The authors demonstrate that collection of urine to measure anastrozole levels is feasible and reliable.
Abstract
Purpose:
Multiple studies have shown that adherence to adjuvant hormonal therapy in women with breast cancer is suboptimal. Measurements of compliance with self-report, pill counts, and/or pharmacy records are susceptible to bias. We assessed the feasibility of using a urine anastrozole assay as an objective biomarker of nonadherence to anastrozole treatment.
Patients and Methods:
We recruited consecutive postmenopausal women, age ≥ 18 years, with hormone-sensitive nonmetastatic breast cancer who were prescribed anastrozole at least 3 months before enrollment. Each completed a short survey to gather information on demographics, anastrozole compliance history, and self-reported medication history, tumor characteristics, and treatment received. A single, random 15-mL urine sample was collected and tested for the presence of anastrozole using a previously validated assay. Patients were told they were part of a study to determine if anastrozole could be detected in the urine.
Results:
Among 96 participants, mean age was 63.7 years (range, 51 to 70 years). The population was diverse, with 56.5% white, 57.6% US born, 59.8% unemployed, and 56.6% college educated. Prior treatment included chemotherapy (50%) and/or radiotherapy (58.7%). Mean duration of anastrozole treatment was 2.2 years (standard deviation, 1.6). Four participants reported nonadherence and declined to submit urine samples, and two had no detectable level of anastrozole (six of 96; 6.3%). Detectable levels among adherent women ranged from 49.3 to 632.8 ng/mL.
Conclusion:
We demonstrated that collection of urine to measure anastrozole levels is feasible and reliable. Identifying biomarkers to measure adherence is critical for studies investigating interventions to improve hormonal therapy compliance.
Introduction
Adjuvant hormonal therapy (tamoxifen and aromatase inhibitors) reduces mortality in women with hormone-sensitive, nonmetastatic breast cancer.1,2 Tamoxifen, in use for more than three decades, has been shown to reduce risk of breast cancer recurrence and mortality by as much as 41% and 34%, respectively.2 In postmenopausal women with breast cancer, aromatase inhibitors are also effective at reducing recurrence and decreasing mortality.1 However, the full benefits of these treatments are best achieved if they are taken for the full 5-year course, as prescribed.3 For many reasons, some women do not initiate adjuvant endocrine treatment,4–9 take the medication < 80% of the time, or discontinue their therapy early.3,6,8–11 This results in a reduction of the full survival benefit.12
Determining adherence to adjuvant hormonal therapy and assessing treatment discontinuation can be methodologically challenging, because many measures result in bias.13 Patient self-reports and medical record reviews are susceptible to misrepresentation and tend to overestimate adherence.14–16 Pill counts and electronic pill bottles have limitations, because they alter behavior, and patients can switch medicine between bottles or throw away the pills to seem adherent.14,17 Electronic pharmacy data abstraction is the least subject to bias; however, the reasons for discontinuation are unknown, and patients can switch pharmacies and seem as if they have discontinued. One possible approach is to use a biomarker such as plasma or urine drug or metabolite levels to determine hormonal treatment adherence.14,18
Athletes use aromatase inhibitors to reverse or prevent gynecomastia, an adverse effect of certain performance-enhancing anabolic androgenic steroids.18 In response to a growing underground movement of aromatase inhibitor misuse, the International Olympic Committee and the World Anti-Doping Agency added hormone antagonists and modulators to their list of prohibited substances.19 As a result, assays for these substances are commercially available through World Anti-Doping Agency–approved laboratories around the world. These tests are highly sensitive at detecting low levels of drug in the urine18 of professional and Olympic athletes,20 but they have not previously been used as a measure of adherence to adjuvant hormonal treatment among patients with breast cancer.
The purpose of this study was to assess the feasibility and reliability of using a urine anastrozole assay as a biomarker for early discontinuation of adjuvant anastrozole therapy in women with breast cancer. We hypothesized that adherence determined by the urine anastrozole level would be less than self-reported adherence.
