptibility to infections observed in this age group(42). Containment of microbial pathogens to sites of initial infection may be an important function of NETs. Indeed, Staphylococci and Streptococci express virulence factors that degrade extracellular traps and may free the bacterium from NET chromatin(29). In cystic fibrosis (CF), pathogens such as P. aeruginosa thrive in the lungs of affected patients and NETs accumulate, promoting chronic lung obstruction. Clinical lung isolates from CF patients acquire a mucoid phenotype that makes P. aeruginosa less sensitive to NET inhibition(35). This suggests that microbes may develop adaptations to resist NET-mediated growth suppression.
Fungi known to elicit NETs include A. nidulans, A. fumigatus and C. albicans(43-45). Patients with chronic granulomatous disease (CGD), who are deficient in the expression of a fully functional NADPH oxidase, are unable to form NETs and often die from infection with A. nidulans. Gene therapy to reconstitute NADPH oxidase function in CGD patients was associated with decreased fungal load and a gain in capacity to form NETs(45). The NET component implicated in immune defense against A. nidulans and C.albicans is calprotectin, a Zn++ chelator and heterodimer of the cytosolic S100A8 and S100A9 proteins present in the NETs. Thus, NETs contain neutrophil proteins that serve specialized functions in fungal immunity and provide high local inhibitor concentrations in close proximity to pathogens(3, 45).
Various protozoan parasites induce NETs(46-48). In vitro, L. amazonensis or its surface lipophosphoglycan induce NETs, while their disruption with DNase I improves pathogen’s survival(46). In the T. gondii infection model, the tachyzoite induces neutrophil influx into the infected tissues and NETs’ release that may limit pathogen viability. In children with malaria, NETs have been observed in fresh blood smears and correlate with specific cytokine profiles and with the development of anti-DNA autoantibodies(48).
Given the diversity of microbes that elicit NETs it is not surprising that, depending on the microbial agent, these lattices may have different effects on the spread and severity of an infection. Just how NETs contribute to immune defense is open to argument. It is important to distinguish microbial death mediated by NETs from failure to recover viable organisms as a result of their entrapment. Histones, the most abundant proteins in NETs, have bactericidal properties. Proteolytic fragments of histones function as defensins in the innate immune system(49). However, some bacteria may survive association with NETs without a loss of viability(50). For these, NETs may represent an important mechanism that limits their spread in the host.
While some pathogens are susceptible to containment or damage by NETs, others may have found ways to counteract these lattices or even to incorporate NET chromatin into their support structures(51). In a surprising twist of evolutionary adaptation, certain bacteria may even benefit from NETs. Colonizing H. influenzae often resist treatment due to their ability to form biofilms that contain structural elements of NETs. Thus, while stimuli produced by H. influenzae can induce NETs, the bacterium avoids damage by these structures. Instead, its lipooligosaccharide coat tightly associates with NETs, and, as result, these bacteria escape phagocytic uptake and clearance(30).
Conflicting reports of bacterial killing by NET chromatin have prompted examination of bactericidal properties of in vitro-generated NETs. Purified NET chromatin alone is not very efficient at killing S. aureus; and MPO present in NETs provides the bactericidal activity needed to kill this pathogen in the presence of hydrogen peroxide(52). Other NET components with antibacterial properties include LL-37, lactoferrin, neutrophil elastase and proteinase-3 (PR-3).
One added level of complexity in studies of the bactericidal and bacteriostatic properties of NETs is that some of the components of these structures, such as MPO, assist in the production of NETs and also directly promote pathogen degradation(53). In addition, proteases that damage captured microbes also may have important roles in shaping the structure of NET chromatin. Histones associated with NETs are “trimmed” by proteases, whereby they lose specific lengths of their amino termini. These histone “tails” have important roles in organizing the higher order structure of chromatin and their cleavage may play a crucial role in producing the “beads on a string” structure of extended NET chromatin(2, 3).
The role of NETs in fighting viral infections remains to be fully characterized, although these web-structures may promote lung injury following influenza infection(7, 54, 55). NETs capture human immunodeficiency virus and promote its elimination through MPO and α-defensin. This virus can develop an anti-NET response mediated by IL-10(56).
