Cost-effectiveness analysis supports EGFR mutation testing in patients with stage IV or recurrent lung adenocarcinoma, rebiopsying if insufficient tissue is available, and first-line erlotinib treatment for those with EGFR mutations.
Abstract
Purpose:
Patients with epidermal growth factor receptor (EGFR) mutation–positive stage IV adenocarcinoma have improved survival with tyrosine kinase inhibitor (TKI) treatments, but the cost effectiveness of personalized first-line therapy using EGFR mutation testing is unknown.
Methods:
We created a decision analytic model comparing the costs and effects of platinum combination chemotherapy with personalized therapy in which patients with EGFR mutation–positive tumors were treated with erlotinib. We used two testing strategies: testing only those with tissue available and performing a repeat biopsy if tissue was not available versus three nontargeted chemotherapy regimens (ie, carboplatin and paclitaxel; carboplatin and pemetrexed; and carboplatin, pemetrexed, and bevacizumab).
Results:
Compared with a carboplatin plus paclitaxel regimen, targeted therapy based on testing available tissue yielded an incremental cost-effectiveness ratio (ICER) of $110,644 per quality-adjusted life year (QALY), and the rebiopsy strategy yielded an ICER of $122,219 per QALY. Probabilistic sensitivity analysis revealed substantial uncertainty around these point estimates. With a willingness to pay of $100,000 per QALY, the testing strategy was cost effective 58% of the time, and the rebiopsy strategy was cost effective 54% of the time. Personalized therapy with an EGFR TKI was more favorable when the nontargeted chemotherapy regimen was more expensive. Compared with carboplatin, pemetrexed, and bevacizumab, ICERs were $25,547 per QALY for the testing strategy and $44,036 per QALY for the rebiopsy strategy.
Conclusion:
Although specific clinical circumstances should guide therapy, our cost-effectiveness analysis supports the strategy of testing for EGFR mutations in patients with stage IV or recurrent adenocarcinoma of the lung, rebiopsying patients if insufficient tissue is available for testing, and treating patients with EGFR mutations with erlotinib as first-line therapy.
Introduction
Lung cancer remains the leading cause of cancer-related death in North America and is the third most costly cancer.1 Non–small-cell lung cancer (NSCLC) accounts for 85.3% of cases,2 and approximately 50% of patients present with incurable metastatic disease (stage IV).3 Standard chemotherapeutic treatments for stage IV NSCLC lengthen expected survival by a few months; however, recent studies have suggested that patients with advanced NSCLC whose tumors are positive for certain epidermal growth factor receptor (EGFR) gene mutations have substantially improved progression-free survival with tyrosine kinase inhibitors (TKIs) such as erlotinib, compared with conventional chemotherapy with a platinum combination regimen.4–12 The National Comprehensive Cancer Network now recommends treating stage IV adenocarcinoma with first-line TKIs for patients with EGFR-activating mutations,13 but debate continues over whether this is appropriate in the first-line setting14,15 and, if so, whether the therapeutic gains are worth the increased cost.
Two analyses have examined the cost effectiveness of TKIs; however, one examined TKIs as second-line therapy, and the other was limited to an East Asian population. One study found that the incremental cost effectiveness of second-line treatment with erlotinib for patients with EGFR-activating mutations (EGFR positive) and docetaxel for patients without such mutations (EGFR negative) compared with docetaxel for all patients was $162,018 per quality adjusted life year (QALY) gained.16 This figure is higher than commonly accepted cost-effective thresholds, and under most circumstances, it would be considered too expensive. However, this study focused on second-line treatment in an unselected population, for whom the survival and quality of life benefits were modest. There may be greater benefit in administering erlotinib as first-line treatment to EGFR mutation–positive patients. This study also evaluated gene copy and protein expression testing, which have mainly been replaced by more predictive mutation testing. The other study compared first-line treatment with the TKI gefitinib with platinum combination chemotherapy using costs and effects from Singapore.17 They found that mutation testing was less costly and more effective than standard chemotherapy, but these results may not hold in a US population. EGFR mutations are much more common in Asian populations than in the general US population,18 and costs may differ substantially between the two countries. In our study, we developed a decision analytic model to evaluate the incremental cost effectiveness (ICER) of EGFR mutation testing to inform first-line treatment in patients with stage IV NSCLC in the United States from a payer's perspective.