Patients and Methods
Study Design and Recruitment
Participants were all under the care of medical oncologists at the Herbert Irving Comprehensive Cancer Center at Columbia University (New York, NY). Patients were consecutively enrolled after being prescreened for eligibility on a daily basis by their treating physician between September 2010 and September 2011. As patients presented in the clinic, each was asked to participate in a study to determine if anastrozole could be detected in the urine. Specimens were collected at the time of enrollment with no advance knowledge of testing. Written informed consent and Health Insurance Portability and Accountability Act authorization were obtained from all participants, and all procedures were approved by the Columbia University Medical Center Institution Review Board and Privacy Office.
Eligibility Criteria
Postmenopausal women age ≥ 18 years diagnosed with stages I to III primary, invasive, or secondary breast cancer, with estrogen receptor– and/or progesterone receptor–positive tumors, were included. Participants had been prescribed anastrozole for at least 3 months before enrollment and were within the 5-year planned course for the use of this medication. Women with noninvasive breast cancer or metastatic disease were excluded, as were women who were unable to provide informed consent because of moderate or severe dementia.
Study Procedures
A short questionnaire was administered at the time of enrollment. Questions included a brief history of anastrozole use (number of years taking anastrozole and days since last anastrozole dose was taken), sociodemographic information (age, race/ethnicity, nativity, educational attainment, employment status, annual household income, and medical insurance and prescription drug coverage information), and breast cancer history (tumor stage, type of surgery, and adjuvant treatment received [radiation and chemotherapy]).
Each participant provided a single, random 15-mL urine sample. All urine samples were delivered to the Biospecimen Repository of the Herbert Irving Comprehensive Cancer Center, then frozen to −70°C, packaged, and shipped in batches overnight on dry ice to the Sports Medicine Research and Testing Laboratory (Salt Lake City, UT) for analysis.
Urine Anastrozole Biomarker and Analysis
Described chemically as 1,3-benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl), anastrozole metabolizes to form triazole, a glucuronide conjugate of hydroxy-anastrozole, and a glucuronide conjugate of anastrozole itself in human plasma and urine. Only tamoxifen and estrogen-containing therapies are known to diminish the pharmacologic action of anastrozole. Food reduces but does not interfere with the overall extent of absorption. The mean elimination half-life of anastrozole is 50 hours.21
Extraction of urine samples was performed by buffering to pH 7 and incubating with β-glucuronidase (Roche, Pleasanton, CA). After incubation, samples were buffered for extraction with 0.75 mL of a 20% (w/v) solution of 1:1 K2CO3/KHCO3, and a liquid-liquid extraction was performed using 6 mL of methyl tert-butyl ether. The methyl tert-butyl ether layer was evaporated to dryness and reconstituted in mobile phase for liquid chromatography–mass spectrometry analysis. Samples were analyzed on a Thermo Finnigan TSQ Quantum mass spectrometer (Thermo Electron, Waltham, MA) coupled to a Finnigan Surveyor LC system (Thermo Electron). The mobile phases were 0.1% formic acid in water and methanol. The linear range of the assay was established at 2.5 to 750 ng/mL. The limits of detection and quantitation were established at 0.5 and 2.5 ng/mL, respectively. For a batch to be considered acceptable, at least 75% of the curve points were required to quantitate within 15% of nominal value and the quality control (QC) reagents to quantitate within 10% of their nominal value. Two QC standards were analyzed with every batch: low QC standard at 50 ng/mL and high QC standard at 500 ng/mL. All QC measurements were recorded and analyzed to determine variability in the assay over time. Over the last year, the coefficients of variation for the low and high QC standards were determined to be 5.9% and 1.8%, respectively. The aromatase inhibitor assay panel included measurement of all three aromatase inhibitors (anastrozole, letrozole, and exemestane); however, only anastrozole was reported for the purpose of this pilot study.
Statistical Analyses
Descriptive statistics were generated. For continuous variables, means and standard deviations (SDs) were computed. For categorical variables, frequency tables were produced. Nonadherence to anastrozole treatment was based on undetectable levels of anastrozole in the urine (0.0 ng/mL). All analyses were performed using PASW version 18 (IBM SPSS, Chicago, IL).