Mechanisms of NETosis
Studies of NETosis are difficult to design and interpret. The use of pharmacologic stimuli for NETosis only compounds the problem. Despite these caveats, important insights have been gained into the mechanisms of NETosis (Figure 2). The stimuli that induce NETosis can be broadly classified into microbe-associated, inflammatory, or endogenous (“sterile”) triggers. Diverse neutrophil receptors can signal to induce NETosis, as binding via TLRs, Fc receptors or complement receptors has been implicated in the induction of NETs(2, 24, 57). Cytokine receptors likely play roles in NETosis signaling, as IL8, TNF, and IFN-γ can trigger of this process(2, 18, 28). More studies are required to address the synergy or cross-talk between different classes of neutrophil receptors in the induction of NETosis.
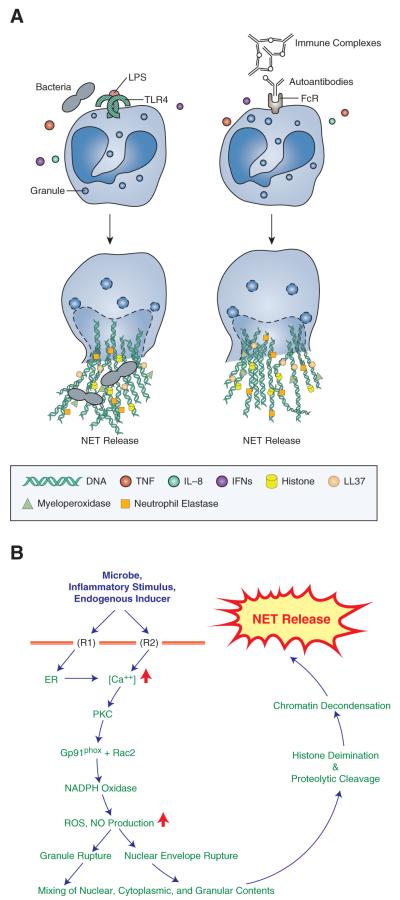
Figure 2. Putative stimuli and steps in NETosis.
A. Microbes and their products, immune complexes, autoantibodies, cytokines and other stimuli (IL8, TNF, and type I and II IFNs) can induce NETosis. Binding via TLRs, Fc receptors or complement receptors have been implicated in NETs’ induction. B. NETosis is initiated by binding of neutrophil surface receptors (R) to microbes or microbial breakdown products, inflammatory stimuli, or endogenous inducers. Binding to receptor(s) (exemplified in diagram as R1 and R2) induces ER calcium store release and opening of membrane channels that lead to cytoplasmic calcium increases. Elevated calcium stimulates PKC activity, phosphorylation of gp91phox, and assembly of functional NADPH Oxidase leading to reactive oxygen species (ROS) and nitric oxide (NO) production. Morphological changes observed during NETosis include breakdown of nuclear and granule membranes and the mixing of nuclear, granular and cytoplasmic contents. Deimination and proteolytic cleavage of histones may initiate before nuclear breakdown, and contribute to chromatin decondensation. A rupture in the plasma membrane allows the release of extracellular chromatin traps.
The most frequently used compound to induce NETosis is PMA, a synthetic activator of the PKC family of enzymes. PKC is directly responsible for activation of NADPH oxidase and ROS production. Many of the natural NET inducers lead to PKC activation, as elevated cytoplasmic calcium and its release from ER stores are direct consequences of receptor activation. The requirement for ROS production is consistent with the inhibition of NETosis by ROS scavengers such as N-acetyl cysteine (NAC), diphenylene iodinium (DPI), or Trolox(15, 58). How ROS contribute to NETosis is controversial. One possibility is that they directly promote the observed morphologic changes occurring in neutrophils during NETosis(39). A proposed alternative is that ROS serve to inactivate caspases, thereby inhibiting apoptosis and instead inducing autophagy, a process leading to dissolution of cellular membranes(17). These alternatives may not be mutually exclusive and they each may occur under different experimental conditions.
How NETosis signals are transduced is suggested by studies on Rac2. Phosphorylation of gp91phox by PKC allows the cytosolic and membrane-bound subunits of NADPH oxidase to assemble into a functional complex that generates ROS, and, in particular, superoxide ions(59). Whereas Rac1 deficient neutrophils release similar quantities of NET chromatin as wild-type neutrophils in response to PMA or LPS, Rac2 deficient cells cannot form a functional enzyme complex and are impaired in NET formation(58). Further steps in the conversion of superoxide to hydrogen peroxide and to perchloric acid are also essential for NET release. Superoxide itself is not essential, as rescue of the NETosis pathway in CGD neutrophils was observed with addition of exogenous peroxide. NETosis may also occur in certain conditions through ROS-independent pathways(42, 60), which suggests that specific stimuli and the surrounding inflammatory milieu may modulate how NETs are generated.