Methods
Testing Strategies
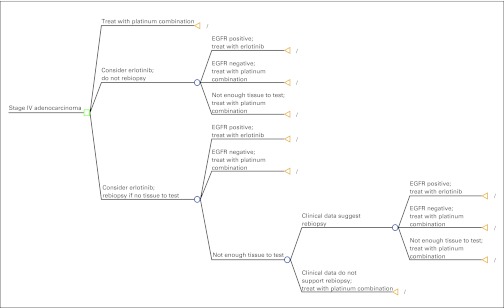
Our decision analytic model estimated the incremental costs and benefits of a theoretic cohort of patients with stage IV adenocarcinoma under three different treatment pathways (Appendix Fig A1, online only). In the base case, all patients were treated with combination chemotherapy with a platinum agent, and none were tested for EGFR mutations. Because a substantial proportion of patients would not have tissue samples available for EGFR testing (44% and 55% in IPASS [Iressa Pan-Asia Study]17 and BR.21 [National Cancer Institute of Canada Clinical Trials Group Study]16 trials, respectively), we examined two EGFR mutation testing strategies: one in which testing was performed only on patients with sufficient tumor tissue (test strategy), and one in which patients without available tissue underwent a repeat biopsy to provide tissue for testing (rebiopsy strategy). In either testing strategy, patients who tested negative or had insufficient tissue for determination were treated with platinum combination chemotherapy. We assumed that 15% of repeat biopsies would yield insufficient tissue for mutation testing. Additionally, we assumed that 50% of rebiopsies would be performed bronchoscopically and 25% via transthoracic needle aspiration and the remaining 25% would be needle aspiration biopsies of metastatic sites. These percentages were based on clinical experience.
Comparator Chemotherapy Regimens
Many platinum combination chemotherapy regimens are available to treat adenocarcinoma. Because these regimens have widely varying costs,19 we evaluated the testing strategies with three commonly used platinum combination regimens that span this variability: carboplatin plus paclitaxel, a relatively inexpensive and widely used treatment option considered standard by many clinicians; carboplatin plus pemetrexed, a more expensive and less toxic regimen; and carboplatin, pemetrexed, and bevacizumab, one of the most expensive and effective regimens available based on data from phase II trials.20 Although a given chemotherapy regimen might not be appropriate for all patients, we framed the model as a choice between each regimen and EGFR testing in a population eligible for the regimen. The base results from each regimen were then compared against alternative treatment pathways whereby the patients underwent EGFR testing, for a total of nine possible treatment pathways.
Because of limited data about later clinical effects, we did not model second- or third-line treatments. We explicitly assumed that after failure of first-line treatment, subsequent treatments would have equal effects unrelated to treatment administered in the first-line setting. Furthermore, we assumed that the proportion of patients receiving additional treatments would be equal across all groups. The model was built in TREEAge Pro 2009 (TREEAge Software, Williamstown, MA).
Clinical Effects
We derived overall and progression-free survival on each medication from median survival times (Table 1) reported in randomized clinical trials conducted in US and European populations.9,10,20–23,32 Although mean survival times would have been preferable, because they are consistent with the decision analytic model39 and better describe average effects in the population, only median survival times were published in the literature.
Table 1.
Model Inputs
| Variable | Base Patient Case | Low | High | Source |
|---|---|---|---|---|
| Overall survival, months | ||||
| Carboplatin plus paclitaxel (EGFR negative or unknown) | 8.1 | 7 | 9.5 | Schiller et al21 |
| Carboplatin plus pemetrexed (EGFR negative or unknown) | 12.0 | 7.6 | 17.1 | Zinner et al,22 Scagliotti et al23 |
| Carboplatin, pemetrexed, and bevacizumab (EGFR negative or unknown) | 14.1 | 10.6 | 19.6 | Patel et al20 |
| Erlotinib (EGFR positive) | 24.0 | 17.5 | 27.0 | Rosell et al,9 Sequist et al10 |
| Progression-free survival, months | ||||
| Carboplatin plus paclitaxel (EGFR negative or unknown) | 3.1 | 2.8 | 3.9 | Schiller et al21 |
| Carboplatin plus pemetrexed (EGFR negative or unknown) | 5.5 | 3.4 | 8.3 | Zinner et al,22 Scagliotti et al23 |
| Carboplatin, pemetrexed, and bevacizumab (EGFR negative or unknown) | 7.8 | 5.2 | 11.5 | Patel et al20 |
| Erlotinib (EGFR positive) | 12.0 | 6.2 | 16.7 | Rosell et al,9 Sequist et al10 |
| Health state utilities | ||||
| Stable disease while receiving oral therapy | 0.670 | 0.335 | 0.080 | Carlson et al16 |
| Stable disease while receiving IV chemotherapy | 0.653 | 0.327 | 0.670 | Carlson et al,16 Nafees et al24 |
| Progressive disease | 0.473 | 0.237 | 0.670 | Carlson et al,16 Nafees et al24 |
| Stable disease plus | ||||
| Rash | 0.640 | 0.320 | 0.670 | Carlson et al,16 Nafees et al24 |
