Whether the United States is able to equitably meet future demand for colorectal cancer screening may depend on access, physician supply, and organization of the health care system.
Abstract
Purpose:
Causes of racial disparities in colorectal cancer (CRC) screening may extend beyond individual-level characteristics. We examined how physician density, beyond socioeconomic factors, affected observed racial disadvantages in recent CRC screening for blacks and Hispanics.
Methods:
We obtained socioeconomic and CRC screening information on adults age ≥ 50 years from the Behavioral Risk Factor Surveillance System (1997 to 2008) and information on the number of primary care physicians and gastroenterologists from the American Medical Association Masterfile (1997 to 2008). We used fixed-effect multivariate logistic regression to model the probability of receiving a fecal occult blood test within the past year or endoscopic screening within the past 5 years as a function of individual-level socioeconomic factors and state-level physician supply.
Results:
In 2008, 60.6% of whites were current on CRC screening (95% CI, 60.6% to 61.0%) compared with 57.9% of blacks (95% CI, 56.7% to 59.2%) and 42.9% of Hispanics (95% CI, 41.0% to 44.8%). Inclusion of socioeconomic variables reversed black-white disparities (odds ratio [OR], 1.17; 95% CI, 1.15 to 1.19) but did not explain disadvantage for Hispanics (OR, 0.89; 95% CI, 0.87 to 0.92). Once interaction of race and physician supply was considered, likelihood of recent CRC screening became statistically indistinguishable for Hispanics and whites of similar socioeconomic status residing in states with high physician supplies.
Conclusion:
Socioeconomic factors and physician supply are key predictors of CRC screening. Adjustment for socioeconomic determinants explained black-white disparities; further adjustment for physician supply explained Hispanic-white disparities. Physician distribution is a potentially remediable contributor to ethnic/racial disparities in CRC screening. Whether the United States is able to equitably meet future demand for screening may depend on access, physician supply, and organization of the health care system.
Introduction
Colorectal cancer (CRC) screening prevents CRC and deaths resulting from CRC by detecting and removing precancerous polyps and identifying early-stage cancers. Despite its net benefits, CRC screening in the United States remains low1,2 and below the uptake of other evidence-based cancer screening tests.3 Over the last decade, approximately 50% of adults age ≥ 50 years underwent CRC screening within recommended time intervals,4–7 and the screening rate is even lower among racial and ethnic minorities.4,8–10 In 2008, for example, 57% of non-Hispanic whites were up to date on CRC screening, yet the proportion was only 51% for non-Hispanic blacks and 39% for Hispanics, according to analyses of the National Health Interview Survey.11
In studies of both pre-Medicare and Medicare-eligible populations, education, income, and insurance have been proven to be important socioeconomic determinants of screening patterns among racial groups over time.3,8,12–15 However, the causes of racial disparities in CRC screening may extend beyond individual-level characteristics. Place of residence may also serve as a key factor in whether an individual receives timely and appropriate screening. The impact of geographic location may result, in part, from both generalist and specialist physician supplies.16–19 However, the degree to which observed racial disparities in CRC screening are associated with physician supply, after adjusting for known socioeconomic determinants, remains an open question.
In this study, we simultaneously examined both individual-level socioeconomic determinants and state-level generalist and specialist physician capacities that may affect racial disparities in screening, both stool based and endoscopic. Our study spanned 1997 to 2008, a time range that includes the Medicare coverage of fecal occult blood testing (FOBT) in 1998 and colonoscopy in 2001 for average-risk beneficiaries. We examined how physician density, beyond socioeconomic factors, affected observed racial disadvantages in recent CRC screening for blacks and Hispanics.
Methods
Data
Individual level.
We collected socioeconomic, behavioral, health, and CRC screening information on adults age ≥ 50 years using the Behavioral Risk Factor Surveillance System, managed by the Centers for Disease Control and Prevention. This system is a national telephone-based survey that collects detailed demographic and household information on the health of a representative sample of community-dwelling adults age ≥ 50 years in the United States. Pertinent information includes year, state, age, sex, race, Hispanic origin, income, educational attainment, health insurance status, marital status, smoking status, obesity status, home-based FOBT history, and endoscopic screening history (which combines sigmoidoscopy and colonoscopy). Nationwide survey questions on both stool-based and endoscopic CRC cancer screening (except in Connecticut, Arizona, Illinois, and Tennessee) began in 1997 and were also asked in 1999, 2001, 2002, 2004, 2006, and 2008. Home-based FOBT was considered recent if it occurred within the past year, and endoscopic screening (either flexible sigmoidoscopy or colonoscopy) was considered recent if it occurred within the past 5 years. (Current US Preventive Services Task Force guidelines recommend colorectal cancer screening by one of the following modalities: annual FOBT, decennial colonoscopy, or quinquennial flexible sigmoidoscopy plus interval FOBT). The total number of survey respondents was 816,881.
