Abstract
Hemorrhage remains a major cause of potentially preventable deaths. Trauma and massive transfusion are associated with coagulopathy secondary to tissue injury, hypoperfusion, dilution, and consumption of clotting factors and platelets. Concepts of damage control surgery have evolved prioritizing early control of the cause of bleeding by non-definitive means, while hemostatic control resuscitation seeks early control of coagulopathy.
Hemostatic resuscitation provides transfusions with plasma and platelets in addition to red blood cells in an immediate and sustained manner as part of the transfusion protocol for massively bleeding patients. Although early and effective reversal of coagulopathy is documented, the most effective means of preventing coagulopathy of massive transfusion remains debated and randomized controlled studies are lacking.
Viscoelastical whole blood assays, like TEG and ROTEM however appear advantageous for identifying coagulopathy in patients with severe hemorrhage as opposed the conventional coagulation assays.
In our view, patients with uncontrolled bleeding, regardless of it´s cause, should be treated with hemostatic control resuscitation involving early administration of plasma and platelets and earliest possible goal-directed, based on the results of TEG/ROTEM analysis. The aim of the goal-directed therapy should be to maintain a normal hemostatic competence until surgical hemostasis is achieved, as this appears to be associated with reduced mortality.
Keywords: Massive transfusion, trauma, hemorrhage, TEG, coagulopathy, FFP, RBC, platelets, rFVIIa, fibrinogen, PCC, antifibrinolytics
Introduction
Hemorrhage requiring massive transfusion remains a major cause of potentially preventable deaths. Trauma and massive transfusion are associated with coagulopathy secondary to tissue injury, hypoperfusion, dilution and consumption of clotting factors and platelets and coagulopathy, together with hypothermia and acidosis, forms a “lethal” triad [1]. Also, in the last 10–15 years there has been some paradigm shift regarding optimal resuscitation of bleeding trauma patients before definitive hemorrhage control is achieved. Aggressive fluid resuscitation increases blood pressure, reverses vasoconstriction, dislodges early formed thrombus, causes dilutional coagulopathy and metabolic acidosis and increases blood loss in experimental studies [2]. Accordingly previous guidelines [3] recommending that fresh frozen plasma (FFP) and platelets (PLT) should be administered only when a whole blood volume or more has been substituted and then according to conventional coagulation analyses is now considered obsolete since this strategy leads to dilutional coagulopathy and compromises hemostatic competence in the most severely bleeding patients [1]. Instead, limiting fluid resuscitation and applying the concept of permissive hypotension with the goal of achieving a palpable radialis pulse in patients has been advocated, whereas in patients with head injury a systolic blood pressure above 110 mmHg is recommended [4-7].
The current transfusion guidelines advocate the concept of hemostatic control resuscitation, i.e., supplementing large transfusions of red blood cells (RBC) with FFP and PLT to critically injured patients in an immediate and sustained manner is proposed [7-9]. The rationale for balanced administration of blood products is that it mimics the composition of circulating blood and, hence, transfusion of RBC, FFP and PLT in a unit-for-unit ratio is likely to both prevent and treat coagulopathy due to massive hemorrhage. This review describes the clinical problems associated with hemorrhage and massive transfusion in trauma.
Coagulopathy in massive hemorrhage
Dilution
The dilution of coagulation factors and platelets is an important cause of coagulopathy in massively transfused trauma patients [10]. The Advanced Trauma Life Support guideline recommends aggressive crystalloid resuscitation but the dilutional effects of such administration on coagulation competence are well described [11,12] and this strategy provokes acidosis, formation of interstitial oedema with tissue swelling, impairment of the microcirculation and hence compromised oxygenation [13,14].
Furthermore, synthetic colloid resuscitation fluids influence coagulation competence more profoundly than crystalloids. Hydroxyethyl starch (HES) causes efflux of plasma proteins from blood to the interstitial space, reduction in plasma concentration of coagulation factor VIII and von Willebrand factor (vWF), inhibition of platelet function and reduced interaction of activated FXIII with fibrin polymers [11,12,15].. This was further corroborated by a recent meta-analysis of 24 studies evaluating the safety of HES 130/0.4 administration in surgical, emergency and intensive care patients, with results demonstrating that HES administration promotes a dose-dependent coagulopathy [16]. Also, administration of blood products such as RBC, FFP and PLT may cause significant dilution since these blood products are stored in anticoagulation solutions reducing coagulation factor concentration to approximately 60% and platelet count to approximately 80x109/l when a hematocrit of 30% is warranted [17].
Hypothermia
Hypothermia is associated with risk of uncontrolled bleeding and death in trauma patients. Hypothermia induced coagulopathy is attributed to platelet dysfunction, reduced coagulation factor activity (significant below 33°C) [14,18], and induction of fibrinolysis [19] and these effects are reversible with normalization of body temperature.
Acidosis
In trauma patients acidosis is often induced by hypoperfusion and excess administration of ionic chloride, i.e. NaCl during resuscitation [20]. Acidosis impairs almost all essential parts of the coagulation process: At pH < 7.4, platelets change their structure and shape [21]. The activity of coagulation factor complexes on the cell surface is reduced and the resulting impaired thrombin generation is a major cause of coagulopathic bleeding. Furthermore, acidosis leads to increased degradation of fibrinogen [22] which further aggravates the coagulopathy.
Trauma
Brohi et al. [23-27] described an early “endogenous” coagulopathy in trauma patients not attributed to dilution and hypothermia with shock and hypoperfusion as the key drivers of acute traumatic coagulopathy through widespread activation of the anticoagulant and fibrinolytic pathways.
