Abstract
Background
Low Molecular Weight Heparins (LMWH) are at least as effective antithrombotic drugs as Unfractionated Heparin (UFH). However, it is still unclear whether the safety profiles of LMWH and UFH differ. We performed a systematic review to compare the bleeding risk of fixed dose subcutaneous LMWH and adjusted dose UFH for treatment of venous thromboembolism (VTE) or acute coronary syndromes (ACS). Major bleeding was the primary end point.
Methods
Electronic databases (MEDLINE, EMBASE, and the Cochrane Library) were searched up to May 2010 with no language restrictions. Randomized controlled trials in which subcutaneous LMWH were compared to intravenous UFH for the treatment of acute thrombotic events were selected. Two reviewers independently screened studies and extracted data on study design, study quality, incidence of major bleeding, patients’ characteristics, type, dose and number of daily administrations of LMWH, co-treatments, study end points and efficacy outcome. Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated using the random effects model.
Results
Twenty-seven studies were included. A total of 14,002 patients received UFH and 14,635 patients LMWH. Overall, no difference in major bleeding was observed between LMWH patients and UFH (OR = 0.79, 95% CI 0.60–1.04). In patients with VTE LMWH appeared safer than UFH, (OR = 0.68, 95% CI 0.47–1.00).
Conclusion
The results of our systematic review suggest that the use of LMWH in the treatment of VTE might be associated with a reduction in major bleeding compared with UFH. The choice of which heparin to use to minimize bleeding risk must be based on the single patient, taking into account the bleeding profile of different heparins in different settings.
Introduction
In daily clinical practice, low molecular weight heparins (LMWH) and unfractionated heparin (UFH) are the most commonly prescribed anticoagulant drugs for the treatment of acute thrombotic conditions, such as venous thromboembolism (VTE) and acute coronary syndromes (ACS).
LMWH have some advantages over UFH, including higher bioavailability and a more predictable anticoagulant effect. These properties allow the use of LMWH at weight-adjusted doses in most patients, without the need for laboratory monitoring. On the other hand, although treatment with UFH needs laboratory monitoring with Activated Partial Thromboplastin Time (aPTT), because its anticoagulant effect is unpredictable, it has the advantage that bleeding complications can be more easily managed, because UFH has a shorter half-life than LMWH, and can be effectively antagonized by protamine [1].
The antithrombotic efficacy of LMWH and UFH in the treatment of VTE and ACS has been evaluated in many randomized clinical trials and analyzed in several meta-analyses. Treatment of VTE with LMWHs is associated with similar or lower rates of recurrences and death as compared to treatment with UFH [2]; [3]. However, there is no evidence that LMWH are more effective than UFH in patients with ACS [4].
Minimizing the bleeding risk in patients treated with anticoagulants is of utmost clinical relevance, considering that major bleeding, anemia and blood transfusion are powerful and independent predictors of morbidity and mortality in patients with VTE or ACS on treatment with antithrombotic drugs [5]–[7]. To date, the systematic reviews comparing LMWH and UFH, focused on drug efficacy as primary end point and considered the incidence of bleeding a secondary end point. This choice could have affected the selection of studies to be included in the analysis, since some studies reporting haemorrhagic events could have been excluded due to the absence of the primary end point considered as inclusion criteria in those meta-analysis.
It has not been established yet whether the incidence of bleeding complications differs between LMWH and UFH.
Aim of our study was to perform a systematic review of randomized clinical trials to compare the incidence of major bleeding associated with the use of subcutaneous LMWH and intravenous UFH for treatment of acute VTE or ACS.
Materials and Methods
Data Sources and Searches
We attempted to identify all relevant published randomized controlled trials (RCT) that compared fixed-dose subcutaneous LMWH with adjusted-dose intravenous UFH in the initial treatment of thrombotic episodes. We searched MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials, using the search terms “randomized controlled trials” and “heparin” in combination with generic and trade names of individual preparations of LMWH. The search was completed in May 2010. We manually searched the references of retrieved publications to look for additional studies. No language restrictions were applied. Two investigators (EC, AMR) independently evaluated the studies for inclusion, and disagreements were resolved by discussion.
Study Selection
In order to be included in this systematic review, published studies had to meet the following criteria: 1) study design: randomized controlled trial; 2) intervention: comparison of subcutaneous weight-adjusted, fixed-dose LMWH with adjusted doses (based on APTT values) intravenous UFH, for the initial treatment of acute thrombotic episodes; 3) availability of outcome data on the incidence of major bleeding. We accepted the definitions of major bleeding that were chosen in each individual trial. Dose-finding studies were excluded. Studies with no events in both LMWH and UFH arms were included in the descriptive analysis, but excluded from the meta-analysis, because calculation of the odds ratio (OR) was not feasible.
Pre-defined subgroup analyses were performed on the basis of the type of disease that was treated (VTE vs ACS), the doses of LMWH that were administered, the number of daily administrations of LMWH (one or two) and the type of LMWH used.
Quality Assessment
In order to evaluate the quality of the included studies, we used the study-quality criteria of Jadad, which take into account proper randomization, allocation of patients and blinding [8].
Data Extraction
Two investigators (EC, AMR) independently extracted the data on study design, study quality, incidence of major bleeding, patients’ characteristics, type, dose and number of daily administrations of LMWH, co-treatments, study end points and efficacy outcome. The data extracted for each trial were confirmed by consensus between the reviewers.
Data Analysis
For each primary study that was included in the meta-analysis, a two-by-two table was constructed and the OR, with their 95% confidence intervals (CI), were calculated to estimate the risk of major bleeding in patients treated with LMWH compared to patients treated with UFH.
Pooled OR and 95% CI were calculated using the random effects model [9]. The homogeneity of the estimates of OR among studies was evaluated using the chi-square statistic test. Given the known difference of clinical settings of primary studies included in our meta-analysis (clinical heterogeneity), random effects analysis was performed for all studies, irrespective of the statistical significance of the heterogeneity chi-square test.
All analyses were performed using STATA 11.0 software [10].