Physician density.
We used the physician characteristics and distribution information in US reports derived from the American Medical Association Masterfile to estimate the total number of primary care physicians (PCPs) in clinical practice and total number of gastroenterologists (GIs) in clinical practice by state and year. We considered PCPs who designated their specialty as general internal medicine, family practice/general practice, and gerontology. We estimated the number of adults age ≥ 50 years from decennial census counts and annual intercensal estimates of the resident population by state and year.
Statistical Analyses
Using fixed-effect multivariate logistic regression, we modeled the probabilities of either receiving FOBT within the past year or endoscopic screening within the past 5 years as a function of the individual-level covariates (age, sex, race, ethnicity, educational attainment, income level, marital status, smoking status, obesity status, health insurance coverage) and state-level covariates (PCP and GI densities). Hereafter, we refer to non-Hispanic whites as whites, non-Hispanic blacks as blacks, and other non-Hispanics as others.
We used R version 2.9.2 (R Project for Statistical Computing; http://www.r-project.org) for all statistical analyses. The Dartmouth College and Dartmouth-Hitchcock Medical Center Committee for the Protection of Human Subjects determined this research met eligibility criteria for review exemption.
Results
Individual-Level Characteristics and Physician Density
Appendix Table A1 (online only) lists individual-level characteristics of the study population in relation to CRC screening. The proportion of adults receiving recent CRC screening grew over time from 38.7% in 1997 (95% CI, 38% to 39.4%) to 55.9% in 2008 (95% CI, 55.6% to 56.2%). Over the entire study period, women were more likely to be up to date for CRC screening: 49.4% (95% CI, 49.2% to 49.6%) compared with 47.5% for men (95% CI, 47.3% to 47.7%). Whites were also more likely to be up to date compared with blacks and Hispanics: 50% (95% CI, 49.9% to 50.2%) versus 46.8% (46.2% to 47.4%) and 36.8% (95% CI, 36.1% to 37.6%), respectively. The proportion of up-to-date adults peaked among those age 70 to 74 years at 56.6% (95% CI, 56.2% to 57%). We also observed an education and income gradient. Finally, the proportion of adults up to date on CRC screening was higher for those with health insurance compared with those without: 50.4% (95% CI, 50.2% to 50.5%) versus 23.9% (95% CI, 23.3% to 24.6%).
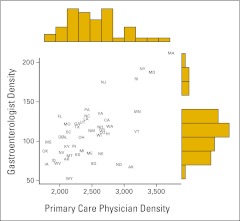
Figure 1 presents the relationship between state-level PCP and GI densities in 2008, along with their marginal distributions. Higher PCP density was associated with higher GI density (r = 0.63). Analyses of earlier years revealed similar patterns of PCP and GI densities (data not shown).
Figure 1.
State-level primary care physician (PCP) and gastroenterologist (GI) densities (physicians per million adults age ≥ 50 years) in the year 2008. Each state is labeled with its two-letter abbreviation. Marginal distributions of PCP and GI densities shown as histograms.
Temporal Patterns in Recent CRC Screening
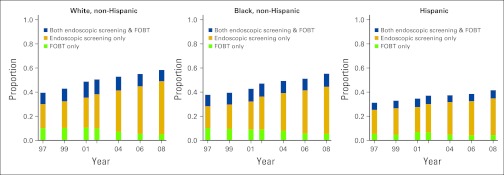
Figure 2 depicts temporal patterns in recent CRC screening. The proportion of adults age ≥ 50 years receiving CRC screening generally increased through the study period for all racial/ethnic groups. The method of screening changed over time, with endoscopic screening increasing and FOBT screening decreasing. Although whites, blacks, and Hispanics experienced similar temporal patterns, levels of screening differed. In 2008, for example, 60.6% of whites were up to date on CRC screening (95% CI, 60.6% to 61.0%) compared with 57.9% of blacks (95% CI, 56.7% to 59.2%) and 42.9% of Hispanics (95% CI, 41.0% to 44.8%).
Figure 2.
Proportion of adults age ≥ 50 years undergoing only fecal occult blood testing (FOBT) within the past year, only endoscopic screening within the past 5 years, and both FOBT within the past year and endoscopic screening within past 5 years.