We recently suggested that the coagulopathy observed in trauma patients, which reflects the state of the fluid phase including its cellular elements i.e., circulating whole blood, is a consequence of the degree of the tissue injury and the thereby generated sympathoadrenal activity and importantly, critically related to the degree of endothelial damage, with a progressively more procoagulant endothelium (solid phase) inducing a gradient of increasing anticoagulation towards the fluid phase (circulating blood) [28]. Though it seems counterintuitive that increasing injury severity is associated with progressive hypocoagulability and hyperfibrinolysis [28,29], this may from an evolutionary perspective exert a survival advantage by preserving blood flow through the progressively more damaged and procoagulant microvasculature [28] In alignment with this, we found that in trauma patients upon hospital admission, a high level of syndecan-1, a marker of endothelial glycocalyx degradation, was associated with high sympathoadrenal activity and increased mortality, even after adjusting for injury severity score [30]. Also, only in patients with high syndecan-1 levels, was increasing injury severity associated with increased tissue and endothelial damage, protein C depletion, hyperfibrinolysis and inflammation [30].
Consumptive coagulopathy
Tissue injury secondary to trauma induce immediate activation of the coagulation system through upregulation of tissue factor (TF) expression and extensive thrombin generation. Tissue injury in association with extensive endothelial injury, massive soft tissue damage, and fat embolization from long bone fractures, may be associated with consumption of coagulation factors and platelets and, hence development of coagulopathy. Disseminated intravascular coagulation (DIC) is the most extreme form of consumptive coagulopathy and is characterized by systemic activation of pathways leading to and regulating coagulation, which can result in the generation of fibrin clots that may cause organ failure with concomitant consumption of platelets and coagulation factors that may result in clinical bleeding [31,32]. We recently reported that disseminated intravascular coagulation (DIC) was not a part of the early coagulopathy secondary to trauma [33] though this may develop later in the course of resuscitation as described by Gando et al. [34].
Hyperfibrinolysis
Increased fibrinolysis is observed in patients with profound endothelial activation and damage secondary to trauma, surgery and ischemia-reperfusion injury where tissue-type plasminogen activator (tPA) is released from the Weibel-Palade bodies of the endothelial cells. In trauma, increased fibrinolysis has been reported in the most severely injured patients and this correlates with poor outcome [29,35-38]. The presence of increased fibrinolysis with increasing injury severity probably reflects an evolutionary mechanism aiming at preventing fatal intravascular coagulation secondary to systemic hypercoagulation induced by the trauma, as reported recently by us [28].
Anticoagulation
Vitamin K antagonists are frequently used by patients with atrial fibrillation or artificial cardiac valves and warfarin has been reported to be associated with about 21,000 visits for bleeding complications per year in the US alone [39], and these data are consistent with reports of major bleeding frequencies for warfarin as high as 10% to 16% [40]. The use of International Normalized Ratio (INR, a plasmatic coagulation analysis) to monitor the degree of anticoagulation by warfarin may in part explain this problem. The lack of adequate hemostatic monitoring is also evident with regards to the newer pharmaceutical agents used for secondary prevention and postoperative thromboprophylaxis such as the direct thrombin inhibitor dabigatran [41], the indirect FXa inhibitors apixaban [42] and rivaroxaban [43]. Furthermore, as for now it is recommended that treatment with these agents do not require hemostatic monitoring [44]. Despite this, however, reports of severe bleedings in patients taking these medications are being reported, including trauma,and since no antidote exists treatment of these patients is a major challenge [45].
Apart from coagulopathy due to iatrogenic heparinization, critically ill patients, including trauma patients, may become endogenously heparinized due to degradation of the endothelial glycocalyx [30,46], the antiadhesive and anticoagulant carbohydrate-rich surface layer that covers and protects the endothelial cells and contains significant amounts of heparin-like substances [47-53].
Platelet inhibitors
An important cause of excessive bleeding in trauma patients is platelet inhibitors, which an increasing proportion of the population today uses as secondary prevention. Currently, the most important are the platelet ADP receptor inhibitors clopidogrel and lately also the even more potent prasugrel that irreversibly inhibits platelet activation through the platelet ADP receptor and confers more potent platelet inhibition than acetylsalicylic acid
Importantly, the enhanced antiplatelet activity and greater efficacy seen with prasugrel when compared to clopidogrel in clinical trials has been accompanied by increased bleeding risk and the FDA advisory committee issued guidance to physicians about increased risk in low-weight or elderly patients [54].
Monitoring hemostasis
Whole blood viscoelastical assays
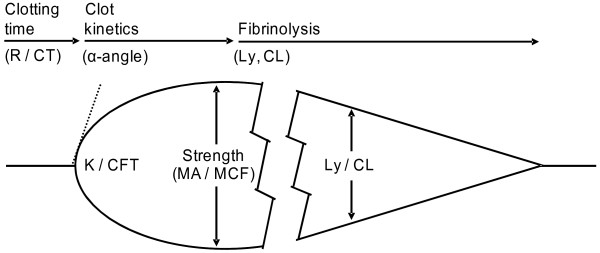
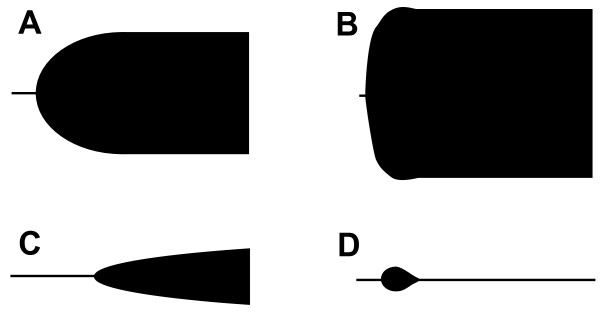
Introduction of the cell-based model of hemostasis emphasizes the role of platelets for intact thrombin generation and highlights the importance of the dynamics of thrombin generation influencing the quality and stability of the thrombus formed [55]. Consequently, hemostatic assays performed on plasma such as activated partial thromboplastin time (APTT) and prothrombin time (PT) are of limited value [56] and they do not correlate with clinically relevant coagulopathies [57,58]. Instead, employing a whole blood assay, such as viscoelastic hemostatic assays (VHA) like TEG/ROTEM that records the viscoelastic changes during clot formation and subsequent lysis is preferable. The TEG reports (Figure 1): R (reaction time), angle (α), the maximum amplitude (MA), the maximal clot strength; and clot lysis (Ly) [29,59-61]. Typical TEG profiles observed in trauma patients are normal, hypercoagulable, hypocoagulable and hyperfibrinolytic profiles (Figure 2), and in actively bleeding patients these are treated according to an algorithm (Table 1).
