Abstract
Cyanine fluorophores exhibit greatly improved photostability when covalently linked to stabilizers, such as cyclooctatetraene (COT), nitrobenzyl alcohol (NBA) or Trolox. However, the mechanism by which photostabilization is mediated has yet to be determined. Here we present spectroscopic evidence that COT, when covalently linked to Cy5, substantially reduces the lifetime of the Cy5 triplet state, and that the degree of triplet state quenching correlates with enhancements in photostability observed in single-molecule fluorescence measurements. By contrast, NBA and Trolox did not quench the Cy5 triplet state under our conditions suggesting that their mechanism of photostabilization is different from COT and does not target the fluorophore triplet state directly. These findings provide insights into the mechanisms of fluorophore photostabilization that may lead to improved fluorophore designs for biological imaging applications.
Keywords: Laser flash photolysis, single-molecule spectroscopy, Cy5, COT
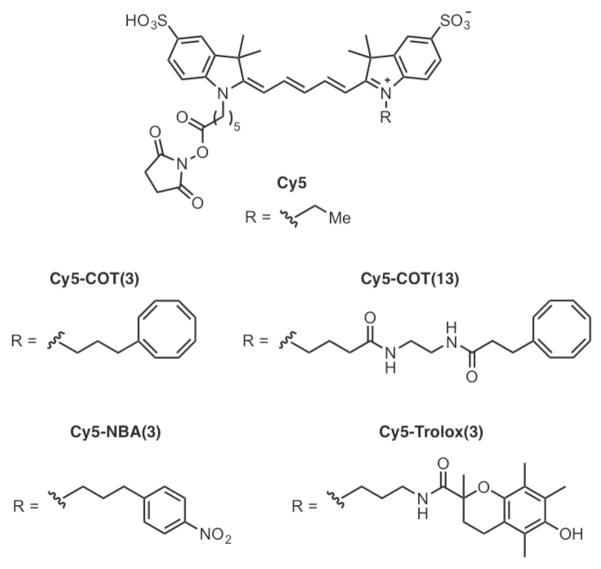
Over the past decade, fluorescence microscopy has seen revolutionary advancements in both sensitivity and resolution, highlighted by the recent development of single-molecule fluorescence.1,2 However, the inherent instabilities and potential toxicities of the fluorophores employed are a current limitation. Organic fluorophores are highly prone to intermittent fluorescence (blinking) and photo-induced degradation (photobleaching), which reduce the amplitude and duration of the experimental signal.2,3 Consequently, there is an increasing demand for the development of new strategies that enable fluorophore photostablization. Recently, we demonstrated that photostabilization of cyanine fluorophores can be achieved by covalently linking the fluorophore to a “protective agent” such as cyclooctatetraene (COT), nitrobenzyl alcohol (NBA) and Trolox (Chart 1).4,5 We observed an up to 70-fold increase in the duration of fluorescence prior to entering a non-fluorescent state.
Chart 1.
Structures of Cy5 derivatives used in this study.
The major pathway for photobleaching of most organic fluorophores is photo-oxidation by reactive oxygen species, such as singlet oxygen and peroxides, which are thought to arise from reactions between molecular oxygen and fluorophore triplet states.6 However, removal of molecular oxygen (a triplet quencher) often induces severe blinking, primarily due to fluorophore triplet state-induced processes.2,3 It had been suggested that the “protective agents” (e.g. COT, NBA and Trolox) quench the triplet states to restore the fluorophore to the singlet ground state (S0).7–9 Several mechanisms for this triplet quenching have been suggested. For example, the quenching of rhodamine fluorophore triplet states by COT present in the solution has been suggested to proceed through an energy transfer mechanism.7 Studies with Trolox and ATTO fluorophores suggested that Trolox restores the fluorophore ground state from the triplet state through a reduction-oxidation (red-ox) mechanism,8 and that a single Trolox molecule may operate through a “ping-pong” red-ox mechanism to enhance photostability.9 The mechanism of NBA-mediated photostabilization is not clear, but may also operate through red-ox cycles.9 However, unambiguous experimental proof of the involved mechanisms are still lacking. Here, we examined whether enhanced photostability of the Cy5 fluorophore, when covalently linked to “protective agents” (COT, NBA or Trolox) (Chart 1) can be specifically attributed to a triplet state quenching mechanism using laser flash photolysis (time-resolved transient absorption spectroscopy).