Results
Study Selection
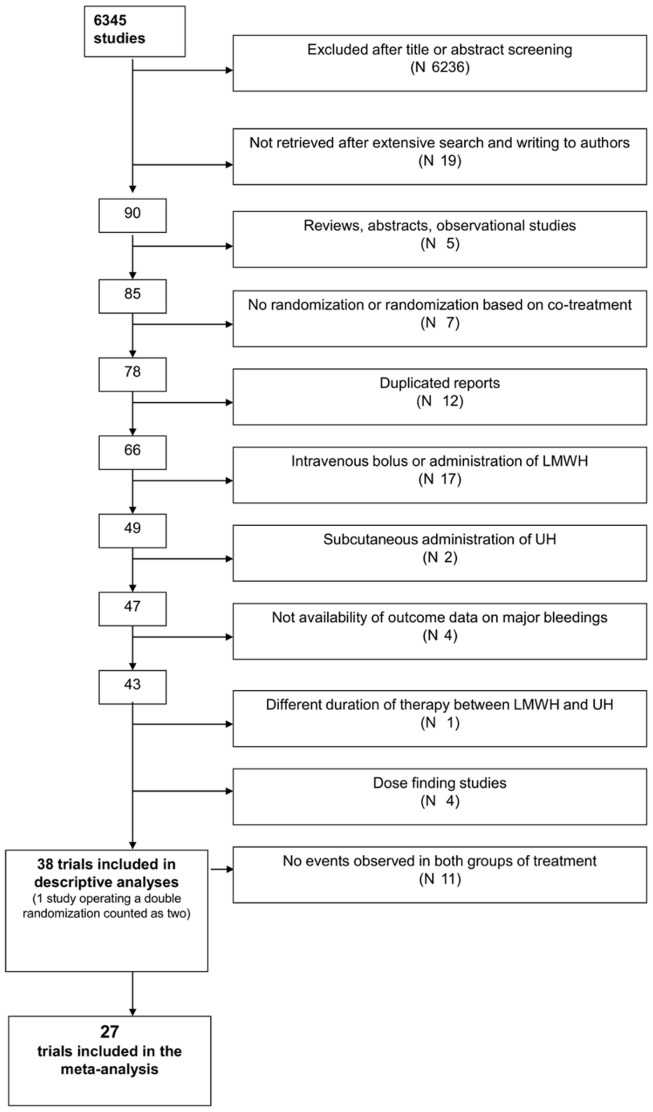
Figure 1 shows the process of study selection. We identified 6513 articles (1498 from Medline, 3205 from Embase, 1810 from the Cochrane Library). Of these, 1515 studies were duplicates, which were therefore eliminated, leaving 4998 articles for consideration. After the exclusion of irrelevant studies, which were identified by reviewing the titles and abstracts of all retrieved articles, 112 publications remained for analysis. We could not obtain the full publication of 19 studies after searching in the British Library and writing to the authors. Fifty-four of the remaining 93 articles were subsequently excluded, based on a more detailed evaluation of the full publications (Fig. 1). No additional studies were identified by reviewing the references from the original studies and other meta-analyses. One study, which operated a double randomization was considered as two data sets [11]. Thus, 37 studies were included in the descriptive analysis [11]–[47]. In 10 of them, no bleeding events were observed in any treatment group: therefore, 27 studies were included in the meta-analysis [12]–[15]; [17]–[38]; [47].
Figure 1. Study selection progression.
Table 1 describes the characteristics of the included studies. Thirty-four studies were published in English, one in French and two in Spanish, in journals of Internal Medicine, Cardiology, Hematology, or, less frequently, Angiology and Pneumology. The years of publication ranged between 1989 and 2006. Twenty-six studies enrolled patients with VTE, 11 studies enrolled patients with ACS (STEMI, NSTEMI, unstable angina). Nine different types of LMWH were used: the most commonly used one was enoxaparin. In all studies, the dose of intravenous UFH was titrated to maintain the APTT ratio between 1.5 and 2.5. The daily doses of subcutaneous LMWH ranged between 90 and 300 anti-Xa U/kg (mean 195 U/kg), in one or two daily administrations (9 and 28 studies respectively); one study allowed both the once daily and the twice daily administration [30]. Treatments lasted between 2 and 28 days (mean, 8 days). Concomitant medications for patients with ACS included Aspirin, Aspirin plus a thienopiridine, Aspirin plus a GPIIb-IIIa inhibitor, or Aspirin plus a thrombolytic agent. Most studies used the TIMI criteria to define major bleeding [48].
Table 1. Characteristics of included studies.
| Clinical indication | Patients n. | Major bleeding n. (%) | Mean age (years) | Type and daily dosage of LMWH | Cotreatments | ||
| Riess H et al. 2003 | VTE | LMWH | 627 | 6 (1.0) | 61 | Certoparin, 8000 IU, bid | None |
| UFH | 593 | 7 (1.3) | |||||
| Decousus et al. 1998 | VTE | LMWH | 195 | 7 (3.6) | 72 | Enoxaparin, 100 IU/Kg, bid | None |
| UFH | 205 | 8 (3.9) | |||||
| Columbus investigators 1997 | VTE | LMWH | 510 | 10 (2.0) | 60 | Reviparin, 3500–6300 IU, bid | None |
| UFH | 511 | 8 (1.6) | |||||
| Zhang Wang et al. 2006 | ACS | LMWH | 96 | 1 (1.0) | 66 | Parnaparin, 4250 IU, bid | ASA, UK |
| UFH | 90 | 3 (3.1) | |||||
| Hull et al. 1992 | VTE | LMWH | 213 | 1 (0.5) | No data | Tinzaparin, 175 IU/Kg, qd | None |
| UFH | 219 | 11 (5.0) | |||||
| Collaborative European Multicentre Study 1991 | VTE | LMWH | 85 | 2 (2.4) | No data | Nadroparin, 12500–17500 IU, bid | None |
| UFH | 81 | 4 (4.9) | |||||
| Campos et a. 2002 | ACS | LMWH | 107 | 1 (0.9) | 60 | Enoxaparin, 80 IU/Kg, bid | ASA |
| UFH | 96 | 9 (9.4) | |||||
| Goldhaber et al. 1998 | VTE | LMWH | 41 | 1 (2.4) | 54 | Ardeparin, 130 /Kg, bid | None |
| UFH | 39 | 1 (2.6) | |||||
| Simonneau et al. 1997 | VTE | LMWH | 304 | 3 (1.0) | 67 | Tinzaparin, 175 IU/Kg, qd | None |
| UFH | 308 | 5 (1.6) | |||||
| PRIME CARE Study Investigators Group 2005 | ACS | LMWH | 451 | 2 (0.4) | 57 | Parnaparin, 6400 IU, qd | ASA |
| UFH | 446 | 2 (0.4) | |||||