Results
A total of 96 eligible women were identified. Four participants declined to participate and provide urine samples because of self-reported nonadherence to prescribed anastrozole. Mean age of participants was 63.7 years (SD, 11.1), and a majority were between the ages of 51 to 70 years (69.5%; Table 1). Only slightly more than half (56.5%) were white, 57.6% were born in the United States, 60% had a college level or higher education, and 59.8% were not currently employed. Nearly one quarter reported an annual household income in excess of $70,000, all had medical health insurance (public or private), and 96.7% had prescription drug coverage. Approximately half the patients had been treated with chemotherapy and radiation therapy (50.0% and 58.7%, respectively).
Table 1.
Patient Sociodemographic and Clinical Characteristics (n = 92)*
| Characteristic | Total Patients |
|
|---|---|---|
| No. | % | |
| Anastrozole level, ng/mL | ||
| 0.0 | 2 | 2.2 |
| 1.0-99.0 | 14 | 15.2 |
| 100.0-199.0 | 38 | 41.3 |
| 200.0-299.0 | 23 | 25.0 |
| 300.0-399.0 | 8 | 8.7 |
| 400.0-499.0 | 3 | 3.3 |
| 500.0-599.0 | 2 | 2.2 |
| 600.0-699.0 | 2 | 2.2 |
| No. of years taking anastrozole | ||
| ≤ 1 | 35 | 38.0 |
| 2 | 15 | 16.3 |
| 3 | 17 | 18.5 |
| 4 | 13 | 14.1 |
| 5 | 11 | 12.0 |
| > 5 | 1 | 1.1 |
| No. of days since last pill taken | ||
| 0 | 58 | 63.7 |
| 1 | 31 | 34.1 |
| > 1 | 2 | 2.2 |
| Age, years | ||
| 41-50 | 9 | 9.8 |
| 51-60 | 30 | 32.6 |
| 61-70 | 34 | 36.9 |
| 71-80 | 9 | 9.8 |
| 81-90 | 9 | 9.8 |
| Unknown | 1 | 1.1 |
| Race | ||
| African American/black | 17 | 18.5 |
| Asian/Pacific Islander | 1 | 1.1 |
| Hispanic | 17 | 18.5 |
| White | 52 | 56.5 |
| Other | 5 | 5.4 |
| Birthplace | ||
| United States | 53 | 57.6 |
| Non–United States | 39 | 42.4 |
| Educational attainment | ||
| ≤ Grade school | 12 | 13.2 |
| High school | 25 | 27.5 |
| College | 28 | 30.8 |
| Graduate school | 26 | 28.6 |
| Employment status | ||
| Employed | 37 | 40.2 |
| Not employed | 55 | 59.8 |
| Annual household income | ||
| ≤ $15,000 | 16 | 17.4 |
| $15,001 to $30,000 | 12 | 13.0 |
| $30,001 to $50,000 | 9 | 9.8 |
| $50,001 to $70,000 | 8 | 8.7 |
| > $70,000 | 22 | 23.9 |
| Medical health insurance | 92 | 100.0 |
| Prescription drug coverage | 89 | 96.7 |
| Disease stage† | ||
| I | 50 | 54.3 |
| II | 25 | 27.2 |
| III | 7 | 7.6 |
| Unknown | 10 | 10.9 |
| Breast cancer treatment† | ||
| Surgery‡ | ||
| Mastectomy | 35 | 38.0 |
| Lumpectomy | 62 | 67.4 |
| Adjuvant treatment‡ | ||
| Chemotherapy | 46 | 50.0 |
| Radiation therapy | 54 | 58.7 |
For postmenopausal women with hormone-sensitive breast cancer for whom anatrozole was prescribed between August 2010 and March 2011.
Self-reported.
Categories were not mutually exclusive; total is greater than 100%.