Because the formation of triplet states of Cy5 is inefficient (triplet quantum yield < 0.003) upon direct excitation,10 a triplet sensitizer was employed to more efficiently populate the Cy5 triplet state (3Cy5*) through an energy transfer mechanism (eq 1). Benzophenone (BP) was selected as a sensitizer, because of its high triplet quantum yield and higher triplet energy (289 kJ/mol)11 compared to Cy5 (154 kJ/mol)12. In addition, BP can be selectively excited at 355 nm, where Cy5 shows negligible absorption.
| (1) |
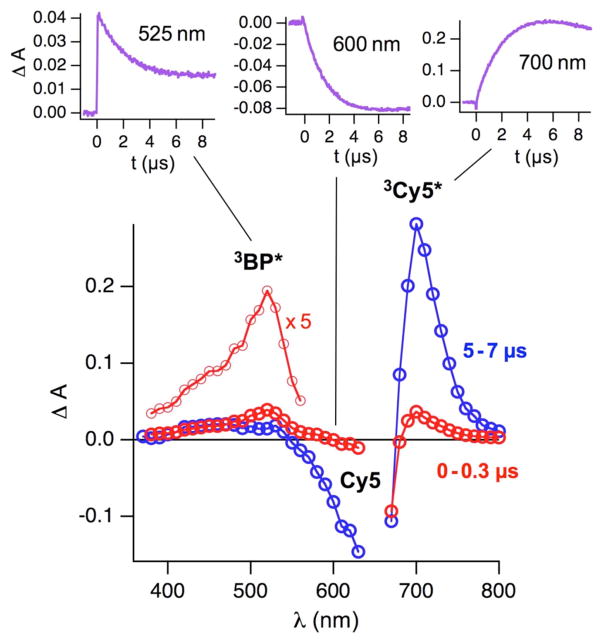
Deoxygenated acetonitrile solutions containing BP and Cy5 were irradiated with light pulses from a Nd-YAG laser at 355 nm (5 ns pulse width) to generate transient absorption kinetic traces across the visible spectrum. From these traces, transient absorption spectra at different times after the laser pulse were constructed (Figure 1). Directly after the laser pulse (Figure 1, red line) the spectrum is dominated by the triplet absorption of BP, which is known to show a peak at 525 nm.11 After several microseconds (Figure 1, blue line) the BP triplet decayed under bleaching of Cy5 ground state absorption (~650 nm) and a new transient absorption at 700 nm appeared. As shown in the insets of Figure 1, the three processes, decay of 3BP* (observed at 525 nm), bleaching of Cy5 (monitored at 600 nm) and growth of the new transient at 700 nm, occur with very similar kinetics. Assignment of this new transient at 700 nm as the triplet state absorption of Cy5 was subsequently confirmed by performing quenching studies in the presence of a small amount of oxygen (0.45 mM; generated by bubbling the acetonitrile solution with a gas mixture of 5% O2 and 95% N2).11 Consistent with its potent, triplet state quenching properties, in the presence of O2 the lifetime of the 700 nm transient was reduced to 1.7 μs compared to ~22 μs in the absence of O2 (Supporting Information, Figure S1). The quenching of the 700 nm transient was paralleled by recovery of Cy5 in the ground state (monitored at 600 nm). In line with this assignment, other cyanine dyes also show triplet state absorption at 700 nm.10,13 Conversely, the cis-conformation of ground state Cy5 is also known to absorb in this spectral region.10,13,14 However, the observed quantitative quenching of the transient by O2 demonstrates that the contribution of the ground state cis-conformer (which is not quenched by O2) to the transient absorption at 700 nm is negligible. Therefore, we conclude that the transient at 700 nm observed under our experimental conditions using the BP sensitization strategy (eq 1) is correctly assigned to 3Cy5* and this transient can be used to investigate Cy5 triplet state quenching by the covalently linked “protective agents”. However, some minor contribution of the cis-conformer to the transient absorption at 700 nm can not be excluded, especially at longer time scales.
Figure 1.
Transient absorption spectra recorded at different delay times after the laser pulse (355 nm, 5 ns pulse width) of deoxygenated acetonitrile solutions of BP (5 mM) and Cy5 (22 μM). The insets show kinetic traces at different observation wavelength.