Figure 1 .
Schematic TEG/ROTEM trace indicating the commonly reported variables reaction time (R)/clotting time (CT), clot formation time (K, CFT), alpha angle (α), maximum amplitude (MA)/maximum clot firmness (MCF) and lysis (Ly)/clot lysis (CL).
Figure 2 .
Schematic presentation of various VHA tracings: A) Normal, B) Hypercoagulability, C) Hypocoagulability (coagulation factor deficiency and thrombocytopenia/pathy and/or low fibrinogen) and D) Primary hyperfibrinolysis.
Table 1.
Recommended TEG algorithm for goal-directed therapy of bleeding patients in the Capital Region of Denmark
| TEG parameter* | Coagulopathy | Intervention |
|---|---|---|
|
R >10 min |
Coagulation factors ↓ |
FFP 10–20 ml/kg (if FFP is without clinical efficacy, consider cryoprecipitate 3–5 ml/kg) |
|
Angle <52 ° |
Hypofibrinogenemia? |
→ Functional Fibrinogen (FF) analysis |
|
MA <49 mm and |
|
|
|
MAFF<14 mm |
Fibrinogen ↓ |
FFP 20–30 ml/kg / |
| Fibrinogen konc. 25–50 mg/kg / |
|
|
| Cryoprecipitate 5 ml/kg |
|
|
|
MA <49 mm and |
|
|
|
MAFF>14 mm |
Platelets ↓ |
Platelets 5–10 ml/kg |
|
Ly30 >8% |
Primary hyperfibrinolysis |
Tranexamic acid 1–2 g IV (adults) |
| Children 10–20 mg/kg IV |
|
|
|
Ly30 >8% and |
|
|
|
Angle and/or MA ↑↑ |
Reactive hyperfibrinolysis |
Tranexamic acid contraindicated |
| Difference in R >2 min between st-TEG and hep-TEG | Heparinization | Protamine sulphate or FFP 20–30 ml/kg |
R, Reaction time; Angle, α-angle; MA, Maximum amplitude; MAFF, Maximum amplitude by Functional Fibrinogen® analysis; Ly30, Lysis after 30 min; st-TEG, standard TEG; hep-TEG, heparinase TEG.
*Reference values (Haemonetics Corp.): R 3–8 min, Angle 55–78 °, MA 51–69 mm, Ly30 0-8%, MAFF=14-24 mm.
Reduced clot stability correlates with clinical bleeding conditions as demonstrated by Plotkin et al. [62] who, in patients with a penetrating trauma, reported TEG to be an accurate indicator of the blood product requirements. Furthermore, TEG is the gold standard for identifying hypercoagulability [63-65] and hyperfibrinolysis [35-38,65,66], the latter a significant cause of bleeding in patients with major trauma [29,35-38,59-61,66]. The use of VHA in trauma is now recommended by recent guidelines [67,68] and text books [69] and its use in the military field is extensive and many Level 1 trauma centers consider use of VHA to monitor and guide hemostatic therapy as routine.
Whole blood platelet function analyzers
Different assays evaluating the degree of platelet inhibition secondary to platelet inhibitors exists. Light transmission aggregometry (LTA), previously considered the ”gold standard” for investigation of platelet aggregation [70], unfortunately relies on artificially manufactured platelet rich plasma suspensions, which do not reflect in vivo conditions and consequently platelet function assays performed on whole blood are today favored. Examples of whole blood platelet function assays are PFA100 (Siemens, Tarrytown, USA), VerifyNow (Accumetrics, San Diego, USA) and Multiplate (Verum Diagnostica GmbH, Munich, Germany), which all have been reported to be able to identify clinically relevant platelet inhibition secondary to pharmacological platelet inhibitors.
Goal-directed hemostatic resuscitation based on VHA
Goal-directed treatment with blood products and antifibrinolytic pharmacological agents based on the result of the whole blood viscoelastic hemostatic assays (VHA), together with the clinical presentation, was introduced for more than 25 years ago in patients undergoing liver transplantation and cardiac surgery and there are validated algorithms for how coagulopathy is identified and treated in patients with ongoing bleeding, based on VHA [71]. More than 25 studies encompassing more than 4,500 patients have evaluated VHA vs. conventional coagulation assays on bleeding and transfusion requirements in patients undergoing cardiac, liver, vascular, or trauma surgery and in patients requiring massive transfusion. These studies demonstrate the superiority of VHA in predicting the need for blood transfusion, and the VHA-based algorithm reduces the transfusion requirements and the need for re-do surgery in contrast to treatment based on conventional coagulation assays [29] and this was further corroborated in a recent Cochrane review [72].
We found that implementation of goal-directed hemostatic resuscitation of massively bleeding patients, including trauma, based on the VHA reduced mortality by approximately 30% in patients requiring more than 10 RBC in the first 24 hours [71]. Systematic use of VHA to monitor and guide transfusion therapy is furthermore endorsed by several recent international transfusion guidelines and teaching books [68,69].
Administration of blood products
Red blood cells
In response to hemorrhage, lowered hematocrit contributes to coagulopathy since erythrocytes promote marginalization of platelets so the platelet concentrations along the endothelium remains almost seven times that of the average blood concentration [73]. In addition, erythrocytes support thrombin generation through exposure of procoagulant membrane phospholipids [1,74] and they activate platelets by liberating ADP [75,76] emphasizing that in vivo thrombus formation is a multicellular event [55,77]. Yet, the optimal hematocrit for platelet-vessel wall interactions remains unknown but it may be as high as 35% [78].
Fresh frozen plasma
It remains controversial when and in what dose plasma should be transfused to massively bleeding trauma patients[79-87].