| Goodman et al. 2003 | ACS | LMWH | 380 | 8 (2.1) | 64 | Enoxaparin, 100 IU/Kg, bid | ASA, EPF |
| UFH | 366 | 20 (5.5) | |||||
| Kakkar et al. 2003 | VTE | LMWH | 126 | 0 (0) | No data | Bemiparin, 115 IU/Kg, qd | None |
| UFH | 126 | 1 (1.0) | |||||
| Prandoni et al. 1992 | VTE | LMWH | 85 | 1 (1.2) | No data | Nadroparin, 12500–17500 IU, bid | None |
| UFH | 85 | 3 (3.5) | |||||
| Levine et al. 1996 | VTE | LMWH | 247 | 5 (2.0) | 58 | Enoxaparin, 100 IU/Kg, bid | None |
| UFH | 253 | 3 (1.2) | |||||
| Gurfinkel et al. 1995 | ACS | LMWH | 68 | 0 (0) | 63 | Nadroparin, 214 IU/Kg, bid | ASA |
| UFH | 70 | 2 (2.9) | |||||
| Harenberg et al. 2000 | VTE | LMWH | 265 | 4 (1.5) | 62 | Certoparin, 8000 IU, bid | None |
| UFH | 273 | 11 (4.0) | |||||
| Cohen et al. 2002 | ACS | LMWH | 315 | 4 (1.3) | 64 | Enoxaparin, 100 IU/Kg, bid | ASA, TFB |
| UFH | 210 | 3 (1.4) | |||||
| Blazing et al. 2004 | ACS | LMWH | 2026 | 18 (0.9) | 61 | Enoxaparin, 100 IU/Kg, bid | ASA, TFB |
| UFH | 1961 | 8 (0.4) | |||||
| Breddin et al. 2001 | VTE | LMWH | 762 | 2 (0.3) | 59 | Reviparin, 7000–12600 IU, qd or bid | None |
| UFH | 375 | 2 (0.6) | |||||
| Perez de Llano et al. 2003 | VTE | LMWH | 29 | 1 (3.4) | No data | Enoxaparin, 100 IU/Kg, bid | None |
| UFH | 21 | 0 (0) | |||||
| Fiessinger et al. 1996 | VTE | LMWH | 120 | 0 (0) | 61 | Dalteparin, subcutaneous bolus 5000 IU, then 200 IU/kg qd | None |
| UFH | 133 | 2 (1.5) | |||||
| Harenberg et al. 1990 | VTE | LMWH | 24 | 3 (12.5) | 61 | Certoparin, 150 IU/Kg, bid | None |
| UFH | 26 | 3 (11.5) | |||||
| Luomanmaki et al. 1996 | VTE | LMWH | 117 | 0 (0) | 59 | Dalteparin, subcutaneous bolus 5000 IU, then 200 IU/kg qd | None |
| UFH | 131 | 1 (0.8) | |||||
| Koopman et al. 1996 | VTE | LMWH | 202 | 1 (0.5) | 60 | Nadroparin, 8200–18400 IU, bid | None |
| UFH | 198 | 2 (1.0) | |||||
| Cohen et al. 1997 | ACS | LMWH | 1607 | 102 (6.3) | 63 | Enoxaparin, 100 IU/Kg, bid | ASA |
| UFH | 1564 | 107 (6.8) | |||||
| SYNERGY Trial Investigators 2004 | ACS | LMWH | 4993 | 453 (9.1) | 68 | Enoxaparin, 100 IU/Kg, bid | ASA clopidogrel/antiIIb/IIIa |
| UFH | 4985 | 379 (7.6) | |||||
| Kirchmaier et al. 1998 | VTE | LMWH | 128 | 1 (0.8) | 61 | Certoparin, 8000 IU, bid | None |
| UFH | 131 | 4 (3.1) | |||||
| Belcaro et al. 1999 | VTE | LMWH | 98 | 0 (0) | 53 | Nadroparin, 100 IU/Kg, bid | None |
| UFH | 97 | 0 (0) | |||||
| Malhotra et al. 2001 | ACS | LMWH | 51 | 0 (0) | 60 | Enoxaparin, 100 IU/Kg, bid | ASA |
| UFH | 42 | 0 (0) | |||||
| Moreno Palomares et al. 2001 | VTE | LMWH | 17 | 0 (0) | 67 | Dalteparin, 200 IU/Kg qd | None |
| UFH | 15 | 0 (0) | |||||
| Simonneau et al. 1993 | VTE | LMWH | 67 | 0 (0) | 62 | Enoxaparin, 100 IU/Kg, bid | None |
| UFH | 67 | 0 (0) | |||||
| Meyer et al. 1995 | VTE | LMWH | 29 | 0 (0) | 61 | Dalteparin, 120 IU/kg, bid | None |
| UFH | 31 | 0 (0) | |||||
| Findik et al. 2002 | VTE | LMWH | 29 | 0 (0) | 50 | Enoxaparin, 100 IU/Kg bid | None |
| UFH | 30 | 0 (0) | |||||
| Lindmarker et al. 1994 | VTE | LMWH | 101 | 0 (0) | 61 | Dalteparin, 200 IU/Kg, qd | None |
| UFH | 103 | 0 (0) | |||||
| Stricker et al. 1999 | VTE | LMWH | 9 | 0 (0) | 66 | Nadroparin, 185 IU/Kg, qd | None |
| UFH | 11 | 0 (0 | |||||
| Kim et al. (a) 2005 | ACS | LMWH | 40 | 0 (0) | 63 | Dalteparin, 120 IU/Kg, bid | ASA, Clopidogrel |
| UFH | 40 | 0 (0) | |||||
| Kim et al. (b) 2005 | ACS | LMWH | 40 | 0 (0) | 59 | Dalteparin, 120 IU/Kg, bid | ASA, Clopidogrel, TFB |
| UFH | 40 | 0 (0) | |||||
| Aiach et al. 1989 | VTE | LMWH | 31 | 0 (0) | 62 | Dalteparin, 100 IU/Kg bid, then in function of antifactor Xa, bid | None |
| UFH | 30 | 0 (0) | |||||
n: numbers; LMWH: low molecular weight heparin; UFH: unfractioned heparin; VTE: venous thromboembolism; ACS: acute coronary syndrome; qd: once daily; bid: twice daily; tid: three times a day; UK: urochinasi; TFB: tirofiban; EPF: eptifibatide; antiIIb/IIIa: GPIIb/IIIa inhibitors; ASA: acetylsalicylic acid.