| Neutropenia | 0.670 | 0.335 | 0.670 | Expert opinion |
| Febrile neutropenia | 0.563 | 0.282 | 0.670 | Carlson et al,16 Nafees et al24 |
| Pneumothorax | 0.630 | 0.315 | 0.670 | Expert opinion |
| Hemorrhage | 0.630 | 0.315 | 0.670 | Expert opinion |
| Nausea/vomiting | 0.605 | 0.303 | 0.670 | Nafees et al24 |
| Neuropathy | 0.620 | 0.310 | 0.670 | Carlson et al16 |
| Thrombocytopenia | 0.650 | 0.325 | 0.670 | Expert opinion |
| Thrombosis | 0.563 | 0.281 | 0.670 | Expert opinion |
| Probabilities | ||||
| EGFR positive | 0.15 | NA | NA | Rosell et al,9 Shigematsu et al,18 Johnson et al25 |
| Not enough tissue for EGFR testing | 0.50 | 0.30 | 0.70 | Mok et al,7 Tsao et al26 |
| Noninformative rebiopsy | 0.15 | 0.10 | 0.25 | Expert opinion |
| Pneumothorax (bronchoscopic biopsy) | 0.01 | 0.01 | 0.05 | Eberhardt et al,27 Facciolongo et al,28 Geraghty et al,29 Hergott et al30 |
| Pneumothorax (transthoracic needle aspiration biopsy) | 0.09 | 0.05 | 0.15 | Geraghty et al,29 Hergott et al30 |
| Hemorrhage resulting from biopsy | 0.01 | NA | NA | Facciolongo et al28 |
| Carboplatin plus pemetrexed AE | ||||
| Neutropenia | 0.197 | 0.080 | 0.310 | Zinner et al,22 Scagliotti et al23 |
| Febrile neutropenia | 0.014 | 0.000 | 0.050 | Zinner et al,22 Scagliotti et al23 |
| Thrombocytopenia | 0.085 | 0.000 | 0.160 | Zinner et al,22 Scagliotti et al23 |
| Erlotinib AE | ||||
| Rash | 0.060 | 0.038 | 0.082 | Genentech31 |
| Carboplatin plus paclitaxel AE | ||||
| Neutropenia | 0.630 | 0.574 | 0.633 | Schiller et al21 |
| Febrile neutropenia | 0.040 | 0.017 | 0.063 | Schiller et al21 |
| Nausea/vomiting | 0.080 | 0.049 | 0.111 | Schiller et al21 |
| Neuropathy | 0.100 | 0.065 | 0.135 | Schiller et al21 |
| Anemia | 0.100 | 0.065 | 0.135 | Schiller et al21 |
| Carboplatin, pemetrexed, and bevacizumab AE | ||||
| Anemia | 0.091 | 0.027 | 0.155 | Patel et al,20 Malhotra et al32 |
| Thrombocytopenia | 0.039 | 0.000 | 0.082 | Patel et al,20 Malhotra et al32 |
| Thrombosis | 0.052 | 0.002 | 0.102 | Patel et al,20 Malhotra et al32 |
| Febrile neutropenia | 0.091 | 0.027 | 0.155 | Patel et al,20 Malhotra et al32 |
| Cost | ||||
| Erlotinib (150 mg per day for 30 days) | $4,336 | $2,168 | $6,505 | Drugstore.com33 |
| Pemetrexed (per cycle) | $4,709 | $2,354 | $7,063 | Centers for Medicare & Medicaid Services34 |
| Carboplatin (per cycle) | $83 | $41 | $124 | Centers for Medicare & Medicaid Services34 |
| Paclitaxel (per cycle) | $86 | $43 | $129 | Drugstore.com33 |
| Bevacizumab (per cycle) | $6,538 | $3,269 | $9,807 | Drugstore.com33 |
| Cleocin-T gel | $105 | $52 | $157 | Drugstore.com33 |
| Neupogen | $3,866 | $1,933 | $5,799 | Drugstore.com33 |
| EGFR gene copy test (CPT-88368) | $243 | $122 | $365 | Centers for Medicare & Medicaid Services35 |
| IV chemotherapy infusion per cycle (CPT-96413) | $335 | $168 | $503 | Centers for Medicare & Medicaid Services35 |
| Transthoracic needle biopsy (CPT-32405) | $733 | $366 | $1,099 | Centers for Medicare & Medicaid Services35 |
| Broncoscopic rebiopsy (CPT-31625) | $840 | $420 | $1,261 | Centers for Medicare & Medicaid Services35 |
| Other biopsy (CPT-47000) | $732 | $366 | $1,098 | Centers for Medicare & Medicaid Services35 |
| Chest tube (CPT-32551) | $527 | $286 | $791 | Centers for Medicare & Medicaid Services35 |
| Outpatient visit (CPT-99233) | $96 | $48 | $143 | Centers for Medicare & Medicaid Services35 |
| Inpatient visit (CPT-99255) | $202 | $101 | $303 | Centers for Medicare & Medicaid Services35 |
| Febrile neutropenia (MS-DRG 814) | $7,057 | $3,528 | $10,585 | Centers for Medicare & Medicaid Services36 |
| RBC transfusion per unit (CPT-36430) | $515 | $258 | $773 | Centers for Medicare & Medicaid Services35 |
| Pyridoxine (per month) | $16 | $8 | $23 | Drugstore.com33 |
| Oral ondansetron (treating one episode of vomiting) | $12 | $6 | $18 | Drugstore.com33 |
| IV ondansetron (treating one episode of vomiting) | $100 | $50 | $150 | Drugstore.com33 |
| Administering IV fluids | $172 | $86 | $258 | Centers for Medicare & Medicaid Services35 |
| Gabapentin | $586 | $243 | $729 | Drugstore.com33 |
| US/dopplers (for diagnosis of DVT CPT-93970) | $254 | $127 | $381 | Centers for Medicare & Medicaid Services35 |
| Enoxaparin (per day) | $100 | $50 | $150 | Drugstore.com33 |
| Rebiopsy pathology (CPT-88305 and -88342) | $271 | $136 | $407 | Centers for Medicare & Medicaid Services35 |
| Disease progression per month | $5,219 | $2,610 | $7,829 | Yabroff et al,37 Bureau of Labor and Statistics38 |
Abbreviations: AE, adverse event; CPT, current procedural terminology; DVT, deep vein thrombosis; EGFR, epidermal growth factor receptor; IV, intravenous; MS-DRG, Medicare severity–diagnosis-related group; NA, not applicable.