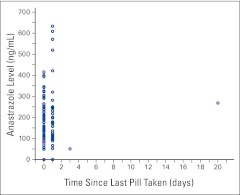
Overall, six (6.3%) of 96 participants were nonadherent to their hormonal therapy, and of these, two (2.1%) were demonstrated to be nonadherent (0.0 ng/mL) by the assay. Detectable levels of anastrozole ranged from 49.3 to 632.8 ng/mL (mean, 204.5 ng/mL; SD, 121.2). More than one third of participants reported taking anastrozole for ≤ 1 year, with a mean duration of 2.2 years (SD, 1.6; Table 1). Nearly all participants (97.8%) reported taking their last anastrozole pill the day of the interview or the day before (Fig 1), including the two participants with undetectable levels of anastrozole in their urine. However, one patient who reported last taking the pill 3 days before and another 20 days before still had detectable levels in the urine. The self-reported mean number of days since the last anastrozole pill was taken was 0.6 (SD, 2.1).
Figure 1.
Comparison of anastrozole level and self-reported number of days since last anastrozole pill was taken (n = 91).
Discussion
Results of this study demonstrate the feasibility and reliability of using urine anastrozole levels to identify women who are noncompliant with their prescribed adjuvant hormonal therapy. Two participants who reported taking their anastrozole either the same day as the interview or the day before, in fact, had no detectable level of anastrozole in their urine, whereas others whose last pill was reportedly taken 3 and 20 days before, respectively, each had detectable level of drug in the urine. This is not surprising, because the elimination half-life of anastrozole is > 2 days. On the basis of these findings, measuring anastrozole levels in the urine of women with hormone-sensitive breast cancer for whom anastrozole has been prescribed may be an unbiased measure of anastrozole adherence.
Medication adherence is an area of concern in many diseases. For some, biomarkers that evaluate clinical response to treatment are also used to measure adherence in clinical trials and in practice. For example, to suppress viral replication and avoid the emergence of resistance to highly active antiretroviral therapy, patients with AIDS need to achieve > 95% adherence to a complex dosing schedule of multiple medications.22 Biomarkers such as CD4 counts and viral load tests are used as to assess adherence and as end points in intervention trials to improve adherence. Although using an indirect method of this kind can be relatively simple and easy to perform, a disadvantage is that factors unrelated to adherence can affect the biomarker irrespective of adherence.14
No other studies to date to our knowledge have used urine aromatase inhibitor levels to assess treatment adherence. One research team in Austria developed and evaluated the usefulness of an assay that simultaneously quantified tamoxifen, anastrozole, and letrozole in human plasma. Of 310 patients with breast cancer undergoing endocrine therapy, eight were found to have unquantifiable levels of the prescribed antiestrogen drug in their system (nonadherence rate, 2.6%).23 In comparison, our rate of nonadherence of 6% was more than twice that reported by Beer et al23 but not inconsistent with other medication adherence studies using pill counts, medical record abstraction, or pharmacy data, given that this was a one-time assessment.3,4,10,11,15,16 These more traditional methods, however, are exquisitely sensitive to measurement error, whereas with urine testing, false-negative tests would be highly unusual.
There are several limitations to our study. Because of genetic and physiologic factors influencing anastrozole pharmokinetics and excretion and limited information related to anastrozole-taking habits, we were unable to interpret the range of anastrozole results that varied widely in our study. We were thus unable to determine exactly when the last pill was taken, and so, we can only state conclusively that the participants with no anastrozole in the urine had discontinued treatment in the past. However, a false-negative test would be highly unusual given the sensitivity of the test. Physicians were not queried to determine if the patient revealed nonadherence during the course of the visit. The small sample size and self-selection, although limited, may have reduced our nonadherence rate. All participants were treated in a university hospital where issues related to compliance and adherence are discussed, so the nonadherence rate may be an underestimation and may not be generalizable to other settings. Furthermore, those patients who discontinue treatment many times leave the care of the oncologist and would not be available for study recruitment. Additionally, we used a strict criterion for determination of nonadherence (0.0 ng/mL). As observed by Beer et al,23 levels at the lower end of the distribution higher than our cutoff value may represent irregular medication intake, but this would require further investigation. This assay is a qualitative test used to detect illegal doping in the sports industry and has not been clinically validated as a measure of aromatase inhibitor compliance among patients with breast cancer.