A total of 28,637 patients had been enrolled in the studies, 14,635 of whom were treated with LMWH and 14,002 with UFH. The mean number of patients enrolled in each trial was 754 (range 20–9,978 ), their mean age was 62 y (range 49–73), and 63% (range 31–83%) were men.
The aim of the majority of the studies was to evaluate combined end-points; less frequently, a single clinical end-point or an instrumental measure were evaluated. Major bleeding ranged from 0 to 12.5%, (mean, 4.4%) in patients treated with LMWH and from 0 to 11.5% (mean, 4.4%) in patients treated with UFH. The mean incidence of bleeding events were lower in VTE (1.1% with LMWH and 1.9% with UFH), compared to ACS (5.8% with LMWH and 5.4% with UFH). Considering the efficacy end-points, 15 studies showed that LMWH were superior to UFH [12]; [15]; [17]–[19]; [21]–[23]; [26]; [27]; [29]; [30]; [36]; [40]; [42], 1 study showed that UFH was superior to LMWH [46], while the remaining 21 showed that there was no statistically significant difference between the two treatments [11]; [13]; [14]; [16]; [20]; [24]; [25]; [28]; [31]–[35]; [37]–[39]; [41]; [43]–[45]; [47].
Assessment of Study Quality
Based on the study-quality criteria of Jadad [8], 3 studies scored 1 point, 17 scored 2 points, 14 scored 3 points, 1 scored 4 points and 2 scored 5 points. Lack of blindness was the most common flaw in the studies with a low Jadad score.
Data Synthesis
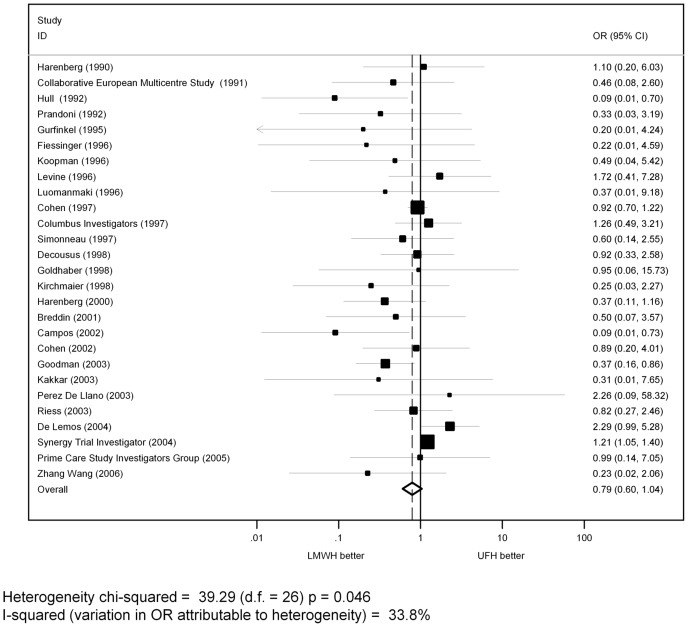
Pooled estimates of ORs for major bleeding showed a non-statistically significant trend in favor of LMWH compared to UFH (OR = 0.79, 95% CI: 0.60–1.04, p = 0.091; n = 27 primary studies) (Figure 2).
Figure 2. Pooled estimates of OR for major bleedings of LMWH versus UFH in all patients.
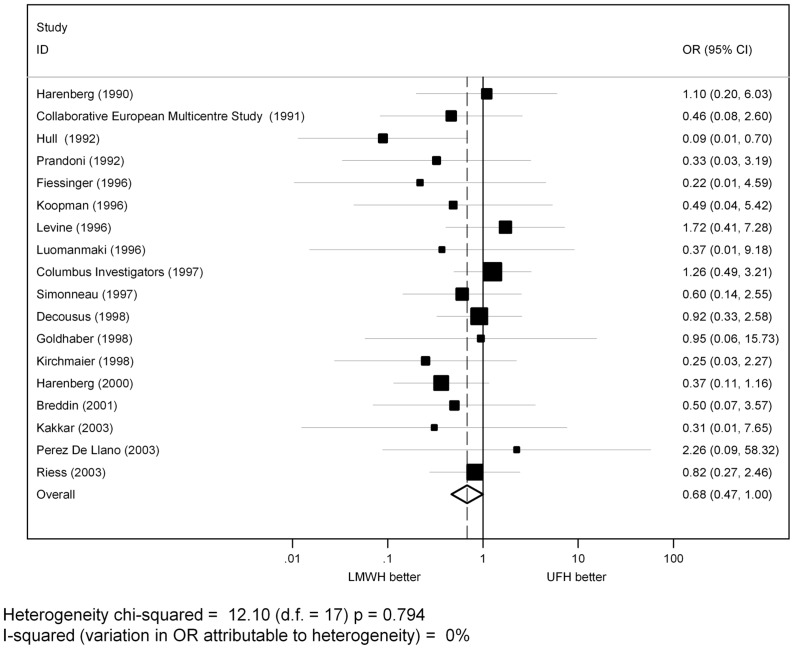
When the analysis was limited to trials that enrolled patients with VTE, pooled estimates of OR was in favor of LMWH (OR = 0.68, 95% CI: 0.47–1.00, p = 0.05) (figure 3).
Figure 3. Pooled estimates of OR for major bleedings of LMWH versus UFH in VTE patients.
Study Characteristics
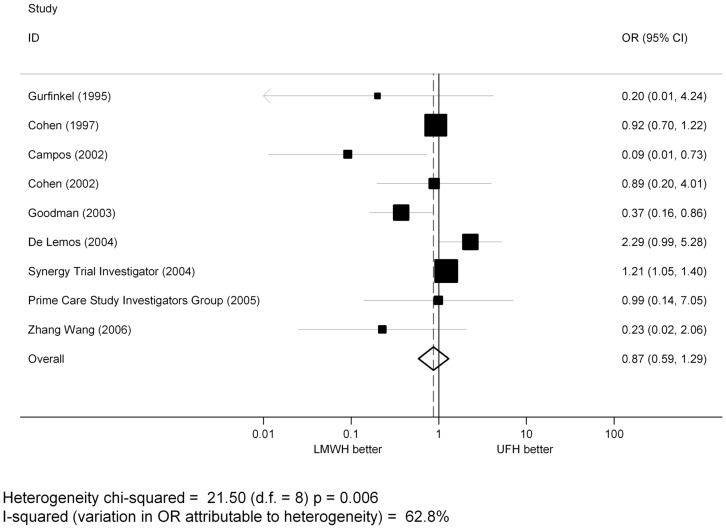
In contrast, when the analysis was limited to trials that enrolled patients with ACS, no statistically significant differences between LMWH and UFH were found (OR = 0.87, 95% CI: 0.59–1.29; p = 0.493) (figure 4).