We determined severe adverse event rates for the different clinical interventions from published results of randomized clinical trials (Table 1).20–23,32 We considered only grades 3 and 4 adverse events, including those requiring hospitalization and those that were disabling, prevented self-care, or were life threatening.40 We included only adverse events with a frequency of ≥ 5% and those requiring hospitalization, if frequency rates were lower.16 We assumed adverse events were treated with one additional physician visit and other standard treatment as appropriate. For example, severe vomiting was treated with 2 hours of intravenous fluids and 24 mg intravenous ondansetron.
We adjusted our estimates for quality of life, because the value of a month of life varies according to severity of disease. We made adjustments using published utility estimates for patients with lung cancer.16,24 The highest possible utility was the state of progression-free survival while receiving oral therapy (erlotinib), estimated at 0.67.24 Disease progression reduced utility, as did adverse events resulting from treatment, with the size of reduction depending on severity of complication or disease state (Table 1). For an uncomplicated rebiopsy, we assumed the reduction in quality of life would be minimal. All treatment benefits were then calculated as QALYs.
Costs
We estimated costs for outpatient medications from retail charges collected from drugstore.com. We estimated inpatient medication costs from the 2010 Medicare Part B Average Selling Price file (Table 1). For carboplatin dosing, we used a standard area under the concentration curve of 6 mg/mL/min41 and assumed male sex, 65 years of age, weight of 70 kg, height of 70 in, and serum creatinine of 1. For pemetrexed, we used 500 mg/m2, assuming average body weight of 70 kg and height of 70 in.42 Dosage of paclitaxel was 200 mg/m3,43,44 and dosage of bevacizumab was 15 mg/kg, each administered once every 3 weeks.20 We assumed that all platinum combination regimens would be administered for six treatment cycles. The dosage for erlotinib was 150 mg daily for all patients, administered until progression. We derived medical resource costs for physician services and hospitalizations based on Medicare inpatient and outpatient reimbursement rates (Table 1).
We determined the cost of time spent in disease progression using the analysis by Yabroff et al,37 which estimated the treatment costs of Medicare patients with lung cancer in the last 12 months of life using Surveillance, Epidemiology, and End Results–Medicare data. We derived a monthly cost from this analysis. We then updated the estimate to 2009 dollars using the consumer price index for medical care services.38 Because of the short survival times, we did not discount costs.
Sensitivity Analysis
We performed univariate and multivariate sensitivity analyses to test the robustness of the model and to determine which parameters most influenced the model findings. We set upper and lower limits for the model inputs based on the literature or expert opinion. Limits for survival times and adverse event probabilities were based on the 95% CIs reported in the literature,9,10,20–23,31,32 and costs were set at ± 50% of their mean point estimates. We also investigated the effect of increasing all survival times by a constant rate, because the median survival would likely underestimate the mean survival.
We were particularly interested in the effects of the pretest probability of EGFR-positive tumors on the cost effectiveness of the treatments. In the US population, approximately 15% of lung adenocarcinomas are EGFR mutation positive.9,25 That figure is higher in women (22%), never smokers (27%), and patients of East Asian origin (30%).18 We hypothesized that alternative strategies including EGFR mutation testing would be more cost effective in patients who were more likely to have an EGFR mutation.
We performed multivariate sensitivity analysis by simultaneously varying the most influential parameters in a Monte Carlo simulation. We defined distributions for the parameters of interest, took repeated random draws from those distributions, and recalculating the model results based on those draws. This process was repeated 10,000 times to provide a distribution of the model results. Costs were assumed to be gamma distributed with the standard deviation set equal to the mean estimate because of the long tails typically associated with health care costs. Survival times were modeled using exponential distribution, and probabilities and utilities were modeled using beta distribution assuming a standard deviation of 0.1.