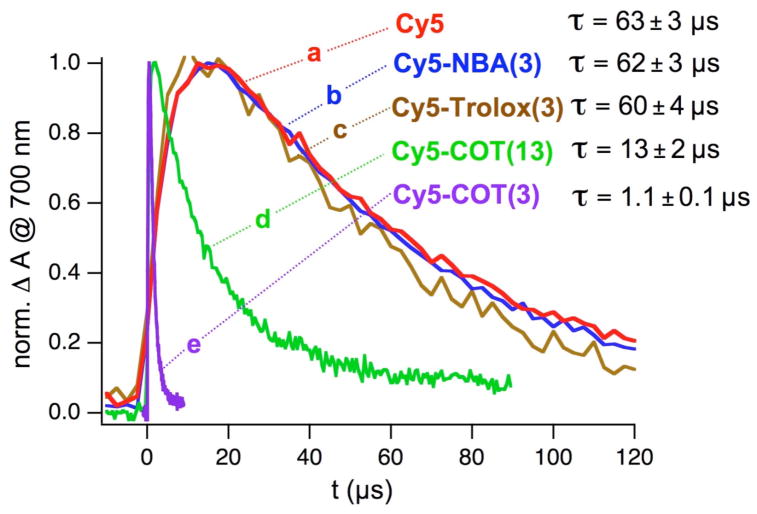
A series of Cy5 derivatives with covalently linked “protective agents” (Chart 1) were synthesized following procedures analogous to those previously described.4,5 In addition to different “protective agents” (COT, NBA and Trolox), the length of the spacer between Cy5 and the “protective agent” was also varied. Laser flash photolysis experiments in argon-saturated acetonitrile solutions using BP as the sensitizer were performed on each of the Cy5 derivatives. Transient absorption bands similar to unsubstituted Cy5 (Figure 1) were observed. However, significant differences were seen in the kinetic features of their triplet absorption at 700 nm (Figure 2). The initial growth in transient absorption is caused by the energy transfer process from 3BP* to the Cy5 chromophore analog eq 1, which then is followed by the decay of the Cy5 triplet state. The concentrations of the Cy5 derivatives were optimized in order to ensure accurate triplet lifetime determination. High concentration, while advantageous by increasing the rate of triplet energy transfer (eq 1), had the negative effect of decreasing the Cy5 triplet lifetime due to self-quenching by ground state Cy5. Exceedingly low concentrations decreased the signal intensity at 700 nm and also substantially reduced the rate at which 3Cy5* was populated. In addition, a low enough laser power was used to eliminate the quenching of 3Cy5* by triplet-triplet annihilation. The growth kinetic was deconvoluted from the decay in order to accurately determine the triplet lifetimes of the Cy5 derivatives (Supporting Information, Figures S2 and S3). The triplet lifetimes obtained are listed in Figure 2. Cy5-NBA(3) (b) and Cy5-Trolox(3) (c) show triplet lifetimes, which are indistinguishable from the lifetime of unsubstituted Cy5 (a) (60–63 μs). However, the COT-linked derivatives (d, e) showed significantly reduced triplet lifetimes. Cy5-COT(3), the derivative with the shortest linker between the cyanine chromophore and COT has the shortest triplet lifetime (1.1 μs), and is approximately 60 times shorter than the triplet lifetime of the unsubstituted Cy5.
Figure 2.
Cy5 triplet absorption traces recorded at 700 nm after pulsed laser excitation (355 nm, 5 ns pulse width) of deoxygenated acetonitrile solutions of BP (a–d: 3 mM; e: 10 mM) and Cy5 derivatives (a–d: 10 ± 1 μM; e: 82 μM). The triplet lifetimes (τ) derived from a kinetic fitting model considering the growth kinetics due to energy transfer from 3BP* to Cy5. Details and the fitted traces are shown in the Supporting Information, Figures S2 and S3.
COT is known to have a low-energy (“relaxed”) triplet state (puckered geometry) with an energy of ~92 kJ/mol15,16 whereas the triplet energy of Cy5 is significantly higher (154 kJ/mol)12. Therefore, energy transfer from 3Cy5* to COT is energetically favorable. The energy transfer mechanism between triplet donors and COT has been investigated in detail.16 The energy transfer process generates COT triplet states and returns the cyanine chromophore to the ground state. The recovery of the cyanine fluorophore to the ground state was directly observable by laser flash photolysis (Figure S2d).