FOBT Within Past Year or Endoscopic Screening Within Past 5 Years
Appendix Table A2 (online only) summarizes results of nested logistic regression models for undergoing either FOBT within the past year or endoscopic screening within the past 5 years (ie, recent FOBT or endoscopic screening). In the baseline model (model one), we adjusted for time, age, sex, and race/ethnicity and observed that blacks (odds ratio [OR], 0.93; 95% CI, 0.92 to 0.95) and Hispanics (OR, 0.69; 95% CI, 0.67 to 0.70) were less likely to undergo recent FOBT or endoscopic screening compared with their white counterparts. In model two, the inclusion of socioeconomic and health variables reversed black-white disparities (OR, 1.17; 95% CI, 1.15 to 1.19) but did not eliminate disparities among Hispanics (OR, 0.89; 95% CI, 0.87 to 0.92). In model three, we further adjusted for physician density and observed a positive gradient between the supply of GIs (GI density) and the likelihood of recent screening (OR, 1.009; 95% CI, 1.007 to 1.01). Finally, in model four, we considered the interaction of physician density and race to examine whether the effect of physician density on recent screening varied by race. We observed a positive and statistically significant interaction term for GI density and Hispanic race/ethnicity, indicating that greater GI density had a larger effect among Hispanics. For blacks, we did not observe any additional impact of greater physician density on recent CRC screening compared with that observed for whites.
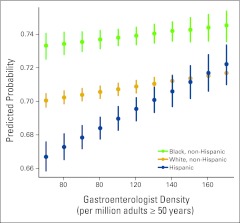
Figure 3 depicts this interaction effect by computing the predicted probability of recent CRC screening by GI density and race. All other covariates are set at their modal values (year 2008, age 65 to 70 years, female sex, at least some college, not obese, nonsmoker, married, income > $50,000, health insurance). At the 10th percentile of GI density (70 GIs per million adults age ≥ 50 years), the probability of recent CRC screening was 66.7% for Hispanics (95% CI, 65.9% to 67.5%), 70.2% for whites (95% CI, 69.8% to 70.6%), and 73.4% for blacks (95% CI, 72.7% to 74.1%). For all three racial/ethnic groups, the probability of recent CRC screening increased as GI density increased. The pace of increase was highest for Hispanics. At the 85th percentile of GI density (140 GIs per million adults age ≥ 50 years) and higher, the probability of recent screening was indistinguishable between whites and Hispanics although still higher for blacks. For example, at a GI density of 170 per million adults age ≥ 50 years, the probability of recent CRC screening was 72.2% for Hispanics (95% CI, 71.0% to 73.3%), 71.7% for whites (95% CI, 71.3% to 72.1%), and 74.5% for blacks (95% CI, 73.6% to 75.4%).
Figure 3.
Predicted probability of recent colorectal cancer screening by racial/ethnic group and gastroenterologist density. All other covariates are set at their modal values (year 2008, age 65 to 70 years, female sex, at least some college, not obese, nonsmoker, married, income > $50,000, health insurance). Solid circles represent the median predicted probability, and vertical lines represent the 95% CI.
Finally, we considered the effect of health insurance on pre–Medicare-age adults 50 to 64 years old (Appendix Table A3, online only). Setting all other covariates at their modal values (ie, year 2008, age 65 to 70 years, female sex, at least some college, not obese, nonsmoker, married, income > $50,000, health insurance) and GI and PCP densities at their median values, the probability of recent CRC screening was higher for those with health insurance compared with those without for all three racial/ethnic groups. The probability equaled 59.6% for whites with health insurance (95% CI, 59.2% to 59.8%) and 37.2% for those without (95% CI, 36.7% to 37.8%), 63.0% for blacks with health insurance (95% CI, 62.4% to 63.5%) and 40.7% for those without (95% CI, 40.0% to 41.4%), and 57.2% for Hispanics with health insurance (95% CI, 56.5% to 58.0%) and 35.0% for those without (95% CI, 34.2% to 35.8%).
Discussion
This study has two main findings. First, socioeconomic determinants and physician density are key predictors of CRC screening. This result confirms previous findings in the literature.4,5,11,12,20 Second, adjustment for socioeconomic determinants eliminated black-white disparities, and further adjustment for physician density eliminated Hispanic-white disparities. Previous research on race and CRC screening has primarily focused on black-white disparities. Here, we also considered Hispanic-white disparities, an important undertaking given the Hispanic population is projected to steadily rise to 20% of the total US population in the next 20 years.21
For both FOBT and endoscopic screening, socioeconomic status has been previously observed to mostly or fully account for observed black-white racial disparities.3,8,15 Socioeconomic factors such as income, educational attainment, occupation, and health insurance directly affect an individual's access and utilization of screening, the quality of screening, and the potential benefits derived from screening. We reached similar conclusions for the black-white disparity in recent CRC screening. Yet for Hispanics, these socioeconomic factors explain some, although not all, of the observed disparities. Higher densities of GIs may be especially important for Hispanics. Further work is needed to determine if these effects are causal and, if so, why Hispanics are particularly sensitive to GI physician density.