Results
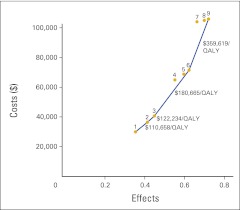
Our first set of analyses compared the three strategies (ie, base, test, and rebiopsy) within each of the three platinum chemotherapy regimens: carboplatin plus paclitaxel; carboplatin plus pemetrexed; and carboplatin, pemetrexed, and bevacizumab. The model estimated the benefits for the three testing strategies as 0.361 (base), 0.420 (test), and 0.454 (rebiopsy) QALYs, with carboplatin plus paclitaxel as the standard regimen (Table 2). Total costs, including direct medication costs as well as ancillary costs, for the three testing strategies with carboplatin plus paclitaxel were $29,987 (base), $36,460 (test), and $40,689 (rebiopsy). As expected, drug acquisition and delivery costs accounted for a substantial proportion of total cost; these varied considerably across regimens. For the duration of first-line treatment, the mean medication costs were $1,995 (carboplatin plus paclitaxel), $30,611 (carboplatin plus pemetrexed), and $69,837 (carboplatin, pemetrexed, and bevacizumab). In all cases, starting patients on first-line erlotinib under the test or rebiopsy strategy increased both QALYs and costs compared with the base strategy (Table 2). Therefore, the ICER comparing carboplatin plus paclitaxel under the test strategy with the same regimen under the base strategy was $110,658 per QALY; comparing carboplatin plus paclitaxel under the rebiopsy strategy with same regimen under the test strategy, it was $122,234 per QALY. For the more expensive chemotherapy regimens (ie, carboplatin plus pemetrexed and carboplatin, pemetrexed, and bevacizumab), the test and rebiopsy strategies had more favorable ICERs than when carboplatin plus paclitaxel was the baseline regimen (Table 2; Fig 1).
Table 2.
Base Patient Case Results
| Strategy | Cost | Incremental Cost | Effect | Incremental Effect | C/E | Incremental Ratio | Final ICER |
|---|---|---|---|---|---|---|---|
| Carboplatin plus paclitaxel | |||||||
| 1. Base | $29,987 | 0.361 | $83,046 | ||||
| 2. Test | $36,460 | $6,474 | 0.420 | 0.058 | $86,892 | $110,658 | $110,658 |
| 3. Rebiopsy | $40,689 | $4,229 | 0.454 | 0.035 | $89,583 | $122,234 | $122,234 |
| Carboplatin plus pemetrexed | |||||||
| 4. Base | $64,962 | $24,273 | 0.555 | 0.101 | $117,067 | $241,303 | Extended dominance* |
| 5. Test | $68,812 | $3,850 | 0.599 | 0.044 | $114,901 | $87,561 | Extended dominance* |
| 6. Rebiopsy | $71,480 | $2,668 | 0.625 | 0.026 | $114,413 | $103,132 | $180,665 |
| Carboplatin, pemetrexed, and bevacizumab | |||||||
| 7. Base | $104,104 | $32,624 | 0.664 | 0.039 | $156,817 | $834,334 | Extended dominance* |
| 8. Test | $105,019 | $915 | 0.700 | 0.036 | $150,100 | $25,547 | Extended dominance* |
| 9. Rebiopsy | $105,940 | $921 | 0.721 | 0.021 | $147,021 | $44,036 | $359,619 |
Abbreviations: C/E, cost/effect; ICER, incremental cost effectiveness ratio.
Strategy had a less favorable ICER than a more effective and expensive strategy.
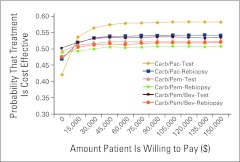
Figure 1.
Cost-effectiveness frontier. Costs and effects of each of the nine potential treatment strategies are plotted. Strategies 4, 5, 7, and 8 lie inside of the cost-effectiveness frontier, because alternative strategies are preferred to them. QALY, quality-adjusted life year.
Our second set of analyses compared the rebiopsy approach across the three platinum chemotherapy strategies. The ICER for carboplatin plus pemetrexed under the rebiopsy strategy versus carboplatin plus paclitaxel under the same strategy was $180,665, and the ICER of carboplatin, pemetrexed, and bevacizumab under rebiopsy versus carboplatin plus pemetrexed under the same strategy was $359,619. Comparing across chemotherapy regimens, the rebiopsy strategy had a more favorable ICER than the base or test strategy for both carboplatin plus pemetrexed and carboplatin, pemetrexed, and bevacizumab (Table 2; Fig 1).
Results of the univariate and multivariate sensitivity analyses are available in Appendix Tables A1 and A2 (online only). We found the results to be largely insensitive to varying the probability that a patient was EGFR positive. Although our results were based on median survival, results were not sensitive to analyses based on alternative approaches designed to provide a proxy for mean survival.
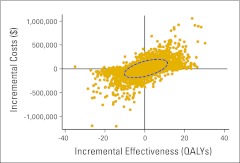
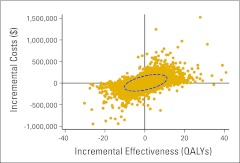
The multivariate sensitivity analysis revealed that for carboplatin plus paclitaxel under test versus the same regimen under base, carboplatin plus paclitaxel under test was more expensive and more effective for 44.3% of the draws. For carboplatin plus paclitaxel under rebiopsy versus the same regimen under test, carboplatin plus paclitaxel under rebiopsy was more expensive and more effective for 39.5% of the draws (Appendix Figs A2 and A3, online only). Using cost-effectiveness acceptability curves, we found that the test and rebiopsy strategies with any of the baseline chemotherapy regimens were cost effective slightly more than 50% of the time for a wide range of commonly used cost-effectiveness thresholds (Appendix Fig A4, online only).