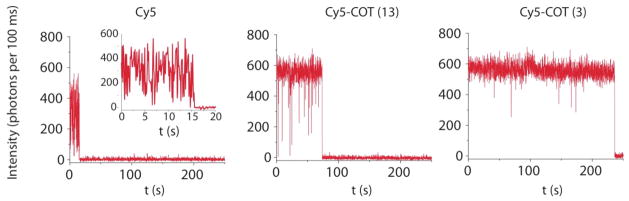
To examine whether this COT-mediated triplet state quenching and rapid ground state recovery correlates with the observed photostability of the cyanine fluorophore, single-molecule fluorescence measurements were performed, as previously described,4 where the Cy5 derivatives were conjugated to double stranded DNA, a model system to study fluorophore stability on biomolecules. Figure S4 shows representative images of these systems using a total internal reflection fluorescence microscope with illumination at 641 nm. By tracking the fluorescence of individual molecules over time, the intensity and duration of fluorescence, as well as the kinetics of blinking and photobleaching could be quantified. Visual inspection of individual fluorescence traces revealed that the time period of fluorescence before blinking or photobleaching was longest for Cy5-COT(3) and shortest for the unsubstituted Cy5 (Figure 3). By quantifying the number of photons detected for each ensemble of single molecules (>500 for each data set; Table 1), we found that the average duration of fluorescence increased from Cy5 to Cy5-COT(13) to Cy5-COT(3) in a manner that was inversely correlated with the triplet lifetime (Figure S5). This finding shows that the triplet state is a key intermediate for fluorophore blinking and photobleaching and that COT photostabilizes the cyanine fluorophore by reducing the duration that the fluorophore spends in the triplet state. A shortened triplet lifetime reduces the probability of fluorophore transformation reactions from the triplet state and reduces the probability of reactive oxygen species production, such as singlet oxygen, which is generated by interaction of triplet excited states with molecular oxygen. It must be noted that the interaction of COT triplet states, which are generated by energy transfer quenching from 3Cy5* to COT, does not lead to singlet oxygen as the energy of the “relaxed” triplet state of COT (~92 kJ/mol)15 is slightly lower than the energy of singlet oxygen (94 kJ/mol).
Figure 3.
Representative single-molecule fluorescence traces for Cy5, Cy5-COT(13) and Cy5-COT(3) covalently linked to DNA oligonucleotides and imaged using a total internal reflection microscope under continuous laser excitation (641 nm).
Table 1.
Average number of photons detected before photobleaching or blinking in single-molecule measurements and triplet lifetime (τtriplet) of Cy5 derivatives.
| Average number of photons (104 photons) | τtriplet (μs) | |
|---|---|---|
| Cy5 | 2.1 ± 0.1 | 63 ± 3 |
| Cy5-COT(13) | 40 ± 4 | 13 ± 2 |
| Cy5-COT(3) | 99 ± 6 | 1.1 ± 0.1 |
| Cy5-NBA(3) | 10 ± 1 | 62 ± 3 |
| Cy5-Trolox(3) | 22 ± 2 | 60 ± 4 |
By contrast, shortening of the triplet lifetime was not observed for Cy5-NBA(3) and Cy5-Trolox(3) under our experimental conditions, but both Cy5 derivatives showed increased photostability compared to unsubstituted Cy5 (Figure 2 and Table 1). This finding suggests that NBA and Trolox operate to stabilize the cyanine fluorophore through different mechanisms, which do not target the Cy5 triplet state directly. Possible stabilization mechanisms of NBA and Trolox could involve passivation of reactive oxygen species and radicals, which can damage the fluorophore. However, a red-ox mechanism where 3Cy5* is deactivated by Trolox and NBA through a electron exchange mechanism (“ping-pong”)9 appears unlikely under our conditions, because no measurable reduction of the triplet lifetime was observed for Cy5-NBA(3) and Cy5-Trolox(3). To test if the short linker between Cy5 and NBA or Trolox might sterically hinder the electron transfer, a larger more flexible 11-atom linker chain was also tested. However, no reduction of the Cy5 triplet lifetime was observed (Figure S6).
In summary, we have observed that Cy5 derivatives containing covalently linked COT have significantly reduced Cy5 triplet lifetimes due to intramolecular energy transfer quenching, which regenerates the Cy5 fluorophore ground state. The triplet lifetimes correlate well with the photostability in single-molecule fluorescence experiments, where Cy5-COT(3), with the shortest triplet lifetime, showed the highest photostability. It also suggests that COT is a robust and potentially general agent that can be used to improve photostability of organic fluorophores especially when covalently linked in close proximity to the fluorogenic center. The central role of the triplet state suggests that reactive oxygen species, which can be generated from the triplet states, significantly reduce the photostability of the fluorophore. Such studies are in progress.