Figure 4. Pooled estimates of OR for major bleedings of LMWH versus UFH in ACS patients.
Once daily LMWH was significantly safer than UFH, (OR = 0.39, 95% CI: 0.16–0.95, p = 0.039), while, for twice daily LMWH, no statistical difference was observed compared to UFH (OR = 0.86, 95% CI: 0.65–1.14, p = 0.296) (figure S1). When the analysis was limited to the subgroup of VTE trials, once daily LMWH was significantly safer than UFH (OR = 0.31, 95% CI: 0.12–0.84), while no significant differences were found when considering the other subgroups (VTE twice, ACS once and ACS twice) (figure S2).
The exclusion of studies in which LMWH was under-dosed (less than 75% of the recommended daily dose) or overdosed (more than 125% of the recommended dose) did not substantially change the results (OR = 0.79, CI 95% 0.58–1.06, p = 0.121) (figure S3).
No statistically significant differences among different LMWH were observed (figure S4).
The results did not change, after Exclusion of the low quality studies (Jadad score <3) from the analysis (OR = 0.88, 95% CI: 0.67–1.15, p = 0.342) (figure S5).
Based on our a priori defined protocol, we excluded dose-finding studies [49]–[52], which had been included in other revisions. In particular, in the VTE setting, the study by Merli et al., which used two different LMWH doses, compared to a single UFH group, was excluded despite the large number of patients enrolled. For the sake of completeness, we repeated our analysis including the dose-finding trials and the results did not change (figure S6).
Discussion
Our systematic review shows a trend toward a non-statistically significant lower incidence of major bleeding with LMWH compared to UFH (OR 0.79), in the treatment of acute thrombotic events, such as VTE and ACS. When we analyzed separately the RCT that enrolled VTE patients and those enrolling ACS patients, the reduction in the bleeding risk associated with LMWH (OR = 0.68, p = 0.05) reached statistical significance for VTE. Some reasons might explain the absence of significant difference in bleeding between LMWH and UFH in ACS patients: i) many ACS patients underwent invasive procedures (coronary angiography and PCI), ii) ACS patients were often in co-treatment with antiplatelet agents; iii) LMWH patients are likely to be more often in the therapeutic range compared to dose adjusted UFH.
Somewhat unexpectedly, the incidence of bleeding complications was significantly lower with once daily administrations of LMWH, compared to twice daily administrations, the total daily doses being similar in the two treatment regimens (figure S1). Although we have not a clear explanation of these results, it could be speculated that bleeding correlates better with the trough drug concentrations (lower in once a day than in twice a day administrations) than with peak concentrations (higher in daily administration).
Anyway, our findings raise the possibility that dosing regimens can be important determinants for bleeding complications. Further studies should clarify this topic.
The use of LMWH and UFH for treatment of VTE and ACS has been evaluated in previous systematic reviews and meta-analyses, which were focused on their antithrombotic efficacy as primary end-point. However, in consideration of the negative impact on mortality and adverse cardiovascular events associated with major bleeding [5]–[7], it is also important to establish which treatment is safer, in terms of incidence of bleeding complications.
As far as treatment of VTE is concerned, only the meta-analysis of Lim et al on LMWH-associated bleeding is available to date [53], which demonstrated a higher incidence of bleeding complications in patients with renal failure, without comparing LMWH with UFH. Information on the difference in bleeding complications between LMWH and UH is retrievable from two other meta-analyses, which were mainly focused on evaluating the differences in efficacy between the two treatments [2]; [3]. Quinlan et at. reported a non-statistically significant trend toward lower frequency of major bleeding associated with LMWH, compared to UFH (1.4% vs 2.3%, OR 0.67, 95% CI 0.36–1.27), in patients with non-massive pulmonary embolism [2]. In the Systematic Review of the Cochrane Collaboration on VTE [3], LMWH were shown to be significantly safer than UFH (incidence of major bleeding, 1% vs 2.1%, OR 0.57; 95% CI 0.39–0.83). While, at a first glance, our review seems to be very similar to the Cochrane review, we think that they are basically different. The recently published Cochrane meta-analysis focused on the comparison between LMWH and unfractionated heparin (UFH in terms of their efficacy for the initial treatment of venous thromboembolism (VTE). In our manuscript we have chosen the safety of these drugs for the initial treatment of acute thrombotic events as primary end point. As a consequence, in our review all the studies reporting haemorrhagic events, irrespective of the efficacy endpoint that was reported, have been included. As a consequence, compared to the Cochrane meta-analysis, 9 additional studies were included in our descriptive analysis [19]; [23]; [30]; [31]; [33]; [41]; [43]; [46]; [47], 6 of which were also included in our meta-analysis [31]; [33]; [41]; [43]; [46]; [47]. These additional studies account for 1344 more VTE patients included in our analysis.
As far as ACS treatment is concerned, three meta-analysis have recently been published, which gave contrasting results. Magee et al [54], consistently with our data, showed a similar rate of major bleeding in LMWH and UFH-treated patients with ACS (RR = 1), despite the fact that only one of the 7 studies included by Magee met our inclusion criteria. In contrast, Murphy et al [55], who analyzed studies comparing enoxaparin and UFH only, found an excess of major bleeding in the enoxaparin group (OR 1.25, p = 0.019). We have no explanation for the contrasting results of the analysis by Murphy et al, compared to those of the study by Magee et al and of our systematic review. The fact that Murphy et al [55] focused on studies that used enoxaparin only does not apparently account for the differences in results, because, when we restricted our analysis to studies that used enoxaparin only, we found no statistically significant differences in the incidence of major bleeding between the enoxaparin group and the UFH group (figure S4). The inclusion of studies that administered enoxaparin either intravenously or subcutaneously in the meta-analysis by Murphy et al might account for the different results obtained in our meta-analysis, which included those studies that administered LMWHs subcutaneously only. This concept seem to be strengthened by the most recent meta-analysis by Silvain et al [56], that enrolled RCT’s and registers studies and included only patients treated with enoxeparin during percutaneous coronary interventions. While they find an important reduction in major bleeding in patients treated with intravenous Enoxeparin, the risk of bleeding using Enoxeparin by the subcutaneous route was the same as UFH.