Histone deimination by peptidylarginine deiminase 4 (PAD4) is a prominent post-translational modification in NETs that is induced by inflammatory or infectious stimuli(18, 61). The conversion of certain arginine residues to citrullines in core histones by PAD4 eliminates the positive charge from that residue and may thereby weaken the binding of histones to DNA. Because the PAD4 substrate arginines are part of histone tails that mediate interactions between nucleosomes, loss of positive charge may unravel the compact nature of nuclear chromatin and allow it to disperse in the form of NETs. Analysis of neutrophils from mice deficient in PAD4 revealed that they were impaired in their capacity to form NETs in vitro(54, 62). In vivo, PAD4-deficient mice had more severe bacterial infections, although the inability to form NETs did not affect viral titers and leukocyte infiltration rates during influenza infections(54).
NETs as sources of autoantigens and immunostimulatory proteins
NETs may play crucial roles in the regulation of immune responses, protease-mediated effector functions and intercellular signal transduction. Until recently, the predominant model explaining the externalization of putative modified nuclear autoantigens (such as DNA) and their access to the immune system has been that apoptotic debris represents the predominant source(63, 64). However, recent evidence that extracellular DNA is a common, and even necessary process in naturally-occurring immune responses mediated by NETosis, has opened new possibilities of better understanding the mechanisms that lead to breaking of tolerance and the promotion of autoimmunity.
Several of the molecules decorating the NETs (e.g. MPO, double-stranded DNA (ds-DNA), histones, etc.) are autoantigens in systemic autoimmune diseases such as antineutrophil cytoplasmic antigen (ANCA)-positive vasculitis and systemic lupus erythematosus (SLE). Thus, various groups have proposed that aberrant NET formation may be important in the generation of autoimmune responses in predisposed individuals(24-26, 65). This hypothesis is supported by the observation that initiation or exacerbation of autoimmune responses often occurs following microbial infections. Various autoantibodies can promote the release of NETs containing antimicrobial proteins including LL37(24-26). This combination of DNA and LL37 is a potent stimulus for pDCs to synthesize type I IFNs. Type I IFNs have antimicrobial roles but also potent immunostimulatory effects in autoimmune diseases such as SLE and psoriasis (11, 24, 26, 65). Importantly, neutrophils from patients with various autoimmune diseases appear more prone to NETose. However, isolated in vivo exposure to NETs may be insufficient to break tolerance and additional factors (genetic influences, inflammatory milieu, etc.) may need to be present to trigger full, sustained autoimmune responses, as recently suggested(66).
Recent evidence also indicates that NETs can shape adaptive immune responses, as these structures can prime T cells and reduce their activation threshold(67). The specific role of NETs in other components of adaptive immune responses remains to be fully characterized. Below, we summarize recent discoveries on the potential role of NETs in the pathogenesis of autoimmune diseases.
Vasculitis
Small vessel vasculitis (SVV) is a systemic autoimmune disease characterized by necrotizing inflammation of small blood vessels. Many patients with this disease develop ANCAs with specific reactivity against PR3 or MPO, which are considered diagnostic of this disease. ANCAs induce NETosis, while NET-derived material is detected in blood and in kidney biopsies from a large proportion of SVV patients(25). Importantly, autoantigens recognized by ANCAs have been found in the NET structure(25). The drug propylthiouracil (PTU) can induce ANCA-vasculitis; this may occur through an effect on the conformation of NETs, leading to their impaired degradation(68) These observations suggest new mechanisms of ANCA generation and induction of organ damage in this autoimmune condition.
Psoriasis
Psoriasis is a common inflammatory disorder of the skin and other organs, where IL-17 may play prominent pathogenic roles. Neutrophils and mast cells were recently reported to represent the predominant cell types expressing IL-17 in human skin in psoriasis and this cytokine is released during ETosis(11). As such, externalization of IL-17 in the extracellular traps of neutrophils and mast cells may be central to psoriasis pathogenesis.