In addition to health care access and usage, the supply and balance of PCPs and GIs have an important effect on CRC screening, detection, and survival. PCPs serve an important role in initiating and overseeing CRC cancer screening.22,23 Over time, colonoscopy has become the more favored method of endoscopic screening compared with sigmoidoscopy. GIs perform two thirds of these procedures,24,25 so it is logical that GI density will affect screening rates. Our results suggest higher GI density may be especially important to higher overall CRC screening and reductions in some disparities. PCP density is also likely important, although its effect may be masked by regional socioeconomic factors and GI density, with which it is correlated. Beyond screening, CRC incidence, late-stage diagnosis, and mortality decreased as PCP and GI densities increased in studies conducted in Florida26,27 and Pennsylvania.28 Similar benefits of high PCP and specialist physician density have been noted in breast cancer,29,30 urologic cancer,31 and melanoma.32
Policy initiatives at the community, state, and national levels have attempted to increase access and physician supply as well as reduce financial, physical, institutional, and organizational barriers. Medicare coverage of annual FOBT and quadrennial sigmoidoscopy began in 1998, and coverage for decennial colonoscopy began in 2001 for average-risk beneficiaries. Results of these initiatives have varied, disparities have persisted,12,13 and modest improvements in screening have been observed most often in the highest socioeconomic groups.33
We acknowledge several limitations in this study. First, we were not able to differentiate between sigmoidoscopy and colonoscopy for the entire time period between 1993 and 2008. Current US Preventive Services Task Force guidelines for endoscopic CRC screening recommend sigmoidoscopy every 5 years or colonoscopy every 10 years for average-risk adults age 50 to 75 years. In considering flexible sigmoidoscopy or colonoscopy within the past 5 years, we may have provided conservative estimates of recent endoscopic screening. Second, we were not able to determine whether the underlying purpose of an individual's endoscopic CRC procedure was screening, diagnosis, or surveillance. In 2008, for example, 40.3% of the adults reporting FOBT within the past year also reported colonoscopy within the past year. Many of these colonoscopies were likely conducted to investigate a positive FOBT result for diagnostic purposes rather than screening. However, our outcome of interest was recent CRC screening, which occurred with the recent FOBT. Third, we relied on self-reported CRC cancer screening, which may be subject to recall bias. Fourth, we were not able to differentiate the type of health insurance carried by respondents. Fifth, reporting lags and other inaccuracies have been identified with the American Medical Association Masterfile, which may have affected our knowledge of physician density levels and the geographic and specialty distributions of the physician workforce.34,35 We may also have underestimated the universe of physicians conducting CRC screening by excluding colon and rectal and general surgeons, some of whom perform screening colonoscopy, and overestimated by including all GIs, including those who do not perform lower endoscopy. Sixth, we did not measure the productivity or number of hours spent on patient care among clinical PCPs and GIs. We treated a state as a unit, yet considerable variation exists within states in the distribution of PCPs and GIs as well as CRC screening levels. Additional physicians in a state may locate in areas where density is already high, and diffusion of care may be limited. Additional analysis at the local or regional health care market level may allow greater understanding of physician supply and utilization of colorectal cancer screening. Finally, the associations we observed may not reflect direct causation.
Future work could expand on this analysis of the joint relationship between individual-level socioeconomic determinants and area-level physician capacity and effect on CRC screening. Focus on smaller geographic areas (eg, hospital referral regions) and more refined calculation of physician capacity (eg, based on full-time equivalents of clinical work) would enable a more nuanced understanding of how local supply might best meet local demand. Additional work could also examine the current distribution of specialist physicians who perform screening colonoscopy (GIs, colon and rectal surgeons, and general surgeons) to determine if future capacity will be able to meet future demand.
As the population ages, and as the modality of screening changes, the demand for CRC cancer screening will likely increase faster than the capacity to perform that screening. First, the proportion of adults age ≥ 50 years is projected to increase by approximately 24% over the next 20 years,36 which may far exceed the rate of growth in physicians.37,37 Second, PCPs serve an important role in initiating and overseeing CRC cancer screening,22,38 and great regional shortages of PCPs currently exist or are anticipated. Third, colonoscopy has become the more favored method of endoscopic screening compared with sigmoidoscopy,39 and GIs—also in limited and variable supply—perform the vast majority of these procedures.24,25 Whether the United States is able to meet the future demand for screening and address historical disparities in screening may depend on access, physician supply, interaction of PCPs and specialists, financial reimbursement of services, and organization of the health care system.