Discussion
Our study has two main findings. First, our analysis showed that targeted first-line treatment with erlotinib for mutation-positive patients with stage IV adenocarcinoma is marginally cost effective when compared within any of the three standard platinum combination chemotherapy regimens. A cutoff of $100,000 per QALY is widely cited in the literature, but somewhat larger ratios are often accepted for treatment of advanced cancer.45,46 Therefore, with an ICER of $110,658 per QALY for the test strategy and $122,234 per QALY for the rebiopsy strategy, EGFR mutation testing with targeted use of erlotinib may be considered cost effective when compared with carboplatin plus paclitaxel as a baseline regimen. However, the results of the probabilistic sensitivity analysis showed that there is uncertainty surrounding this conclusion. Furthermore, others may have higher or lower thresholds for cost effectiveness, which could lead to different conclusions.
Second, our analysis showed that the more expensive platinum combination regimens do not necessarily meet conventional cost-effectiveness acceptability thresholds when compared with carboplatin plus paclitaxel. It should be noted that the choice of chemotherapy platform regimen is not molecularly driven but instead based on toxicity, convenience, and historic efficacy. The carboplatin, pemetrexed, and bevacizumab regimen had a particularly unfavorable incremental cost-effectiveness ratio of $359,619 per QALY for the rebiopsy strategy. Although these regimens generate suboptimal cost effectiveness, they are commonly used in current clinical practice. Hence, one might question the use of these more expensive platinum combination regimens because of their relatively poor cost effectiveness, but if a clinician were to choose to use these regimens, cost effectiveness would be enhanced by following the rebiopsy strategy.
Our analysis has several limitations. First, we were unable to model the full course of therapy across a patient's lifetime, which often includes second- and even third-line treatments. Ideally, we would want to fully account for both the costs and effects of these treatments, but inputs for such a model are not readily available in the existing literature, particularly in patients who receive a TKI first line. Virtually no randomized trial assigns both first- and second-line treatments, and the distinctions among second- and third-line therapies are relatively small with respect to survival. A study of European patients with EGFR mutations showed no difference in survival when TKIs were administered in the first or second line,9 but this study was observational and potentially subject to selection bias for patients treated in the second line.
This analysis is also limited by our use of a model-based approach rather than a randomized trial. As such, our conclusions are dependent on the validity of the assumptions used to develop the model. For example, we used estimates of costs and effects from available sources, such as clinical trial results and Medicare reimbursement rates, which may not be reflective of costs and effects in the entire treatment population. To assess the effects of any violations of our assumptions, we used sensitivity analyses, in which we showed that our conclusions were fairly robust over plausible ranges. Because EGFR mutation testing and first-line use of erlotinib have only recently been adopted, we were not able to gather costs and effects from claims and registry data. Ideally, our results should be validated with such data as they become available. Finally, the current analyses are subject to modifications in cost. Drug costs, in particular, are typically reduced with time, and those reductions will make all strategies more favorable.
Despite these limitations, our model reveals that personalized therapy with TKIs for advanced adenocarcinoma is cost effective using thresholds acceptable in current US practice. This was especially true when we compared the personalized strategy with newer, more expensive chemotherapy regimens. When comparing treatment strategies with different chemotherapy regimens, the strategy including a repeat biopsy for patients without tissue available for EGFR testing was favorable, indicating that the additional biopsy adds value. This analysis supports the current clinical recommendation to test for EGFR mutations early in the course of treatment for advanced adenocarcinoma.
Acknowledgment
We thank Henry Glick, PhD, who provided helpful guidance in constructing the model. Supported by Award No. UC2CA148310 from the National Cancer Institute and in part by a grant from the Pennsylvania Department of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Appendix
Table A1.
One-Way Sensitivity Analysis* of the Most Influential Inputs (cost per QALY)
| Variable | Least Favorable |
Most Favorable |
||
|---|---|---|---|---|
| Test v Base | Rebiopsy v Test | Test v Base | Rebiopsy v Test | |
| Carboplatin plus paclitaxel as reference regimen | ||||
| Utility of stable disease during oral therapy | $221,316 | $244,656 | $74,136 | $81,852 |
| Cost of erlotinib | $144,012 | $155,784 | $77,304 | $88,680 |
| Cost of progression per month | $121,884 | $133,524 | $83,184 | $94,596 |
| PFS with erlotinib | $133,344 | $146,568 | $95,940 | $106,452 |
| Utility of disease progression | $134,436 | $148,644 | $96,444 | $106,476 |
| Carboplatin plus pemetrexed as reference regimen | ||||
| Utility of stable disease during oral therapy | $175,122 | $206,396 | $73,332 | $86,351 |
| Cost of erlotinib | $131,938 | $148,014 | $43,184 | $58,249 |
| PFS with carboplatin plus pemetrexed | $121,097 | $138,907 | $65,380 | $79,679 |
| Cost of pemetrexed | $111,659 | $127,504 | $63,468 | $78,764 |
| PFS with erlotinib | $114,756 | $133,572 | $71,202 | $84,852 |
| Carboplatin, pemetrexed, and bevacizumab as reference regimen | ||||
| OS with erlotinib | $46,764 | $61,848 | Base dominated† | Test dominated† |
| OS with carboplatin, pemetrexed, and bevacizumab | $48,432 | $63,228 | Base dominated† | Test dominated† |
| Cost of erlotinib | $80,052 | $99,540 | Base dominated† | Test dominated† |
| Cost of bevacizumab | $66,636 | $85,872 | Base dominated† | $2199 |
| PFS with carboplatin, pemetrexed, and bevacizumab | $73,944 | $96,432 | Base dominated† | $13,967 |
Abbreviations: OS, overall survival; PFS, progression-free survival; QALY, quality-adjusted life year.