SLE
SLE is an autoimmune syndrome that affects many organs and is characterized by autoantibodies against DNA, chromatin and DNA-associated proteins, including NET components. Recent evidence points to an imbalance between NET formation and NET clearance in this disease(24, 26, 65, 69). As such, patients with aberrantly elevated NETosis, in conjunction with impaired degradation of these structures by nucleases, may be particularly prone to NET-associated tissue damage. Decreased NET degradation occurs in a subset of SLE patients, correlating with renal disease, and secondary to DNase1 inhibitors and anti-NET Abs(69). Self-DNA in lupus immune complexes contains LL37, which triggers TLR9 in pDCs, and consequent IFN-α synthesis, and protects nucleic acids from degradation by nucleases(26). These DNA-antimicrobial complexes are released during NETosis, a process enhanced in SLE, and mediated in part by autoantibodies present in serum from a subset of lupus patients(24, 26). NETs may also activate complement, thereby amplifying disease(70). A subset of aberrant low-density granulocytes present in SLE displays enhanced capacity to form NETs and kill ECs in a NET-dependent manner. Further, netting lupus neutrophils are detected in blood and infiltrate affected organs, where they expose immunostimulatory molecules(65). There is also preliminary evidence that nuclear material that is externalized on NETs promotes autoaAb formation in SLE(26).
Felty’s syndrome (FS)
FS is characterized as rheumatoid arthritis (RA), splenomegaly and neutropenia. In FS, autoantibodies were found to be directed against PAD-4-deiminated histones and to induce NETosis(71). Netting neutrophils were found to be reactive with autoantibodies from FS, SLE and RA patients, suggesting a common cellular mechanism in these disorders. The role that NETs play more broadly in RA pathogenesis, remains to be determined.
Gout
Gout is characterized by acute neutrophilic joint inflammation triggered by inflammatory responses to uric acid crystals. Activation of neutrophils in gout is associated with the formation of proinflammatory NETs, a phenomenon that was linked to both autophagy and IL-1β release(23).
Additional studies are required to assess the exact role of aberrant NETosis in induction and perpetuation of the diseases mentioned above and in other chronic inflammatory diseases.
NETs in vascular damage and atherothrombosis
EC activation can elicit NETosis and, in turn, NETs induce vascular damage. Furthermore, netting neutrophils may play important roles in the promotion of atherosclerosis, thrombosis and other vascular complications(21, 65).
NETs and thrombosis
NETs can provide a stimulus and scaffold for thrombus formation, by promoting platelet and RBC adhesion and by concentrating effector proteins and coagulation factors involved in clotting(72-74). Thrombus-resident neutrophils are indispensable for deep venous thrombosis (DVT) propagation by binding factor XII and supporting its activation through NETosis. Interestingly, the anticoagulant heparin can remove histones from NETs’ chromatin fibers, leading to their destabilization, suggesting an additional mechanism by which this compound is antithrombotic(72-74). Ligation of TLR4 by LPS in narrow vessels induces platelet binding to adherent neutrophils, promoting NETosis. Human ß-defensin1 is an antimicrobial component of platelets that can induce NETs. Platelet-neutrophil interactions leading to NETs may play important roles in EC and tissue damage not only during sepsis but, also, in chronic inflammatory disorders(22).
NETs and atherosclerosis
Neutrophil-derived CRAMP (the murine equivalent of LL37) is proatherogenic by recruiting inflammatory monocytes into arteries. CRAMP/DNA complexes stimulate pDC in plaque lesions and contribute to generation of autoAbs and aggravation of atherosclerosis in Apolipoprotein E−/− mice(75, 76). These observations suggest a putative role for NETs components in atheroma formation that requires further investigation.
Pregnancy
In preeclampsia, a pregnancy-related inflammatory and vasculopathic disorder, massive numbers of NETs have been reported in vivo in the intervillious space, apparently triggered by trophoblast microdebris and IL-8. A pathogenic role for NETs in preeclampsia and pregnancy outcomes remains to be proven(19).