Acknowledgment
Supported by Grant No. RC2CA148259 from the National Cancer Institute and by the Robert Wood Johnson Foundation Health and Society Scholars and Robert Wood Johnson Clinical Scholars programs. We thank Robert Aronowitz, MD, David Goodman, MD, Valerie Lewis, PhD, and Jason Schnittker, PhD, and two anonymous reviewers for helpful comments and suggestions.
Appendix
Table A1.
Distribution of Demographic and Socioeconomic Characteristics of Study Population
| Characteristic | FOBT Within Past 1 Year or Endoscopic Screening Within Past 5 Years |
||
|---|---|---|---|
| No. | % | 95% CI | |
| Total | 415,080 | 48.4 | 48.2 to 48.5 |
| Year | |||
| 1997 | 18,098 | 38.7 | 38.0 to 39.4 |
| 1999 | 23,835 | 41.8 | 41.1 to 42.4 |
| 2001 | 36,918 | 46.6 | 46.1 to 47.1 |
| 2002 | 48,475 | 48.7 | 48.3 to 49.2 |
| 2004 | 67,433 | 50.8 | 50.4 to 51.2 |
| 2006 | 92,805 | 52.9 | 52.6 to 53.2 |
| 2008 | 127,516 | 55.9 | 55.6 to 56.2 |
| Sex | |||
| Male | 255,174 | 47.5 | 47.3 to 47.7 |
| Female | 159,906 | 49.4 | 49.2 to 49.6 |
| Race/ethnicity | |||
| White, non-Hispanic | 356,092 | 50 | 49.9 to 50.2 |
| Black, non-Hispanic | 30,391 | 46.8 | 46.2 to 47.4 |
| Hispanic | 15,046 | 36.8 | 36.1 to 37.6 |
| Other, non-Hispanic | 19,518 | 42.7 | 42.0 to 43.4 |
| Age group, years | |||
| 50-54 | 60,348 | 35.8 | 35.5 to 36.2 |
| 55-59 | 70,845 | 46.6 | 46.2 to 46.9 |
| 60-64 | 67,776 | 51.5 | 51.2 to 51.9 |
| 65-69 | 64,946 | 55 | 54.6 to 55.4 |
| 70-74 | 58,128 | 56.6 | 56.2 to 57.0 |
| 75-79 | 47,605 | 56.5 | 56.1 to 56.9 |
| 80-84 | 30,325 | 52.4 | 51.8 to 53.0 |
| ≥ 85 | 15,107 | 42 | 41.2 to 42.8 |
| Education | |||
| Less than high school | 46,935 | 38 | 37.6 to 38.5 |
| High school graduate | 130,047 | 45.7 | 45.4 to 45.9 |
| At least some college | 239,568 | 53.1 | 52.9 to 53.3 |
| Annual household income | |||
| < $25,000 | 109,885 | 42.3 | 42.0 to 42.6 |
| $25,0000-$49,999 | 107,259 | 49 | 48.7 to 49.3 |
| > $50,000 | 197,942 | 51.5 | 51.3 to 51.7 |
| Marital status | |||
| Married | 239,183 | 50.7 | 50.5 to 50.9 |
| Separated/divorced | 67,912 | 41.7 | 41.3 to 42.0 |
| Single | 24,396 | 40.5 | 39.9 to 41.1 |
| Widowed | 95,733 | 47.2 | 46.9 to 47.5 |
| Obese | |||
| No | 314,190 | 48.8 | 48.7 to 49.0 |
| Yes | 114,667 | 49.1 | 48.9 to 49.4 |
| Current smoker | |||
| No | 201,781 | 48.2 | 48.0 to 48.4 |
| Yes | 214,841 | 48.5 | 48.3 to 48.7 |
| Health insurance | |||
| No | 15,909 | 23.9 | 23.3 to 24.6 |
| Yes | 399,655 | 50.4 | 50.2 to 50.5 |
Abbreviation: FOBT, fecal occult blood testing.
Table A2.