In the one-way sensitivity analysis, the value of a single input is set to the end of its hypothesized range, and the ICER is recalculated.
A strategy is dominated if it is more costly and less effective than the alternative.
Table A2.
Expected Cost Per QALY for Population Subgroups
| Probability EGFR Positive (%) | Population | Carboplatin Plus Paclitaxel As Reference |
Carboplatin Plus Pemetrexed As Reference |
Carboplatin, Pemetrexed, and Bevacizumab As Reference |
|||
|---|---|---|---|---|---|---|---|
| Test v Base | Rebiopsy v Test | Test v Base | Rebiopsy v Test | Test v Base | Rebiopsy v Test | ||
| 6 | Men, smokers (low estimate) | $113,784 | $142,620 | $91,712 | $131,028 | $30,645 | $78,184 |
| 10 | Men, smokers | $111,696 | $129,000 | $88,945 | $112,343 | $27,246 | $55,231 |
| 15 | General population | $110,664 | $122,232 | $87,561 | $103,132 | $25,547 | $44,036 |
| 20 | Women (low estimate) | $110,136 | $118,860 | $86,869 | $98,558 | $24,697 | $38,508 |
| 26 | Eastern Asian (low estimate) | $109,776 | $116,532 | $86,225 | $94,319 | $24,109 | $34,707 |
| 32 | Eastern Asian, nonsmoker | $109,548 | $115,068 | $86,091 | $93,438 | $23,741 | $32,342 |
| 38 | Women | $109,404 | $114,084 | $85,886 | $92,096 | $23,489 | $30,728 |
| 59 | Women (high estimate) | $109,104 | $112,188 | $85,497 | $89,552 | $23,012 | $27,676 |
| 69 | Nonsmokers (high estimate) | $109,032 | $111,696 | $85,395 | $88,885 | $22,887 | $26,878 |
Abbreviations: EGFR, epidermal growth factor receptor; QALY, quality-adjusted life year.
Figure A1.
Baseline decision tree reflecting three testing strategies. Each path contained possible adverse events, which are not shown here. EGFR, epidermal growth factor receptor.
Figure A2.
Probabilistic sensitivity analysis of carboplatin plus paclitaxel under the test strategy versus the same regimen under the base strategy. The ellipse represents the 95% CI, and it spans all four quadrants of the cost-effectiveness plane. QALY, quality-adjusted life year.
Figure A3.
Probabilistic sensitivity analysis of carboplatin plus paclitaxel under the rebiopsy strategy versus the same regimen under the test strategy. The ellipse represents the 95% CI, and it spans all four quadrants of the cost-effectiveness plane. QALY, quality-adjusted life year.
Figure A4.
Cost-effectiveness acceptability curve. On the basis of the probabilistic sensitivity analysis, all strategies are cost effective slightly more than 50% of the time over a wide range of cost-effectiveness thresholds. Bev, bevacizumab; Carb, carboplatin; Pac, paclitaxel; Pem, pemetrexed.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Corey J. Langer, Genentech (C), Eli Lilly (C), OSI Pharmaceuticals/Genentech (C), Bristol-Myers Squibb/Imclone (C) Stock Ownership: None Honoraria: None Research Funding: Corey J. Langer, Eli Lilly, OSI Pharmaceuticals/Genentech Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Elizabeth A. Handorf, Sean McElligott, Anil Vachani, Corey J. Langer, Katrina Armstrong, David A. Asch
Administrative support: Mirar Bristol Demeter
Collection and assembly of data: Elizabeth A. Handorf, Sean McElligott, Mirar Bristol Demeter
Data analysis and interpretation: Elizabeth A. Handorf, Sean McElligott, Anil Vachani, David A. Asch
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.National Cancer Institute. Costs of cancer care. http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2007&chid=75&coid=726&mid=
- 2.National Cancer Institute. SEER cancer statistics review 1975-2008. http://seer.cancer.gov/csr/1975_2008/index.html.
- 3.National Cancer Institute. SEER fast stats. http://seer.cancer.gov/faststats/selections.php?#Output.