The optimal ratio of FFP to RBCs remains to be established although collectively the data indicate that a FFP:RBC ratio greater than 1:2 is associated with improved survival compared to one lower than 1:2 [79,80,83-85]. This is further supported by a review and meta-analysis from 2010 reporting that in patients undergoing massive transfusion, high FFP to RBC ratios was associated with a significant reduction in the risk of death (odds ratio (OR) 0.38 (95%CI 0.24-0.60) and multiorgan failure (OR 0.40 (95%CI 0.26-0.60) [88], and a meta-analysis from 2012 reports of reduced mortality in trauma patients treated with the highest FFP or PLT to RBC ratios [89].
Platelets
Platelets are also pivotal for hemostasis [55,77] and several retrospective studies report an association between thrombocytopenia and postoperative bleeding and mortality [8,90,91]. Holcomb et al. [85] found that the highest survival was established in patients who received both a high PLT:RBC and a high FFP:RBC ratio. Inaba et al. recently reported from a retrospective study of massively transfused patients that as the apheresis platelet to RBC ratio increased, a stepwise improvement in survival was seen and a high apheresis PLT:RBC ratio was independently associated with improved survival [92]. This is in alignment with Brown et al. who reported that admission platelet count was inversely correlated with 24-hour mortality and transfusion of RBCs and that a normal platelet count may be insufficient after severe trauma suggesting these patients may benefit from a higher platelet transfusion threshold [93]. In a recent meta-analysis, a high PLT:RBC ratio in massively bleeding trauma patients was reported to reduce mortality [89].
Massive transfusion protocols and ratios
A recent meta-analysis of retrospective observational studies evaluating the effect of FFP:RBC and/or PLT:RBC ratios and survival in massively bleeding trauma patients recently reported a significant survival benefit in patients receiving high FFP:RBC and PLT:RBC ratios [89]. A potential confounder of these results are survivorship bias relating to that those surviving long enough will receive FFP and PLT whereas those dying early will not, as reported by Snyder et al. [79]. This was recently corroborated by Ho et al. who employed mathematical modeling of the observational trauma studies performed involving FFP:RBC ratios concluding that some of these probably included survivorship bias in favor of a high FFP:RBC ratio [94].
Survivorship bias, however, does not explain the improved survival in studies concerning the introduction of transfusion packages where both FFP and PLT are immediately available i.e. where pre-thawed FFP are available. Cotton et al. [95] implemented a trauma exsanguination protocol (TEP) involving 10 RBC, 4 FFP and 2 apheresis PLT for trauma patients and used it to evaluate 211 trauma patients of who 94 received TEP and 117 were historic controls. The TEP patients received more RBC (16 vs. 11), FFP (8 vs. 4), and PLT (2 vs. 1) intraoperatively than the controls and displayed lower 30-day mortality (51% vs. 66%). After controlling for age, sex, mechanism of injury, Trauma and Injury Severity Score (TRISS), and 24-hour blood product usage, a 74% reduction in the odds ratio of mortality was found among patients in the TEP group. In a later study involving additionally 53 patients
Cotton also [96] reported that not only was 30-day survival higher in the TEP group compared to the controls, but the incidence of pneumonia, pulmonary failure and abdominal compartment syndrome was lower in the TEP patients. Also, the incidence of sepsis or septic shock and multi-organ failure was lower in TEP patients. Although the TEP group received more blood products intraoperatively, the 24 h transfusion requirements were lower than in controls, supporting that early and aggressive administration of plasma and platelets improves hemostasis and this is in alignment with the result of a recent meta-analysis of trauma patients requiring massive transfusion [89]. In alignment with this, Duchesne et al. reported that in trauma patients undergoing damage control laparotomy introduction of damage control resuscitation encompassing early administration of plasma and platelets together with RBC was associated with improved 30-day survival, (73.6% vs. 54.8%, p<0.009) when compared to patients treated with conventional resuscitation efforts [97]. Similarly, we evaluated 832 massively transfused patients, of whom 20% were trauma patients, two years prior toand two years after implementation of hemostatic control resuscitation in 2004 [71]. The concept involved pre-emptive use of PLT and FFP organized into transfusion packages (5 RBC, 5 pre-thawed FFP and 2 PLT; each a pool of 4 buffy coat platelets) to be administered to patients with uncontrollable bleeding. Compared to the controls, the intervention group received displayed a reduction in 30-day mortality (20% vs. 32% in controls, p=0.002).
A multicentre randomized control study evaluating the effect of different blood product ratios on survival in massively bleeding trauma patients will commence in the USA this year and hopefully this will result in evidence for how best to resuscitate these patients with blood products (http://www.uth.tmc.edu/cetir/PROPPR/index.html).
Fresh whole blood
With the implementation of fractionated blood components, routine use of fresh whole blood (FWB) for resuscitation of bleeding patients was abandoned in the civilian setting. In the combat setting, however, FWB has been used in situations where fractionated blood products, and especially platelets were not available. In a report of US military patients in Iraq and Afghanistan from January 2004 to October 2007, those with hemorrhagic shock, a resuscitation strategy that included FWB was associated with improved 30-day survival (95% vs. 82%, p=0.002) [98] and an ongoing trial is currently addressing this issue (www.clingovtrial.com/ NCT01227005)..
It should be noted that administration of any blood product carries potential risks for the patient including viral and bacterial transmission, hemolytic transfusion reactions, transfusion related acute lung injury and immunomodulation and consequently transfusion of blood products should be reserved to patients who actually needs this therapy [99].
Hemostatic agents
Antifibrinolytics
Hyperfibrinolysis contribute significantly to coagulopathy and antifibrinolytics agents reduce the blood loss in patients with both normal and exaggerated fibrinolytic responses to surgery by preventing plasmin(ogen) from binding to fibrin and by preventing plasmin degradation of platelet glycoprotein Ib receptors [100,101]. In a placebo controlled randomized study (CRASH-2) including 20,211 adult trauma patients, tranexamic acid as compared to placebo significantly decreased all-cause mortality from 16.0% to 14.5%, p=0.0035 [102]. We recommend monitoring of hemostasis with TEG to identify hyperfibrinolytic states in trauma patients [35-38,65,66] and, consequently, targeted treatment with antifibrinolytic agents.