Nested Logistic Regression Model Results for Recent Colorectal Cancer Screening
| Parameter | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Intercept | −1.07 | −1.091 to −1.048 | −1.931 | −1.963 to −1.899 | −1.708 | −1.745 to −1.671 | −1.715 | −1.752 to −1.677 |
| Year (ref: 1997) | ||||||||
| 1999 | 0.158 | 0.133 to 0.183 | 0.147 | 0.121 to 0.173 | 0.144 | 0.118 to 0.17 | 0.144 | 0.118 to 0.17 |
| 2001 | 0.425 | 0.401 to 0.448 | 0.395 | 0.371 to 0.42 | 0.394 | 0.37 to 0.419 | 0.394 | 0.37 to 0.419 |
| 2002 | 0.475 | 0.453 to 0.498 | 0.445 | 0.421 to 0.468 | 0.45 | 0.426 to 0.473 | 0.45 | 0.427 to 0.474 |
| 2004 | 0.598 | 0.577 to 0.62 | 0.561 | 0.538 to 0.583 | 0.564 | 0.542 to 0.587 | 0.565 | 0.542 to 0.588 |
| 2006 | 0.689 | 0.668 to 0.71 | 0.639 | 0.617 to 0.661 | 0.632 | 0.61 to 0.654 | 0.632 | 0.61 to 0.654 |
| 2008 | 0.806 | 0.786 to 0.827 | 0.746 | 0.725 to 0.768 | 0.736 | 0.714 to 0.757 | 0.736 | 0.715 to 0.758 |
| Age, years (ref: 50-54) | ||||||||
| 55-59 | 0.444 | 0.43 to 0.459 | 0.473 | 0.457 to 0.488 | 0.473 | 0.457 to 0.488 | 0.473 | 0.457 to 0.488 |
| 60-64 | 0.64 | 0.625 to 0.655 | 0.714 | 0.698 to 0.73 | 0.714 | 0.698 to 0.73 | 0.714 | 0.698 to 0.73 |
| 65-69 | 0.799 | 0.784 to 0.815 | 0.848 | 0.831 to 0.865 | 0.849 | 0.832 to 0.865 | 0.849 | 0.832 to 0.866 |
| 70-74 | 0.88 | 0.864 to 0.897 | 0.969 | 0.952 to 0.987 | 0.969 | 0.951 to 0.987 | 0.97 | 0.952 to 0.987 |
| 75-79 | 0.826 | 0.808 to 0.843 | 0.956 | 0.937 to 0.975 | 0.955 | 0.936 to 0.974 | 0.955 | 0.936 to 0.974 |
| 80-84 | 0.607 | 0.587 to 0.626 | 0.779 | 0.757 to 0.801 | 0.777 | 0.756 to 0.799 | 0.778 | 0.756 to 0.8 |
| ≥ 85 | 0.192 | 0.168 to 0.216 | 0.411 | 0.384 to 0.438 | 0.409 | 0.383 to 0.436 | 0.409 | 0.383 to 0.436 |
| Male sex (ref: female) | 0.083 | 0.074 to 0.092 | 0.01 | 0 to 0.02 | 0.01 | 0 to 0.02 | 0.01 | 0 to 0.02 |
| Race/ethnicity (ref: white, non-Hispanic) | ||||||||
| Black, non-Hispanic | −0.071 | −0.089 to −0.054 | 0.159 | 0.14 to 0.178 | 0.152 | 0.133 to 0.17 | 0.232 | 0.151 to 0.313 |
| Hispanic | −0.374 | −0.397 to −0.35 | −0.113 | −0.138 to −0.087 | −0.095 | −0.121 to −0.07 | −0.062 | −0.155 to 0.032 |
| Other, non-Hispanic | −0.263 | −0.284 to −0.242 | −0.182 | −0.204 to −0.16 | −0.179 | −0.201 to −0.157 | −0.143 | −0.238 to −0.048 |
| Education (ref, HS graduate) | ||||||||
| Less than HS graduate | −0.216 | −0.232 to −0.201 | −0.216 | −0.232 to −0.201 | −0.217 | −0.232 to −0.201 | ||
| Some college | 0.27 | 0.259 to 0.281 | 0.272 | 0.262 to 0.283 | 0.272 | 0.262 to 0.283 | ||
| Obese (ref: not obese) | 0.076 | 0.065 to 0.087 | 0.075 | 0.064 to 0.086 | 0.075 | 0.064 to 0.085 | ||
| Marital status (ref: married) | ||||||||
| Separated/divorced | −0.21 | −0.223 to −0.197 | −0.21 | −0.224 to −0.197 | −0.211 | −0.224 to −0.197 | ||
| Single | −0.276 | −0.297 to −0.255 | −0.28 | −0.301 to −0.259 | −0.28 | −0.301 to −0.259 | ||
| Widowed | −0.207 | −0.22 to −0.193 | −0.207 | −0.221 to −0.194 | −0.207 | −0.221 to −0.194 | ||
| Income (ref: $25,000-$50,000) | ||||||||
| > $50,000 | 0.078 | 0.066 to 0.089 | 0.077 | 0.065 to 0.088 | 0.077 | 0.065 to 0.088 | ||
| < $25,000 | −0.12 | −0.133 to −0.107 | −0.119 | −0.133 to −0.106 | −0.119 | −0.133 to −0.106 | ||
| Smoker (ref: nonsmoker) | 0.016 | 0.007 to 0.026 | 0.016 | 0.007 to 0.026 | 0.016 | 0.007 to 0.026 | ||
| Health insurance (ref: none) | 0.866 | 0.846 to 0.887 | 0.862 | 0.841 to 0.883 | 0.862 | 0.841 to 0.883 | ||
| Density* | ||||||||
| PCP, units 100 | −0.012 | −0.013 to −0.011 | −0.012 | −0.013 to −0.01 | ||||
| GI, units 10 | 0.009 | 0.007 to 0.01 | 0.008 | 0.006 to 0.01 | ||||
| Black, non-Hispanic × PCP | −0.002 | −0.007 to 0.003 | ||||||
| Hispanic × PCP | −0.009 | −0.014 to −0.004 | ||||||
| Other, non-Hispanic × PCP | −0.001 | −0.007 to 0.004 | ||||||
| Black, non-Hispanic × GI | −0.002 | −0.009 to 0.006 | ||||||
| Hispanic × GI | 0.018 | 0.011 to 0.025 | ||||||
| Other, non-Hispanic × GI | 0 | −0.008 to 0.007 | ||||||
| AIC | 1,094,410 | 1,008,824 | 1,008,271 | 1,008,253 | ||||
| Residual deviance | 1,094,374 | 1,008,768 | 1,008,211 | 1,008,181 | ||||
| df | 812,089 | 764,685 | 764,683 | 764,677 | ||||