The results of our study may have important clinical implications. Patients at high risk for thrombotic events are often also at high risk for bleeding [57]; [58]. Iron deficiency anemia and/or hemorrhagic diathesis are common co-morbidities. In this context, the choice of the best anticoagulant treatment should be done taking into account the risk of adverse events more than the therapeutic efficacy. As a matter of fact, minimizing the bleeding risk in patients treated with anticoagulants is of utmost clinical relevance, considering that major bleeding, anemia and blood transfusion are powerful and independent predictors of morbidity and mortality in patients with VTE or ACS on treatment with antithrombotic drugs [5]–[7]. LMWH has the advantages of subcutaneous administration, more predictable anticoagulant response, lack of the need for laboratory monitoring and probably less risk of major bleeding in the VTE. On the other hand the lack of complete antagonization by antidotes and the long acting profile can be a disadvantage in active bleeding patients. UFH is less easy to handle, but the presence of an antidote and its brief half life could be arguments to consider in choosing the type of heparin to use in high bleeding risk patients who need anticoagulation. The choice must be based on the single patient and on the clinical and practical context, taking into account the bleeding profile of different heparins in different settings.
Limitations
The major limitation of our systematic review is the clinical heterogeneity between studies, especially considering all the trials together. Sources of clinical heterogeneity are several.
The primary outcome of our systematic review, major bleeding, was the secondary end-point of the original RCT that we considered in our analysis. As we included all the RCT that compared subcutaneous LMWH with UFH, we considered different clinical scenarios and consequently different study designs, in which heparins were used at different doses and with different co-therapies. However, our results should not be affected by these factors, since we considered randomized studies only. Moreover, trying to take into account clinical heterogeneity, we used a random effect model for the analysis, which is known to be more conservative.
We chose the criteria that had been used in the original studies as criteria for major bleeding and this could account for some of the heterogeneity present in our results. However, most studies considered the following major bleeding events, which are undoubtedly clinically relevant: i) the presence of intracranial or retroperitoneal haemorrhage, ii) haemorrhage that led directly to death, necessitated transfusion or led to the interruption of antithrombotic treatment, iii) a ≥2 g/dl decrease in the haemoglobin concentration.
Finally, in the arterial setting clinical and statistical heterogeneity does not allow to derive definitive conclusions; vice versa, in venous setting the sources of clinical heterogeneity are lower as confirmed by the absence of statistical heterogeneity.
In conclusion, the results of our systematic review suggest that LMWH might have a better safety profile than UFH in the treatment of VTE, while no differences between the two treatments was detected in ACS. The choice of which heparin to use to minimize bleeding risk must be based on the single patient and on the clinical and practical context, taking into account the bleeding profile of different heparins in different settings.
Supporting Information
Subgroup analysis: number of daily LMWH administrations (once daily vs twice daily).
(DOC)
Subgroup analysis: number of daily LMWH administrations (once daily vs twice daily) in VTE and ACS studies.
(DOC)
Subgroup analysis, after the exclusion of studies in which LMWH was was under-dosed (less than 75% of the recommended daily dose) or overdosed (more than 125% of the recommended dose).
(DOC)
Subgroup analysis by type of LMWH.
(DOC)
Subgroup analysis, after exclusion of low quality studies (Jadad Score<3).
(DOC)
Overall meta-analysis, including dose-finding studies (studies grouped by VTE or ACS patients).
(DOC)
Funding Statement
The authors have no funding or support to report.
References
- 1. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, et al. (2008) Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 454S–545S. [DOI] [PubMed] [Google Scholar]
- 2. Quinlan DJ, McQuillan A, Eikelboom JW (2004) Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med 140: 175–83. [DOI] [PubMed] [Google Scholar]
- 3.Erkens PM, Prins MH (2010) Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev CD001100. [DOI] [PubMed]
- 4. Harrington RA, Becker RC, Cannon CP, Gutterman D, Lincoff AM, et al. (2008) Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 670S–707S. [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, et al. (2005) Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 111: 2042–9. [DOI] [PubMed] [Google Scholar]
- 6. Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, et al. (2007) Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 49: 1362–8. [DOI] [PubMed] [Google Scholar]
- 7. Jimenez D, Escobar C, Marti D, Diaz G, Cesar J, et al. (2009) Association of anaemia and mortality in patients with acute pulmonary embolism. Thromb Haemost 102: 153–8. [DOI] [PubMed] [Google Scholar]
- 8. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 9. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 10.Stata statistical software: release 8.0 [computer program]. Version; 2003.