Recombinant Factor VIIa
Recombinant factor VIIa (rFVIIa) acts in pharmacological doses by enhancing thrombin generation on the activated platelets independent of factor VIII and IX and is currently approved for episodes of severe hemorrhage or perioperative management of bleeding in patients with congenital factor VII deficiency and hemophilia A or B with inhibitors [103,104]. Data from the CONTROL trial, a phase 3 randomized clinical trial evaluating efficacy and safety of rFVIIa as an adjunct to direct hemostasis in major trauma [105], rFVIIa did not change mortality in patients with blunt (11.0% (rFVIIa) vs. 10.7% (placebo)) or penetrating (18.2% (rFVIIa) vs. 13.2% (placebo)) trauma. [106]In a recent review reporting on the rate of thromboembolic events in all published randomized placebo controlled trials of rFVIIa use [107]reported that the rates of arterial thromboembolic events among all subjects were higher among those who received rFVIIa than among those who received placebo (5.5% vs. 3.2%, p=0.003) [105].
Fibrinogen concentrate
Conversion of sufficient amounts of fibrinogen to fibrin is a prerequisite for clot formation and reduction in the circulating level of fibrinogen due to consumption [32] and/or dilution by resuscitation fluids [108] induces coagulopathy [109]. Fibrinogen is the first hemostatic component that declines to pathologically low levels following trauma and/or hemodilution [108]. It is, therefore, important to maintain an adequate fibrinogen level when continued bleeding is bridged by saline and/or colloid infusion or blood products (primarily RBC) without fibrinogen. Recent data indicate that coagulopathy induced by synthetic colloids such as HES may be reversed by the administration of fibrinogen concentrate [110]. A recent review only found four studies of poor quality assessing fibrinogen concentrate to bleeding surgical and trauma patients and concluded that randomized controlled trials of sufficient size and long-term follow-up needs to be performed before such a practice can be recommended routinely [111]. The use of fibrinogen concentrate in patients with established hypofibrinogenemia, diagnosed by VHA (Functional Fibrinogen® or FibTEM®), in addition to a balanced administration of RBC, FFP and platelets, may however contribute to faster achievement of a normal hemostasis in massively bleeding patients, and we recommend the use of fibrinogen concentrate according to TEG guided algorithms in such patients (Table 1).
Prothrombin complex concentrate
The four-factor PCC encompasses coagulation factors II, VII, IX and X that all are essential for thrombin generation. Administration of PCC is indicated for the treatment of congenital coagulation disorders and to reverse oral administered anticoagulation by vitamin K antagonists [112], whereas experience with treatment of massive bleeding with PCC is lacking. Recently, Carvalho et al. reported that administration of PCC to patients with massive bleedings had beneficial effect on hemostasis and this warrants further investigation in a randomized controlled setting [113].
Factor XIII
Factor XIII is important for clot firmness by binding to platelets through the GPIIb/IIIa receptor and by cross-linking fibrin and increasing the resistance of the formed clot against fibrinolysis [114]. Notably, patients with “unexplained” intraoperative coagulopathies and, hence, bleeding demonstrate significantly less FXIII per unit thrombin available both before, during and after surgery [115]. An increased tendency to postoperative bleeding has been observed, even at factor XIII activities as high as 60% [116]. The role of FXIII administration to bleeding trauma patients however remains to be investigated in randomized clinical trials.
Teragnostic approach
Recently, a new approach to resuscitation of patients with massive blood loss was introduced, employing goal-directed administration of pharmacological agents such as fibrinogen concentrate, prothrombin complex concentrate, recombinant factor VIIa and factor XIII as alternatives to FFP and platelet concentrates together with volume resuscitation with synthetic colloids. Observational studies of limited size of such an approach in injured patients with massive blood loss indicate that it may be feasible to achieve hemostasis with the use of these procoagulant agents [117].
It should be noted, however, and especially in the light of the disappointing results from the trial regarding the use of recombinant factor VII in trauma, that adequately powered double-blind randomized studies are required before routine use of goal-directed administration of procoagulant agents to injured patients with bleeding is recommended. This is especially important with regard to the safety of these agents, which, as opposed to FFP, do not contain relevant concentrations of natural anticoagulant factors such as antithrombin, protein C and protein S, and this may be of importance in the later phase after hemostatic control has been established [118].
Additionally, it remains elusive which fluid that should be used to support the hemodynamic system during massive bleeding treated with the teragnostic approach when plasma not is included in the resuscitation regimen. Crystalloids clearly have not sufficient volume expanding effects and based on the findings of increased bleeding, transfusion requirements and mortality in septic patients receiving synthetic colloids [119] it could be feared that similar results would surfer in severely injured trauma patients.
Conclusions
Viscoelastic whole blood assays, such as TEG and ROTEM are advantageous for identifying coagulopathy, and guide ongoing transfusion therapy. From the result of these assays, implementation of a hemostatic control resuscitation strategy to massively bleeding patients seems both reasonable and lifesaving although data from prospective randomized controlled trials are lacking. Until definite proof from such trials is available, retrospective data support a shift in transfusion medicine in regard to early and aggressive administration of plasma and platelets.
Competing interest
PJO has received support with analyzers and reagents from Hemonetic Corp, TEM International and Viber Int. PJO has received unrestricted research grants from Novo Nordisk AS and Octaparma GmbH. JS and SRO declare no conflicts of interest.
Author contribution
PJ, JS and SRO contributed to the conception and design of the manuscript, literature search, and writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Pär I Johansson, Email: per.johansson@rh.regionh.dk.
Jakob Stensballe, Email: jakob.stensballe@rh.regionh.dk.
Sisse R Ostrowski, Email: sisse.ostrowski@gmail.com.