Abbreviations: AIC, Akaike information criterion; GI, gastroenterologist; HS, high school; PCP, primary care physician; ref, reference.
PCP density measured in units of 100 PCPs per million adults age ≥ 50 years; GI density measured in units of 10 GIs per million adults age ≥ 50 years.
Table A3.
Predicted Probability of Recent Colorectal Cancer Screening of Pre-Medicare Adults*
| Racial/Ethnic Group | No Health Insurance |
Health Insurance |
||
|---|---|---|---|---|
| Point Estimate (%) | 95% CI | Point Estimate (%) | 95% CI | |
| Non-Hispanic white | 37.3 | 36.3 to 37.8 | 59.9 | 59.2 to 59.9 |
| Non-Hispanic black | 40.7 | 39.9 to 41.3 | 63.0 | 62.5 to 63.5 |
| Hispanic | 35.0 | 34.3 to 35.8 | 57.2 | 56.5 to 58.0 |
Age 50 to 64 years, by racial/ethnic group and health insurance status. All other covariates set at modal values (year 2008, age 65-70 years, female sex, at least some college, not obese, nonsmoker, married, income > $50,000, 110 gastroenterologists per million adults age ≥ 50 years, 2,462 primary care physicians per million adults age ≥ 50 years).
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: All authors
Financial support: Samir Soneji
Administrative support: Samir Soneji, Katrina Armstrong
Collection and assembly of data: Samir Soneji
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Mitka M. Colorectal cancer screening rates still fall far short of recommended levels. JAMA. 2008;299:622. doi: 10.1001/jama.299.6.622. [DOI] [PubMed] [Google Scholar]
- 2.Breen N, Wagener DK, Brown ML, et al. Progress in cancer screening over a decade: Results of cancer screening from the 1987, 1992, and 1998 National Health Interview surveys. J Natl Cancer Inst. 2001;93:1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 3.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168:1317–1324. doi: 10.1001/archinte.168.12.1317. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 5.Liang SY, Phillips KA, Nagamine M, et al. Rates and predictors of colorectal cancer screening. Prev Chronic Dis. 2006;3:A117. [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro JA, Seeff LC, Thompson TD, et al. Colorectal cancer test use from the 2005 National Health Interview survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital signs: Colorectal cancer screening, incidence, and mortality—United States, 2002-2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–889. [PubMed] [Google Scholar]
- 8.Doubeni CA, Laiyemo AO, Klabunde CN, et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010;38:184–191. doi: 10.1016/j.amepre.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100:418–424. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 10.Adams-Campbell L, Makambi K, Mouton C, et al. Colonoscopy utitlization in the Black Women's Health study. J Natl Med Assoc. 2010;102:237–242. doi: 10.1016/s0027-9684(15)30530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde CN, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doubeni CA, Laiyemo AO, Reed G, et al. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18:2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White A, Vernon SW, Franzini L, et al. Racial and ethnic disparities in colorectal cancer screening persisted despite expansion of Medicare's screening reimbursement. Cancer Epidemiol Biomarkers Prev. 2011;20:811–817. doi: 10.1158/1055-9965.EPI-09-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton JJ, Tancredi DJ, Green P, et al. Persistent racial and ethnic disparities in up-to-date colorectal cancer testing in Medicare enrollees. J Am Geriatr Soc. 2009;57:412–418. doi: 10.1111/j.1532-5415.2008.02143.x. [DOI] [PubMed] [Google Scholar]
- 15.Schenck AP, Klabunde CN, Davis WW. Racial differences in colorectal cancer test use by Medicare consumers. Am J Prev Med. 2006;30:320–326. doi: 10.1016/j.amepre.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: Data from the National Cancer Institute survey of colorectal cancer screening practices. Am J Med. 2003;115:129–133. doi: 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 17.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 18.Vijan S, Inadomi J, Hayward RA, et al. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Aliment Pharmacol Ther. 2004;20:507–515. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]