- 11. Kim JH, Jeong MH, Rhew JY, Lim JH, Yun KH, et al. (2005) Long-term clinical outcomes of platelet glycoprotein IIb/IIIa inhibitor combined with low molecular weight heparin in patients with acute coronary syndrome. Circ J 69: 159–64. [DOI] [PubMed] [Google Scholar]
- 12. Riess H, Koppenhagen K, Tolle A, Kemkes-Matthes B, Grave M, et al. (2003) Fixed-dose, body weight-independent subcutaneous low molecular weight heparin Certoparin compared with adjusted-dose intravenous unfractionated heparin in patients with proximal deep venous thrombosis. Thromb Haemost 90: 252–9. [DOI] [PubMed] [Google Scholar]
- 13. Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, et al. (1998) A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med 338: 409–15. [DOI] [PubMed] [Google Scholar]
- 14. The Columbus Investigators (1997) Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. N Engl J Med 337: 657–62. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Wang XK, Yang CM, Liu GY (2004) [Use of unfractionated heparin and a low-molecular-weight heparin following thrombolytic therapy for acute ST-segment elevation myocardial infarction]. Di Yi Jun Yi Da Xue Xue Bao 24: 81–4. [PubMed] [Google Scholar]
- 16. Hull RD, Raskob GE, Pineo GF, Green D, Trowbridge AA, et al. (1992) Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med 326: 975–82. [DOI] [PubMed] [Google Scholar]
- 17. A randomised trial of subcutaneous low molecular weight heparin (CY 216) compared with intravenous unfractionated heparin in the treatment of deep vein thrombosis. A collaborative European multicentre study. Thromb Haemost65: 251–6. [PubMed] [Google Scholar]
- 18. Campos JV, Juarez HU, Rosas PM, Lupi HE, Gonzalez PH, et al. (2002) [Decrease of total hemorrhage with reduced doses of enoxaparin in high risk unstable angina. ENHNFAI study. (Enoxaparin vs non-fractionated heparin in unstable angina). Preliminary report]. Arch Cardiol Mex 72: 209–19. [PubMed] [Google Scholar]
- 19. Goldhaber SZ, Morrison RB, Diran LL, Creager MA, Lee TH (1998) Abbreviated hospitalization for deep venous thrombosis with the use of ardeparin. Arch Intern Med 158: 2325–8. [DOI] [PubMed] [Google Scholar]
- 20. Simonneau G, Sors H, Charbonnier B, Page Y, Laaban JP, et al. (1997) A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l'Embolie Pulmonaire. N Engl J Med 337: 663–9. [DOI] [PubMed] [Google Scholar]
- 21. Comparative efficacy of once daily parnaparin and unfractionated heparin in unstable angina pectoris: PRIME CARE study. Indian Heart J 57: 648–54. [PubMed] [Google Scholar]
- 22. Goodman SG, Fitchett D, Armstrong PW, Tan M, Langer A (2003) Randomized evaluation of the safety and efficacy of enoxaparin versus unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes receiving the glycoprotein IIb/IIIa inhibitor eptifibatide. Circulation 107: 238–44. [DOI] [PubMed] [Google Scholar]
- 23. Kakkar VV, Gebska M, Kadziola Z, Saba N, Carrasco P (2003) Low-molecular-weight heparin in the acute and long-term treatment of deep vein thrombosis. Thromb Haemost 89: 674–80. [PubMed] [Google Scholar]
- 24. Prandoni P, Lensing AW, Buller HR, Carta M, Cogo A, et al. (1992) Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet 339: 441–5. [DOI] [PubMed] [Google Scholar]
- 25. Levine M, Gent M, Hirsh J, Leclerc J, Anderson D, et al. (1996) A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 334: 677–81. [DOI] [PubMed] [Google Scholar]
- 26. Gurfinkel EP, Manos EJ, Mejail RI, Cerda MA, Duronto EA, et al. (1995) Low molecular weight heparin versus regular heparin or aspirin in the treatment of unstable angina and silent ischemia. J Am Coll Cardiol 26: 313–8. [DOI] [PubMed] [Google Scholar]
- 27. Harenberg J, Schmidt JA, Koppenhagen K, Tolle A, Huisman MV, et al. (2000) Fixed-dose, body weight-independent subcutaneous LMW heparin versus adjusted dose unfractionated intravenous heparin in the initial treatment of proximal venous thrombosis. EASTERN Investigators. Thromb Haemost 83: 652–6. [PubMed] [Google Scholar]
- 28. Cohen M, Theroux P, Borzak S, Frey MJ, White HD, et al. (2002) Randomized double-blind safety study of enoxaparin versus unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes treated with tirofiban and aspirin: the ACUTE II study. The Antithrombotic Combination Using Tirofiban and Enoxaparin. Am Heart J 144: 470–7. [DOI] [PubMed] [Google Scholar]
- 29. de Lemos JA, Blazing MA, Wiviott SD, Brady WE, White HD, et al. (2004) Enoxaparin versus unfractionated heparin in patients treated with tirofiban, aspirin and an early conservative initial management strategy: results from the A phase of the A-to-Z trial. Eur Heart J 25: 1688–94. [DOI] [PubMed] [Google Scholar]
- 30. Breddin HK, Hach-Wunderle V, Nakov R, Kakkar VV (2001) Effects of a low-molecular-weight heparin on thrombus regression and recurrent thromboembolism in patients with deep-vein thrombosis. N Engl J Med 344: 626–31. [DOI] [PubMed] [Google Scholar]
- 31. Perez de Llano LA, Baloira VA, Veres RA, Veiga F, Golpe GR, et al. (2003) [Multicenter, prospective study comparing enoxaparin with unfractionated heparin in the treatment of submassive pulmonary thromboembolism]. Arch Bronconeumol 39: 341–5. [DOI] [PubMed] [Google Scholar]
- 32. Fiessinger JN, Lopez-Fernandez M, Gatterer E, Granqvist S, Kher A, et al. (1996) Once-daily subcutaneous dalteparin, a low molecular weight heparin, for the initial treatment of acute deep vein thrombosis. Thromb Haemost 76: 195–9. [PubMed] [Google Scholar]
- 33. Harenberg J, Huck K, Bratsch H, Stehle G, Dempfle CE, et al. (1990) Therapeutic application of subcutaneous low-molecular-weight heparin in acute venous thrombosis. Haemostasis 20 Suppl 1205–19. [DOI] [PubMed] [Google Scholar]
- 34. Luomanmaki K, Grankvist S, Hallert C, J Jauro I, Ketola K, et al. (1996) A multicentre comparison of once-daily subcutaneous dalteparin (low molecular weight heparin) and continuous intravenous heparin in the treatment of deep vein thrombosis. J Intern Med 240: 85–92. [DOI] [PubMed] [Google Scholar]
- 35. Koopman MM, Prandoni P, Piovella F, Ockelford PA, Brandies DP, et al. (1996) Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 334: 682–7. [DOI] [PubMed] [Google Scholar]
- 36. Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, et al. (1997) A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med 337: 447–52. [DOI] [PubMed] [Google Scholar]