Supplementary Material
Acknowledgments
SCB, QZ, RBA and ZZ thank the National Institutes of Health (GM079238) and the Tri-Institutional Training Program in Chemical Biology for financial support. NJT and SJ thank the National Science Foundation for support through Grant NSF-CHE-11-11398. QZ thanks Mr. Cong Mai for fruitful discussions and suggestions.
Footnotes
Supporting Information. Origin of materials, experimental details, additional laser flash photolysis data and single-molecule fluorescence images. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Michalet X, Weiss S, Jaeger M. Single-molecule fluorescence studies of protein folding and conformational dynamics. Chem Rev. 2006;106:1785–1813. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T. Advances in single-molecule fluorescence methods for molecular biology. Annu Rev Biochem. 2008;77:51–76. doi: 10.1146/annurev.biochem.77.070606.101543. [DOI] [PubMed] [Google Scholar]
- 3.Ha T, Tinnefeld P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annu Rev Phys Chem. 2012;63:595–617. doi: 10.1146/annurev-physchem-032210-103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altman RB, Terry DS, Zhou Z, Zheng Q, Geggier P, Kolster RA, Zhao Y, Javitch JA, Warren JD, Blanchard SC. Cyanine fluorophore derivatives with enhanced photostability. Nat Methods. 2012;9:68–71. doi: 10.1038/nmeth.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman RB, Zheng Q, Zhou Z, Terry DS, Warren JD, Blanchard SC. Enhanced photostability of cyanine fluorophores across the visible spectrum. Nat Methods. 2012;9:428–429. doi: 10.1038/nmeth.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitus M, Ranjit S. Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments. Q Rev Biophys. 2011;44:123–151. doi: 10.1017/S0033583510000247. [DOI] [PubMed] [Google Scholar]
- 7.Widengren J, Chmyrov A, Eggeling C, Loefdahl PA, Seidel CA. Strategies to improve photostabilities in ultrasensitive fluorescence spectroscopy. J Phys Chem A. 2007;111:429–440. doi: 10.1021/jp0646325. [DOI] [PubMed] [Google Scholar]
- 8.Cordes T, Vogelsang J, Tinnefeld P. On the mechanism of Trolox as antiblinking and antibleaching reagent. J Am Chem Soc. 2009;131:5018–5019. doi: 10.1021/ja809117z. [DOI] [PubMed] [Google Scholar]
- 9.Tinnefeld P, Cordes C. ‘Self-healing’ dyes: intramolecular stabilization of organic fluorophores. Nat Methods. 2012;9:426–427. doi: 10.1038/nmeth.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chibisov AK, Zakharova GV, Goerner H. Effects of substituents in the polymethine chain on the photoprocesses in indodicarbocyanine dyes. J Chem Soc Faraday Trans. 1996;92:4917–4925. [Google Scholar]
- 11.Montalti M, Credi A, Prodi L, Gandolfi MT. Handbook of Photochemistry. CRC Press LLC; Boca Raton: 2006. [Google Scholar]
- 12.Huang Z, Ji D, Xia A, Koberling F, Patting M, Erdmann R. Direct observation of delayed fluorescence from a remarkable back-isomerization in Cy5. J Am Chem Soc. 2005;127:8064–8066. doi: 10.1021/ja050050+. [DOI] [PubMed] [Google Scholar]
- 13.Chibisov AK, Shvedov SV, Goerner H. Photosensitized processes in dicarbocyanine dyes induced by energy transfer: delayed fluorescence, trans-cis isomerization and electron transfer. J Photochem Photobiol A. 2001;141:39–45. [Google Scholar]
- 14.Huang Z, Ji D, Wang S, Xia A, Koberling F, Patting M, Erdmann R. Spectral identification of specific photophysics of Cy5 by means of ensemble and single molecule measurements. J Phys Chem A. 2005;110:45–50. doi: 10.1021/jp0562936. [DOI] [PubMed] [Google Scholar]
- 15.Wenthold PG, Hrovat DA, Borden WT, Lineberger WC. Transition-state spectroscopy of cyclooctatetraene. Science. 1996;272:1456–1459. doi: 10.1126/science.272.5267.1456. [DOI] [PubMed] [Google Scholar]
- 16.Frutos LM, Castano O, Andres JL, Merchan M, Acuna AU. A theory of nonvertical triplet energy transfer in terms of accurate potential energy surfaces: The transfer reaction from π, π* triplet donors to 1,3,5,7-cyclooctatetraene. J Chem Phys. 2004;120:1208–1216. doi: 10.1063/1.1631418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.