References
- Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65:951–960. doi: 10.1097/TA.0b013e318187e15b. [DOI] [PubMed] [Google Scholar]
- Stern SA, Dronen SC, Birrer P, Wang X. Effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med. 1993;22:155–163. doi: 10.1016/S0196-0644(05)80195-7. [DOI] [PubMed] [Google Scholar]
- Anesthesiology. 2006. pp. 198–208. [DOI] [PubMed]
- Dries DJ. Hypotensive resuscitation. Shock. 1996;6:311–316. doi: 10.1097/00024382-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Krausz MM. Fluid resuscitation strategies in the Israeli army. J Trauma. 2003;54:S39–S42. doi: 10.1097/01.TA.0000064506.47688.51. [DOI] [PubMed] [Google Scholar]
- Soreide E, Deakin CD. Pre-hospital fluid therapy in the critically injured patient–a clinical update. Injury. 2005;36:1001–1010. doi: 10.1016/j.injury.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dutton RP. Resuscitative strategies to maintain homeostasis during damage control surgery. Br J Surg. 2012;99(Suppl 1):21–8. doi: 10.1002/bjs.7731.:21-28. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Hansen MB, Sorensen H. Transfusion practice in massively bleeding patients: time for a change? Vox Sang. 2005;89:92–96. doi: 10.1111/j.1423-0410.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–740. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- Mittermayr M, Streif W, Haas T, Fries D, Velik-Salchner C, Klingler A, Oswald E, Bach C, Schnapka-Koepf M, Innerhofer P. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg. 2007;105:905–17. doi: 10.1213/01.ane.0000280481.18570.27. table. [DOI] [PubMed] [Google Scholar]
- Nielsen VG. Colloids decrease clot propagation and strength: role of factor XIII-fibrin polymer and thrombin-fibrinogen interactions. Acta Anaesthesiol Scand. 2005;49:1163–1171. doi: 10.1111/j.1399-6576.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- Knotzer H, Pajk W, Maier S, Dunser MW, Ulmer H, Schwarz B, Salak N, Hasibeder WR. Comparison of lactated Ringer's, gelatine and blood resuscitation on intestinal oxygen supply and mucosal tissue oxygen tension in haemorrhagic shock. Br J Anaesth. 2006;97:509–516. doi: 10.1093/bja/ael208. [DOI] [PubMed] [Google Scholar]
- Thorsen K, Ringdal KG, Strand K, Soreide E, Hagemo J, Soreide K. Clinical and cellular effects of hypothermia, acidosis and coagulopathy in major injury. Br J Surg. 2011;98:894–907. doi: 10.1002/bjs.7497. [DOI] [PubMed] [Google Scholar]
- Mortier E, Ongenae M, De Baerdemaeker L, Herregods L, Den Blauwen N, Van Aken J, Rolly G. In vitro evaluation of the effect of profound haemodilution with hydroxyethyl starch 6%, modified fluid gelatin 4% and dextran 40 10% on coagulation profile measured by thromboelastography. Anaesthesia. 1997;52:1061–1064. doi: 10.1111/j.1365-2044.1997.220-az0354.x. [DOI] [PubMed] [Google Scholar]
- Hartog CS, Kohl M, Reinhart K. A Systematic Review of Third-Generation Hydroxyethyl Starch (HES 130/0.4) in Resuscitation: Safety Not Adequately Addressed. Anesth Analg. 2011;112:635–645. doi: 10.1213/ANE.0b013e31820ad607. [DOI] [PubMed] [Google Scholar]
- Hess JR. Massive transfusion for trauma: opportunities and risks. ISBT Science Series. 2008;3:197–201. [Google Scholar]
- Wolberg AS, Meng ZH, Monroe DM. Hoffman M: A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221–1228. doi: 10.1097/01.TA.0000064328.97941.FC. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Yamamoto T, Mihara H. Changes in coagulation and fibrinolysis occurring in dogs during hypothermia. Thromb Res. 1985;37:503–512. doi: 10.1016/0049-3848(85)90096-9. [DOI] [PubMed] [Google Scholar]
- Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- Djaldetti M, Fishman P, Bessler H, Chaimoff C. pH-induced platelet ultrastructural alterations. A possible mechanism for impaired platelet aggregation. Arch Surg. 1979;114:707–710. doi: 10.1001/archsurg.1979.01370300061009. [DOI] [PubMed] [Google Scholar]
- Martini WZ, Holcomb JB. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann Surg. 2007;246:831–835. doi: 10.1097/SLA.0b013e3180cc2e94. [DOI] [PubMed] [Google Scholar]
- Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003. pp. 1127–1130. [DOI] [PubMed]
- Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Curr Opin Anaesthesiol. 2012;25:229–234. doi: 10.1097/ACO.0b013e3283509675. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Ostrowski SR. Acute coagulopathy of trauma: Balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med Hypotheses. 2010;75:564–567. doi: 10.1016/j.mehy.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A High Admission Syndecan-1 Level, A Marker of Endothelial Glycocalyx Degradation, Is Associated With Inflammation, Protein C Depletion, Fibrinolysis, and Increased Mortality in Trauma Patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- Hess JR, Lawson JH. The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma. 2006;60:S12–S19. doi: 10.1097/01.ta.0000199545.06536.22. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Sørensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, Ostrowski SR. Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? An observational study. Crit Care. 2011;15:R272. doi: 10.1186/cc10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost. 2001;27:585–592. doi: 10.1055/s-2001-18864. [DOI] [PubMed] [Google Scholar]
- Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, David JS. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100:792–797. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, Dakin PA, Lawson CM, Enderson BL, Kurek SJ. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154:34–39. doi: 10.1016/j.trsl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67:125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252:434–442. doi: 10.1097/SLA.0b013e3181f09191. [DOI] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- Oldgren J, Alings M, Darius H, Diener HC, Eikelboom J, Ezekowitz MD, Kamensky G, Reilly PA, Yang S, Yusuf S, Wallentin L, Connolly SJ. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med. 2011;155:660–7. doi: 10.7326/0003-4819-155-10-201111150-00004. W204. [DOI] [PubMed] [Google Scholar]
- Huang J, Cao Y, Liao C, Wu L, Gao F. Apixaban versus enoxaparin in patients with total knee arthroplasty. A meta-analysis of randomised trials. Thromb Haemost. 2011;105:245–253. doi: 10.1160/TH10-08-0552. [DOI] [PubMed] [Google Scholar]