Testing for the presence of aromatase inhibitors in the urine is a reliable, feasible, and objective alternative to patient self-report and methods of determining adjuvant hormonal treatment adherence. Identification of early discontinuation may provide researchers with an unbiased outcome measure on which to base interventions that may increase the completion rate of this treatment and reduce breast cancer recurrence and mortality. Future clinical validation of this test may also provide physicians with a valuable tool with which to guide breast cancer treatment.
Acknowledgment
We thank Regina Santella, PhD, and Irina Gurvich, MS, of the Biospecimen Repository of the Herbert Irving Comprehensive Cancer Center for their assistance with this project. Supported by grants from Women at Risk (A.I.N., D.L.H.) and the Breast Cancer Research Foundation (D.L.H.), and by Department of Defense Breast Cancer Center of Excellence Award No. BC 043120 (A.I.N.).
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Grace Clarke Hillyer, Alfred I. Neugut, Dawn L. Hershman
Financial support: Alfred I. Neugut, Dawn L. Hershman
Administrative support: Grace Clarke Hillyer, Demetra Z. Rotsides
Provision of study materials or patients: Katherine D. Crew, Kevin Kalinsky, Matthew A. Maurer, Jonathan Danaceau, Dawn L. Hershman
Collection and assembly of data: Grace Clarke Hillyer, Demetra Z. Rotsides, Jonathan Danaceau
Data analysis and interpretation: Grace Clarke Hillyer, Demetra Z. Rotsides, Jonathan Danaceau, Alfred I. Neugut, Dawn L. Hershman
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickell NA, LePar F, Wang JJ, et al. Lost opportunities: Physicians' reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25:2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 5.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 6.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pini T, Griggs J, Hamilton A, et al. Patterns and correlates of adjuvant breast cancer endocrine therapy use. J Clin Oncol. 2011;29(suppl):47s. abstr 510. [Google Scholar]
- 8.Quinn V, Strauss J, Schottinger J, et al. When to intervene to increase use of adjuvant hormone therapy among with with hormone-sensitive breast cancer. J Clin Oncol. 2011;29(suppl):47s. abstr 511. [Google Scholar]
- 9.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: Predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 10.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 11.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith H, Hankins M, Hodson A, et al. Measuring the adherence to medication of elderly patients with heart failure: Is there a gold standard? Int J Cardiol. 2010;145:122–123. doi: 10.1016/j.ijcard.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 15.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 16.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 17.Rudd P, Byyny RL, Zachary V, et al. Pill count measures of compliance in a drug trial: Variability and suitability. Am J Hypertens. 1988;1:309–312. doi: 10.1093/ajh/1.3.309. [DOI] [PubMed] [Google Scholar]
- 18.Mareck U, Geyer H, Guddat S, et al. Identification of the aromatase inhibitors anastrozole and exemestane in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1954–1962. doi: 10.1002/rcm.2545. [DOI] [PubMed] [Google Scholar]
- 19.World Anti-Doping Agency. Montreal, Canada: 2011. The World Anti-Doping Code: The 2011 Prohibited List, International Standard. www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/To_be_effective/WADA_Prohibited_List_2011_EN.pdf. [Google Scholar]
- 20.Mazzarino M, Botrè F. A fast liquid chromatographic/mass spectrometric screening method for the simultaneous detection of synthetic glucocorticoids, some stimulants, anti-oestrogen drugs and synthetic anabolic steroids. Rapid Commun Mass Spectrom. 2006;20:3465–3476. doi: 10.1002/rcm.2729. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. Arimidex (anastrozole) tablet label. www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lb.pdf.
- 22.Simoni JM, Amico KR, Smith L, et al. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7:44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beer B, Schubert B, Oberguggenberger A, et al. Development and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of tamoxifen, anastrozole, and letrozole in human plasma and its application to a clinical study. Anal Bioanal Chem. 2010;398:1791–1800. doi: 10.1007/s00216-010-4075-z. [DOI] [PubMed] [Google Scholar]