- 4.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non–small-cell lung cancer: Data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Phase III study (Tarceva) vs. chemotherapy to treat advanced non-small-cell lung cancer (NSCLC) in patients with mutations in the TK domain of EGFR: NCT00446225. http://clinicaltrials.gov/ct2/show/NCT00446225?term=NCT00446225&rank=1.
- 9.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non–small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C, Wu YL, Chen GY, et al. Efficacy results from the randomised phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine(GEM), in Chinese advanced non-small-cell lung cancer (NSCLC) patients (pts) with EGFR activatingmutations. Presented at the European Society for Medical Oncology Congress; October 8-12, 2010; Milan, Italy. [Google Scholar]
- 12.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Non-small-cell lung cancer. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 14.Pirker R. Stage IV: Targeted therapy as first line treatment—Yes. Presented at the 2nd European Lung Cancer Conference; April 28-May 1, 2010; Geneva, Switzerland. [Google Scholar]
- 15.Scagliotti GV. Stage IV: Targeted therapy as first line treatment—No. Presented at the 2nd European Lung Cancer Conference; April 28-May 1, 2010; Geneva, Switzerland. [Google Scholar]
- 16.Carlson JJ, Garrison LP, Ramsey SD, et al. The potential clinical and economic outcomes of pharmacogenomic approaches to EGFR-tyrosine kinase inhibitor therapy in non-small-cell lung cancer. Value Health. 2009;12:20–27. doi: 10.1111/j.1524-4733.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 17.de Lima Lopes G, Jr, Segel JE, Tan DS, et al. Cost-effectiveness of epidermal growth factor receptor mutation testing and first-line treatment with gefitinib for patients with advanced adenocarcinoma of the lung. Cancer. 2011;118:1032–1039. doi: 10.1002/cncr.26372. [DOI] [PubMed] [Google Scholar]
- 18.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Muehlenbein C, Liepa AM, et al. Cost-effectiveness of pemetrexed plus cisplatin as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2009;4:1404–1414. doi: 10.1097/JTO.0b013e3181ba31e0. [DOI] [PubMed] [Google Scholar]
- 20.Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer. J Clin Oncol. 2009;27:3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 21.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 22.Zinner RG, Fossella FV, Gladish GW, et al. Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer. 2005;104:2449–2456. doi: 10.1002/cncr.21480. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti GV, Kortsik C, Dark GG, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: A multicenter, randomized, phase II trial. Clin Cancer Res. 2005;11:690–696. [PubMed] [Google Scholar]
- 24.Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson BE, Ryan AJ, Heymach J, et al. Tumor biomarker analyses from the phase III ZODIAC study of docetaxel (D) plus or minus vandetanib (VAN) in second-line advanced NSCLC. J Clin Oncol. 2010;28(suppl):542s. abstr 7516. [Google Scholar]
- 26.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer: Molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 27.Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 28.Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy: Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71:8–14. doi: 10.4081/monaldi.2009.370. [DOI] [PubMed] [Google Scholar]
- 29.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: Needle size and pneumothorax rate. Radiology. 2003;229:475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- 30.Hergott CA, Tremblay A. Role of bronchoscopy in the evaluation of solitary pulmonary nodules. Clin Chest Med. 2010;31:49–63. doi: 10.1016/j.ccm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Genentech. Tarceva prescribing information. www.gene.com/gene/products/information/pdf/tarceva-prescribing.pdf.
- 32.Malhotra B, Evans T, Weiss J, et al. Carboplatin/pemetrexed/bevacizumab in the treatment of patients with advanced non-small-cell lung cancer: A single-institution experience. Clin Lung Cancer. 2010;11:192–197. doi: 10.3816/CLC.2010.n.025. [DOI] [PubMed] [Google Scholar]
- 33.Drugstore.com. Online pharmacy. www.drugstore.com/prescriptions/qxc10663.
- 34.Centers for Medicare and Medicaid Services. Medicare Part B drug average sales price, April 2010. http://www.cms.gov/McrPartBDrugAvgSalesPrice/01a19_2010aspfiles.asp#TopOfPage.
- 35.Centers for Medicare and Medicaid Services. FY 2009 physician fee schedule. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. [PubMed]
- 36.Centers for Medicare and Medicaid Services. FY 2009 inpatient prospective payment system final rule. www.cms.gov/AcuteInpatientPPS/IPPS2009/list.asp#TopOfPage.
- 37.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 38.Bureau of Labor and Statistics. Inflation and prices. http://data.bls.gov.
- 39.Willan AR, Briggs AH. Chichester, England: John Wiley; 2006. Statistical analysis of cost-effectiveness data. [Google Scholar]
- 40.National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 41.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 42.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 43.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: Results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. CHOosing Interventions that are Cost Effective (WHO-CHOICE) www.who.int/choice/costs/CER_levels/en/index.html. [DOI] [PMC free article] [PubMed]
- 46.Appleby J, Devlin N, Parkin D. NICE's cost effectiveness threshold. BMJ. 2007;335:358–359. doi: 10.1136/bmj.39308.560069.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]