- Duggan ST. Rivaroxaban: a review of its use for the prophylaxis of venous thromboembolism after total hip or knee replacement surgery. Am J Cardiovasc Drugs. 2012;12:57–72. doi: 10.2165/11208470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Levi M, Eerenberg ES, Kampuisen PW. Anticoagulants. Old and new. Hamostaseologie. 2011;31:229–235. doi: 10.5482/ha-1153. [DOI] [PubMed] [Google Scholar]
- Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med. 2011;365:2039–2040. doi: 10.1056/NEJMc1111095. [DOI] [PubMed] [Google Scholar]
- Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012. In press. [DOI] [PubMed]
- Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- Senzolo M, Coppell J, Cholongitas E, Riddell A, Triantos CK, Perry D, Burroughs AK. The effects of glycosaminoglycans on coagulation: a thromboelastographic study. Blood Coagul Fibrinolysis. 2007;18:227–236. doi: 10.1097/MBC.0b013e328010bd3d. [DOI] [PubMed] [Google Scholar]
- Zambruni A, Thalheimer U, Coppell J, Riddell A, Mancuso A, Leandro G, Perry D, Burroughs AK. Endogenous heparin-like activity detected by anti-Xa assay in infected cirrhotic and non-cirrhotic patients. Scand J Gastroenterol. 2004;39:830–836. doi: 10.1080/00365520410004433. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Senzolo M, Melikian C, Burroughs A, Mallett SV. The prevalence of a heparin-like effect shown on the thromboelastograph in patients undergoing liver transplantation. Liver Transpl. 2008;14:855–860. doi: 10.1002/lt.21437. [DOI] [PubMed] [Google Scholar]
- Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, Weitz J, Hofmann U, Weigand MA. Sepsis and Major Abdominal Surgery Lead to Flaking of the Endothelial Glycocalix. J Surg Res. 2011;165:136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Spinler SA, Rees C. Review of prasugrel for the secondary prevention of atherothrombosis. J Manag Care Pharm. 2009;15:383–395. doi: 10.18553/jmcp.2009.15.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts HR, Hoffman M, Monroe DM. A cell-based model of thrombin generation. Semin Thromb Hemost. 2006;32(Suppl 1):32–38. doi: 10.1055/s-2006-939552. [DOI] [PubMed] [Google Scholar]
- Fries D, Innerhofer P, Schobersberger W. Time for changing coagulation management in trauma-related massive bleeding. Curr Opin Anaesthesiol. 2009;22:267–274. doi: 10.1097/ACO.0b013e32832678d9. [DOI] [PubMed] [Google Scholar]
- Murray D, Pennell B, Olson J. Variability of prothrombin time and activated partial thromboplastin time in the diagnosis of increased surgical bleeding. Transfusion. 1999;39:56–62. doi: 10.1046/j.1537-2995.1999.39199116895.x. [DOI] [PubMed] [Google Scholar]
- Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–1425. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327–337. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106:1366–1375. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–S68. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, Mann KG, Holcomb JB. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Ostrowski SR, Sorensen AM, Larsen CF, Johansson PI. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: A prospective study. Scand J Trauma Resusc Emerg Med. 2011;19:64. doi: 10.1186/1757-7241-19-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- Gaarder C, Naess PA, Frischknecht CE, Hakala P, Handolin L, Heier HE, Ivancev K, Johansson P, Leppaniemi A, Lippert F, Lossius HM, Opdahl H, Pillgram-Larsen J, Roise O, Skaga NO, Soreide E, Stensballe J, Tonnessen E, Tottermann A, Ortenwall P, Ostlund A. Scandinavian Guidelines–"The massively bleeding patient". Scand J Surg. 2008;97:15–36. doi: 10.1177/145749690809700104. [DOI] [PubMed] [Google Scholar]
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Stahel PF, Vincent JL, Spahn DR. Task Force for Advanced Bleeding Care in Trauma. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JR, Johansson PI, Holcomb JB. Transfusion Therapy: Clinical Principles and Practice (3rd Edition) Mintz PD (Ed.). AABB Press 2011, Bethesda, MD, USA; 2011. Massive transfusion and transfusion therapy in trauma; pp. 305–321. [Google Scholar]
- Hayward CP, Moffat KA, Raby A, Israels S, Plumhoff E, Flynn G, Zehnder JL. Development of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometry. Am J Clin Pathol. 2010;134:955–963. doi: 10.1309/AJCP9V3RRVNZMKDS. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96:111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD007871.pub2. CD007871.:CD007871. [DOI] [PubMed] [Google Scholar]
- Uijttewaal WS, Nijhof EJ, Bronkhorst PJ, Den Hartog E, Heethaar RM. Near-wall excess of platelets induced by lateral migration of erythrocytes in flowing blood. Am J Physiol. 1993;264:H1239–H1244. doi: 10.1152/ajpheart.1993.264.4.H1239. [DOI] [PubMed] [Google Scholar]
- Peyrou V, Lormeau JC, Herault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost. 1999;81:400–406. [PubMed] [Google Scholar]
- Joist JH, Bauman JE, Sutera SP. Platelet adhesion and aggregation in pulsatile shear flow: effects of red blood cells. Thromb Res. 1998;92:S47–S52. doi: 10.1016/S0049-3848(98)00160-1. [DOI] [PubMed] [Google Scholar]
- Valles J, Santos MT, Aznar J, Marcus AJ, Martinez-Sales V, Portoles M, Broekman MJ, Safier LB. Erythrocytes metabolically enhance collagen-induced platelet responsiveness via increased thromboxane production, adenosine diphosphate release, and recruitment. Blood. 1991;78:154–162. [PubMed] [Google Scholar]
- Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26:41–48. doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- Hardy JF, de Moerloose P, Samama CM. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2006;53:S40–S58. doi: 10.1007/BF03022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CW, Weinberg JA, McGwin G, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, Wright MJ, McSwain NE. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, Wolf SE, Wade CE, Holcomb JB. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–S85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- Teixeira PG, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, Browder T, Green D, Rhee P. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66:693–697. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–570. doi: 10.1016/j.amjsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, Moore EE. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50:1370–1383. doi: 10.1111/j.1537-2995.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Oliveri R, Ostrowski SR. Hemostatic resuscitation with plasma and platelets in trauma. A meta-analysis. J Emerg Trauma Shock. 2012;5:120–125. doi: 10.4103/0974-2700.96479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam DJ, Haggart PC, Ludlam CA, Bradbury AW. von Willebrand factor and platelet count in ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2003;26:412–417. doi: 10.1016/S1078-5884(03)00013-3. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Stensballe J, Rosenberg I, Hilslov TL, Jorgensen L, Secher NH. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47:593–598. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PG, Shulman I, Nelson J, Demetriades D. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010;210:957–965. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Brown LM, Call MS, Margaret KM, Cohen MJ, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, Dutton RP, Hess JR, Duchesne JC, McSwain NE, Muskat P, Johannigamn J, Cryer HM, Tillou A, Pittet JF, De Moya MA, Schreiber MA, Tieu B, Brundage S, Napolitano LM, Brunsvold M, Brunsvold M, Beilman G, Peitzman AB, Zenait MS, Sperry J, Alarcon L, Croce MA, Minei JP, Kozar R, Gonzalez EA, Stewart RM, Cohn SM, Mickalek JE, Bulger EM, Cotton BA, Nunez TC, Ivatury R, Meredith JW, Miller P, Pomper GJ, Marin B. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71:S337–S342. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AM, Dion PW, Yeung JH, Joynt GM, Lee A, Ng CS, Chang A, So FL, Cheung CW. Simulation of survivorship bias in observational studies on plasma to red blood cell ratios in massive transfusion for trauma. Br J Surg. 2012;99(Suppl 1):132–9. doi: 10.1002/bjs.7732.:132-139. [DOI] [PubMed] [Google Scholar]
- Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA. St Jacques P, Young PP: Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–48. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, Wells JE, Islam TM, Meade P, Stuke L, Barbeau JM, Hunt JP, Baker CC, McSwain NE. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. 2010;69:46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66:S69–S76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–3417. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- Porte RJ, Leebeek FW. Pharmacological strategies to decrease transfusion requirements in patients undergoing surgery. Drugs. 2002;62:2193–2211. doi: 10.2165/00003495-200262150-00003. [DOI] [PubMed] [Google Scholar]
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, McClelland B, Laupacis A, Fergusson D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;16 doi: 10.1002/14651858.CD001886.pub2. CD001886. [DOI] [PubMed] [Google Scholar]
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S. CRASH-2 Trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- Hedner U. Factor VIIa and its potential therapeutic use in bleeding-associated pathologies. Thromb Haemost. 2008;100:557–562. [PubMed] [Google Scholar]
- Johansson PI, Ostrowski SR. Evidence supporting the use of recombinant activated factor vii in congenital bleeding disorders. Drug Design Dev Ther. 2010;4:107–116. doi: 10.2147/dddt.s11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ, Dimsits J, Bouillon B. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1879;1999:354. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of Recombinant Activated Factor VII in Randomized Clinical Trials. N Engl J Med. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360–365. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- Nielsen VG, Cohen BM, Cohen E. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiol Scand. 2005;49:222–231. doi: 10.1111/j.1399-6576.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Fenger-Eriksen C, Jensen TM, Kristensen BS, Jensen KM, Tonnesen E, Ingerslev J, Sorensen B. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- Warmuth M, Mad P, Wild C. Systematic review of the efficacy and safety of fibrinogen concentrate substitution in adults. Acta Anaesthesiol Scand. 2011;56:539–5348. doi: 10.1111/j.1399-6576.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- Pabinger-Fasching I. Warfarin-reversal: results of a phase III study with pasteurised, nanofiltrated prothrombin complex concentrate. Thromb Res. 2008;122(Suppl 2):S19–22. doi: 10.1016/S0049-3848(08)70005-7. S19-S22. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Rodrigues AG, Conceicao LM, Galvao ML, Ribeiro LC. Prothrombin complex concentrate (Octaplex): a Portuguese experience in 1152 patients. Blood Coagul Fibrinolysis. 2012;23:222–228. doi: 10.1097/MBC.0b013e328351250f. [DOI] [PubMed] [Google Scholar]
- Lorand L. Factor III: structure, activation, and interactions with fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:291–311. doi: 10.1111/j.1749-6632.2001.tb03516.x. [DOI] [PubMed] [Google Scholar]
- Wettstein P, Haeberli A, Stutz M, Rohner M, Corbetta C, Gabi K, Schnider T, Korte W. Decreased factor XIII availability for thrombin and early loss of clot firmness in patients with unexplained intraoperative bleeding. Anesth Analg. 2004;99:1564–1569. doi: 10.1213/01.ANE.0000134800.46276.21. [DOI] [PubMed] [Google Scholar]
- Chandler WL, Patel MA, Gravelle L, Soltow LO, Lewis K, Bishop PD, Spiess BD. Factor XIIIA and clot strength after cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2001;12:101–108. doi: 10.1097/00001721-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Sorensen B, Fries D. Emerging treatment strategies for trauma-induced coagulopathy. Br J Surg. 2012;99(Suppl 1):40–50. doi: 10.1002/bjs.7770.:40-50. [DOI] [PubMed] [Google Scholar]
- Johansson PI. Emerging treatment strategies for trauma-induced coagulopathy. Br J Surg. 2012;99(Suppl 1):40–50. doi: 10.1002/bjs.7770. Br J Surg 2012, 99 Suppl 1:51. doi: 10.1002/bjs.7792.:51. [DOI] [PubMed] [Google Scholar]
- Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, Madsen KR, Møller MH, Elkjær JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Søe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K K, Kjældgaard AL, Fabritius ML, Mondrup F, Pott FC, Møller TP, Winkel P, Wetterslev J. 6S Trial Group; Scandinavian Critical Care Trials Group. . Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;12:101–108. [Google Scholar]