Therapeutics
There is already some precedent in manipulating NETosis for therapeutic purposes, as mentioned above in the case of CGD(45). Various ROS scavengers are effective at reducing NETs’ release(15) and similar avenues may be available to repress NETosis in vivo in chronic inflammatory disorders. MPO inhibitors, such as 4-aminobenzoic acid hydrazide, and various PAD4 inhibitors may have similar effects(39, 77). The role of colchicine or other drugs that destabilize the cytoskeleton, a structure implicated in NETosis, should be explored (78) (79). Inhibition of the effects of histones contained in NETs may prove beneficial in various inflammatory conditions or in EC damage. Clearly, a better understanding of how NETs are generated and whether NETs that form upon microbial exposure are distinct from those that form under “sterile” conditions, as exist in autoimmune or vasculopathic disorders, may allow the development of compounds that selectively target the deleterious aspects triggered by these lattices.
Conclusions
The biological significance of NETs is just beginning to be explored. In addition to playing a central part in antimicrobial innate immunity, NETs are generated upon non-infectious stimuli in various clinical settings. In acute or chronic inflammatory disorders, aberrantly enhanced NET formation and/or decreased NET degradation may play key roles in initiation and perpetuation of autoimmune responses and organ damage. Various areas pertinent to NET biology need to be better defined, including the molecular mechanisms governing their generation and the downstream pathways leading to their formation. At this point, it is unclear if all NETs are created equal and if traps from different disease conditions or species will be phenotypically and functionally identical. Identification of endogenous stimuli leading to NETosis has been incomplete and needs to be better understood. For example, a more accurate distinction between the effects of NET trapping and NET killing on different types of microbes, and the kinetics of NET release in different tissues or in the bloodstream are needed. Better characterizing NET formation and their role in biological processes in vivo remains a priority. Unraveling the NET properties unique to specific disease states or microbial infections will be important to further the understanding on the role of these matrices in health and disease. Current and future research holds promise to open avenues for the development of tools to modulate inflammation.
Acknowledgments
We thank Tim Higgins and Ritika Khandpur for support with figures.
Footnotes
Supported by the National Institutes of Health through PHS grant HL088419 (to MJK) and by the Lupus Research Institute of New York (to MR).
References
- 1.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, Garlanda C, Romani L, Gascan H, Bellocchio S, Bozza S, Cassatella MA, Jeannin P, Mantovani A. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuammitri P, Ostojic J, Andreasen CB, Redmond SB, Lamont SJ, Palic D. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet Immunol Immunopathol. 2009;129:126–131. doi: 10.1016/j.vetimm.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Wardini AB, Guimaraes-Costa AB, Nascimento MT, Nadaes NR, Danelli MG, Mazur C, Benjamim CF, Saraiva EM, Pinto-da-Silva LH. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J Gen Virol. 2010;91:259–264. doi: 10.1099/vir.0.014613-0. [DOI] [PubMed] [Google Scholar]
- 8.Palic D, Ostojic J, Andreasen CB, Roth JA. Fish cast NETs: neutrophil extracellular traps are released from fish neutrophils. Dev Comp Immunol. 2007;31:805–816. doi: 10.1016/j.dci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Altincicek B, Stotzel S, Wygrecka M, Preissner KT, Vilcinskas A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol. 2008;181:2705–2712. doi: 10.4049/jimmunol.181.4.2705. [DOI] [PubMed] [Google Scholar]
- 10.Wen F, White GJ, VanEtten HD, Xiong Z, Hawes MC. Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol. 2009;151:820–829. doi: 10.1104/pp.109.142067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 13.Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–1266. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010 doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 17.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, Verma A, Mitra K, Barthwal MK, Krishnamurthy H, Bajpai VK, Dikshit M. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide. 2010;22:226–234. doi: 10.1016/j.niox.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 23.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krautgartner WD, Klappacher M, Hannig M, Obermayer A, Hartl D, Marcos V, Vitkov L. Fibrin mimics neutrophil extracellular traps in SEM. Ultrastruct Pathol. 2010;34:226–231. doi: 10.3109/01913121003725721. [DOI] [PubMed] [Google Scholar]
- 28.Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, Dummer R, Simon HU, Yousefi S. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279:44123–44132. doi: 10.1074/jbc.M405883200. [DOI] [PubMed] [Google Scholar]