- 19.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 20.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168:1317–1324. doi: 10.1001/archinte.168.12.1317. [DOI] [PubMed] [Google Scholar]
- 21.Niner D, Rios M. Washington, DC: US Census Bureau; 2009. Hispanics in the United States, Puerto Rico, and the U.S. Virgin Islands: 2000. [Google Scholar]
- 22.Fletcher RH, Nadel MR, Allen JI, et al. The quality of colonoscopy services: Responsibilities of referring clinicians—A consensus statement of the Quality Assurance Task Group, National Colorectal Cancer Roundtable. J Gen Intern Med. 2010;25:1230–1234. doi: 10.1007/s11606-010-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christie J, Itzkowitz S, Lihau-Nkanza I, et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 24.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: Data from the National Cancer Institute Survey of Colorectal Cancer Screening Practices. Am J Med. 2003;115:129–133. doi: 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 25.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 26.Roetzheim RG, Pal N, Gonzalez EC, et al. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48:850–858. [PubMed] [Google Scholar]
- 27.Roetzheim RG, Gonzalez EC, Ramirez A, et al. Primary care physician supply and colorectal cancer. J Fam Pract. 2001;50:1027–1031. [PubMed] [Google Scholar]
- 28.Ananthakrishnan AN, Hoffmann RG, Saeian K. Higher physician density is associated with lower incidence of late-stage colorectal cancer. J Gen Intern Med. 2010;25:1164–1171. doi: 10.1007/s11606-010-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrante JM, Gonzalez EC, Pal N, et al. Effects of physician supply on early detection of breast cancer. J Am Board Fam Pract. 2000;13:408–414. doi: 10.3122/15572625-13-6-408. [DOI] [PubMed] [Google Scholar]
- 30.Gorey KM, Luginaah IN, Fung KY, et al. Physician supply and breast cancer survival. J Am Board Fam Med. 2010;23:104–108. doi: 10.3122/jabfm.2010.01.090064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odisho AY, Cooperberg MR, Fradet V, et al. Urologist density and county-level urologic cancer mortality. J Clin Oncol. 2010;28:2499–2504. doi: 10.1200/JCO.2009.26.9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Durme DJ, Ullman R, Campbell RJ, et al. Effects of physician supply on melanoma incidence and mortality in Florida. South Med J. 2003;96:656–660. doi: 10.1097/01.SMJ.0000053569.81565.19. [DOI] [PubMed] [Google Scholar]
- 33.Phillips KA, Liang SY, Ladabaum U, et al. Trends in colonoscopy for colorectal cancer screening. Med Care. 2007;45:160–167. doi: 10.1097/01.mlr.0000246612.35245.21. [DOI] [PubMed] [Google Scholar]
- 34.Kletke PR. Physician workforce data: When the best is not good enough. Health Serv Res. 2004;39:1251–1255. doi: 10.1111/j.1475-6773.2004.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CH, Stukel TA, Flood AB, et al. Primary care physician workforce and Medicare beneficiaries' health outcomes. JAMA. 2011;305:2096–2104. doi: 10.1001/jama.2011.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Economics and Statistics Administration. Washington, DC: US Department of Commerce; 1996. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. [Google Scholar]
- 37.Mitka M. Looming shortage of physicians raises concerns about access to care. JAMA. 2007;297:1045–1046. doi: 10.1001/jama.297.10.1045. [DOI] [PubMed] [Google Scholar]
- 38.Robinson CM, Cassells AN, Greene MA, et al. Barriers to colorectal cancer screening among publicly insured urban women: No knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011;103:746–753. doi: 10.1016/s0027-9684(15)30414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fenton JJ, Cai Y, Green P, et al. Trends in colorectal cancer testing among Medicare subpopulations. Am J Prev Med. 2008;35:194–202. doi: 10.1016/j.amepre.2008.05.029. [DOI] [PubMed] [Google Scholar]