- 37. Ferguson JJ, Califf RM, Antman EM, Cohen M Grines CL, et al. (2004) Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 292: 45–54. [DOI] [PubMed] [Google Scholar]
- 38. Kirchmaier CM, Wolf H, Schafer H, Ehlers B, Breddin HK (1998) Efficacy of a low molecular weight heparin administered intravenously or subcutaneously in comparison with intravenous unfractionated heparin in the treatment of deep venous thrombosis. Certoparin-Study Group. Int Angiol 17: 135–45. [PubMed] [Google Scholar]
- 39. Belcaro G, Nicolaides AN, Cesarone MR, De Sanctis MT, Laurora G, et al. (1999) Comparison of low-molecular-weight heparin, administered primarily at home, with unfractionated heparin, administered in hospital, and subcutaneous heparin, administered at home for deep-vein thrombosis. Angiology 50: 781–7. [DOI] [PubMed] [Google Scholar]
- 40. Malhotra S, Bhargava VK, Grover A, Pandhi P, Sharma YP (2001) A randomized trial to compare the efficacy, safety, cost and platelet aggregation effects of enoxaparin and unfractionated heparin (the ESCAPEU trial). Int J Clin Pharmacol Ther 39: 110–5. [DOI] [PubMed] [Google Scholar]
- 41. Moreno-Palomares JJ, Fisac-Herrero RM, Herrero-Domingo A, Ferreira-Pasos EM, Grasa J, et al. (2001) [Low molecular weight heparin versus unfractionated heparin in the treatment of deep vein thrombosis]. An Med Interna 18: 364–8. [PubMed] [Google Scholar]
- 42. Simonneau G, Charbonnier B, Decousus H, Planchon B, Ninet J, et al. (1993) Subcutaneous low-molecular-weight heparin compared with continuous intravenous unfractionated heparin in the treatment of proximal deep vein thrombosis. Arch Intern Med 153: 1541–6. [PubMed] [Google Scholar]
- 43. Meyer G, Brenot F, Pacouret G, Simonneau G Gillet Juvin K, et al. (1995) Subcutaneous low-molecular-weight heparin fragmin versus intravenous unfractionated heparin in the treatment of acute non massive pulmonary embolism: an open randomized pilot study. Thromb Haemost 74: 1432–5. [PubMed] [Google Scholar]
- 44. Findik S, Erkan ML, Selcuk MB, Albayrak S, Atici AG, et al. (2002) Low-molecular-weight heparin versus unfractionated heparin in the treatment of patients with acute pulmonary thromboembolism. Respiration 69: 440–4. [DOI] [PubMed] [Google Scholar]
- 45. Lindmarker P, Holmstrom M, Granqvist S, Johnsson H, Lockner D (1994) Comparison of once-daily subcutaneous Fragmin with continuous intravenous unfractionated heparin in the treatment of deep vein thrombosis. Thromb Haemost 72: 186–90. [PubMed] [Google Scholar]
- 46. Stricker H, Marchetti O, Haeberli A, Mombelli G (1999) Hemostatic activation under anticoagulant treatment: a comparison of unfractionated heparin vs. nadroparin in the treatment of proximal deep vein thrombosis. Thromb Haemost 82: 1227–31. [PubMed] [Google Scholar]
- 47. Aiach M (1989) Treatment of deep venous thrombosis. Comparative study of a low molecular weight heparin fragment (Fragmin) by the subcutaneous route and standard heparin by the continuous intravenous route. A multicenter study. 10: 375–381. [PubMed] [Google Scholar]
- 48. Antman EM, Morrow DA, McCabe CH, Jiang F, White HD, et al. (2005) Enoxaparin versus unfractionated heparin as antithrombin therapy in patients receiving fibrinolysis for ST-elevation myocardial infarction. Design and rationale for the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment-Thrombolysis In Myocardial Infarction study 25 (ExTRACT-TIMI 25). Am Heart J 149: 217–26. [DOI] [PubMed] [Google Scholar]
- 49. Correia LC, Neubauer C, Azevedo A (1995) O papel da heparina de baixo peso molecular na angina instavel, infarto agudo do miocardio e pos angioplastia percutanea transluminal coronaria eletiva. Arq Bras Cardiol 65: 475–78. [PubMed] [Google Scholar]
- 50. Montalescot G, Bal-dit-Solier C, Chibedi D, Collet JP, Soulat T, et al. (2003) Comparison of effects on markers of blood cell activation of Enoxeparin, Dalteparin, and Unfractionated Heparin in patients with unstable angina pectoris or non-ST-segment elevation acute myocardial infarction (the ARMADA study). Am J Cardiol 91: 925–930. [DOI] [PubMed] [Google Scholar]
- 51. Thery C, Simmoneau G, Meyer G, Helenon O, Bridey F, et al. (1992) Randomized trial of subcutaneous low-molecular-weight heparin CY 216 (Fraxiparine) compared with intravenous unfractionated heparin in the curative treatment of submassive pulmonary embolism. A dose ranging study. Circulation 85: 1380–1389. [DOI] [PubMed] [Google Scholar]
- 52. Merli G, Spiro T, Olsson CG, Abilgaard U, Davidson BL, et al. (2001) Subcutaneous Enoxeparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med 134: 191–202. [DOI] [PubMed] [Google Scholar]
- 53. Lim W, Dentali F, Eikelboom JW, Crowther MA (2006) Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med 144: 673–84. [DOI] [PubMed] [Google Scholar]
- 54.Magee KD, Sevcik W, Moher D, Rowe BH (2003) Low molecular weight heparins versus unfractionated heparin for acute coronary syndromes. Cochrane Database Syst Rev CD002132. [DOI] [PubMed]
- 55. Murphy SA, Gibson CM, Morrow DA, Van de Werf F, Menown IB, et al. (2007) Efficacy and safety of the low-molecular weight heparin enoxaparin compared with unfractionated heparin across the acute coronary syndrome spectrum: a meta-analysis. Eur Heart J 28: 2077–86. [DOI] [PubMed] [Google Scholar]
- 56. Silvain J, BeyguiF, Barthelemy O, Pollack C, Cohen M, et al. (2012) Efficacy and sefatey of enoxeparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis.BMJ 2012. 344: e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loke YK, Price D, Herxheimer A (2007) Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DeEugenio D, Kolman L, DeCaro M, Andrel J, Chervoneva I, et al. (2007) Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 27: 691–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis: number of daily LMWH administrations (once daily vs twice daily).
(DOC)
Subgroup analysis: number of daily LMWH administrations (once daily vs twice daily) in VTE and ACS studies.
(DOC)
Subgroup analysis, after the exclusion of studies in which LMWH was was under-dosed (less than 75% of the recommended daily dose) or overdosed (more than 125% of the recommended dose).
(DOC)
Subgroup analysis by type of LMWH.
(DOC)
Subgroup analysis, after exclusion of low quality studies (Jadad Score<3).
(DOC)
Overall meta-analysis, including dose-finding studies (studies grouped by VTE or ACS patients).
(DOC)