- 29.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 30.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun. 2011;79:431–438. doi: 10.1128/IAI.00660-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos-Kichik V, Mondragon-Flores R, Mondragon-Castelan M, Gonzalez-Pozos S, Muniz-Hernandez S, Rojas-Espinosa O, Chacon-Salinas R, Estrada-Parra S, Estrada-Garcia I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89:29–37. doi: 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delbosc S, Alsac JM, Journe C, Louedec L, Castier Y, Bonnaure-Mallet M, Ruimy R, Rossignol P, Bouchard P, Michel JB, Meilhac O. Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS One. 2011;6:e18679. doi: 10.1371/journal.pone.0018679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin-Esteban V, Turbica I, Dufour G, Semiramoth N, Gleizes A, Gorges R, Beau I, Servin AL, Lievin-Le Moal V, Sandre C, Chollet-Martin S. Afa/Dr Diffusely Adherent Escherichia coli Strain C1845 Induces Neutrophil Extracellular Traps that Kill Bacteria and Damage Human Enterocyte-Like Cells. Infect Immun. 2012 doi: 10.1128/IAI.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 37.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 38.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munafo DB, Johnson JL, Brzezinska AA, Ellis BA, Wood MR, Catz SD. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J Innate Immun. 2009;1:527–542. doi: 10.1159/000235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casutt-Meyer S, Renzi F, Schmaler M, Jann NJ, Amstutz M, Cornelis GR. Oligomeric coiled-coil adhesin YadA is a double-edged sword. PLoS One. 2010;5:e15159. doi: 10.1371/journal.pone.0015159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, Chandler NB, Rodesch CK, Albertine KH, Petti CA, Weyrich AS, Zimmerman GA. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 44.McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, Heesemann J, Ebel F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010;12:928–936. doi: 10.1016/j.micinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127:1243–1252. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, Saraiva EM. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect Immun. 2012;80:768–777. doi: 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, Sagay SA, Egah DZ, Iya D, Afolabi BB, Baker M, Ford K, Ford R, Roux KH, Keller TC., 3rd. Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J. 2008;7:41. doi: 10.1186/1475-2875-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho JH, Sung BH, Kim SC. Buforins: histone H2A-derived antimicrobial peptides from toad stomach. Biochim Biophys Acta. 2009;1788:1564–1569. doi: 10.1016/j.bbamem.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 50.Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 51.Cole JN, Pence MA, von Kockritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, Nizet V. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. MBio 1. 2010 doi: 10.1128/mBio.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol. 2012;91:369–376. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 53.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6:e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 57.Munks MW, McKee AS, Macleod MK, Powell RL, Degen JL, Reisdorph NA, Kappler JW, Marrack P. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood. 2010;116:5191–5199. doi: 10.1182/blood-2010-03-275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim MB, Kuiper JW, Katchky A, Goldberg H, Glogauer M. Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol. 2011;90:771–776. doi: 10.1189/jlb.1010549. [DOI] [PubMed] [Google Scholar]
- 59.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012 doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 64.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 65.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, Gozani OP, Alizadeh AA, Utz PJ. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188:3150–3159. doi: 10.4049/jimmunol.1103414. [DOI] [PubMed] [Google Scholar]
- 68.Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, Nishio S, Kasahara M, Ishizu A. Abnormal conformation and impaired degradation of NETs induced by propylthiouracil: Implication of disordered NETs in MPO-ANCA-associated vasculitis. Arthritis Rheum. 2012 doi: 10.1002/art.34619. [DOI] [PubMed] [Google Scholar]
- 69.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, Bengtsson AA, Blom AM. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 71.Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, Kirou KA, Hellmich B, Knuckley B, Thompson PR, Crow MK, Mikuls TR, Csernok E, Radic M. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular traps. Arthritis Rheum. 2011 doi: 10.1002/art.33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brill A, Fuchs TA, Savchenko A, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil Extracellular Traps Promote Deep Vein Thrombosis in Mice. J Thromb Haemost. 2011 doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doring Y, Manthey H, Drechsler M, Lievens D, Megens R, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, Daemen MJ, Lutgens E, Zenke M, Binder CJ, Weber C, Zernecke A. Auto-Antigenic Protein-DNA Complexes Stimulate Plasmacytoid Dendritic Cells to Promote Atherosclerosis. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 76.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 77.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang LP. Oral colchicine (Colcrys): in the treatment and prophylaxis of gout. Drugs. 2010;70:1603–1613. doi: 10.2165/11205470-000000000-00000. [DOI] [PubMed] [Google Scholar]