Abstract
Background
Prospective cohort studies in relation to the associations between n-3 polyunsaturated fatty acids (PUFA) and risk of type 2 diabetes (T2D) were inconsistent. Differences in tissue n-3 PUFA compositions in subjects with and without T2D were also inconsistent in both cohort and case-control studies. We conducted a systematic review and meta-analysis of prospective cohort studies to examine the associations of fish and n-3 PUFA intake with T2D risk. The differences in tissue n-3 PUFA compositions in subjects with and without T2D were investigated based on cohort and case-control studies.
Methods and Findings
PubMed, Embase, Cochrane library, China National Knowledge Infrastructure (CNKI) and Chinese VIP database up to January 2012 was used to identify relevant studies, and reference lists from retrieved studies were reviewed. Two authors independently extracted the data. Random-effects models were used to pool the summary relative risk (RR). Twenty-four studies including 24,509 T2D patients and 545,275 participants were identified. For cohort studies, the summary RR of T2D for the highest vs lowest categories of total fish, marine n-3 PUFA and alpha-linolenic acid intake was 1.07 (95% CI: 0.91, 1.25), 1.07 (95% CI: 0.95, 1.20) and 0.93 (95% CI: 0.81, 1.07), respectively. Subgroup analyses indicated that summary RR (highest vs lowest category) of T2D for fish and marine n-3 PUFA intake was 0.89 (95% CI: 0.81, 0.98) and 0.87 (95% CI: 0.79, 0.96) for Asian populations, and 1.20 (95% CI: 1.01, 1.44) and 1.16 (95% CI: 1.04, 1.28) for Western populations. Asian subjects with T2D had significantly lower tissue compositions of C22∶6n-3 (SMD: −1.43; 95% CI: −1.75, −1.12) and total n-3 PUFA (SMD: −1.41; 95% CI: −2.23, −0.59) compared with those without T2D.
Conclusion
This systematic review and meta-analysis provides evidence that marine n-3 PUFA have beneficial effects on the prevention of T2D in Asian populations.
Introduction
Type 2 diabetes (T2D) is one of the most common chronic diseases in the world, leading to a huge economic burden for society [1]. Dietary factors were postulated to play an important role in the prevention of T2D [2], [3]. N-3 polyunsaturated fatty acids (PUFA), especially marine n-3 PUFA (eicosapentaenoic acid (C20∶5n-3, EPA) and docosahexaenoic acid (C22∶6n-3, DHA)) intake, had been demonstrated to improve insulin sensitivity in animal models [4]. However, observational studies in relation to the association of n-3 PUFA intake with risk of T2D were inconsistent [3], [5]–[11]. In addition, fish, rich in marine n-3 PUFA, also showed inconsistent associations with risk of T2D in observational studies [2], [6]–[10], [12]–[15]. An ecological study of 41 countries revealed that fish and seafood intake might reduce the risk of T2D in populations with a high prevalence of obesity [12]. Fish consumption was also associated with lower risk of glucose intolerance from the Seven Countries Study [2] and an elderly population [13]. But prospective cohort studies had reported inverse [10], [14], [15], positive [6], [9], or null associations [7], [8] between fish intake and risk of T2D.
Tissue (plasma/serum/erythrocytes) n-3 PUFA compositions were reported to be significantly lower in subjects with T2D compared with control subjects in case-control studies [16], [17]. Nevertheless, many other case-control or cohort studies showed inconsistent results [5], [18]–[22].
Therefore, the aim of the present meta-analysis was to investigate the associations of fish and n-3 PUFA intake with risk of T2D based on prospective cohort studies. The differences of tissue (plasma/serum/erythrocytes) n-3 PUFA compositions in subjects with and without T2D were also investigated based on prospective cohort and case-control studies. Stratified analyses were conducted to examine sources of heterogeneity.
Methods
Search Strategy
Our report followed the Meta-analysis of Observational Studies in Epidemiology Guidelines [23]. Results were reported according to PRISMA guidelines (http://www.prisma-statement.org; Text S1). Our protocol was available in Text S2. PubMed, Embase, Cochrane library, China National Knowledge Infrastructure (CNKI) and Chinese VIP database up to January 2012 was searched. Following Medical Subject Headings terms or key words were searched: “fish” OR “fish oils” OR “seafood” OR “fatty acids” OR “docosahexaenoic acid” OR “eicosapentaenoic acid” OR “alpha-linolenic acid” AND “diabetes mellitus, type 2”. The search was restricted to human studies and had no language restriction. References from the retrieved articles were reviewed to identify potential bibliographies. Authors were not contacted for detailed information of primary studies.
Selection Criteria
Two authors (JZ and TH) independently conducted the search and discrepancies were resolved through group discussion. To investigate the associations of fish and n-3 PUFA intake with risk of T2D, the inclusion criteria were: 1) prospective cohort study design; 2) the exposure of interest was dietary intake of fish, fatty fish, shellfish, n-3 PUFA, marine n-3 PUFA or alpha-linolenic acid (C18∶3n-3, ALA); 3) the endpoint of interest was T2D incidence; 4) relative risk (RR) or hazard ratio (HR) with the corresponding 95% confidence interval (CI) of T2D for each category of fish or n-3 PUFA intake were provided; and 5) if the same population or cohort was duplicated, the most recent and complete study was included. To investigate the differences in tissue (plasma/serum/erythrocytes) n-3 PUFA compositions in subjects with and without T2D, the inclusion criteria were: 1) prospective cohort or case-control study design; 2) tissue compositions of C22∶6n-3, C20∶5n-3, C18∶3n-3 or total n-3 PUFA in subjects with and without T2D were provided; 3) both the cases and controls in each study were from the same population. The exclusion criteria were: studies with cross-sectional, ecologic or intervention study design; duplicate studies; studies without detailed risk estimates of T2D for dietary fish or n-3 PUFA intake, or without tissue n-3 PUFA compositions in T2D subjects and controls.
Data Extraction
The following information was extracted from the included studies: first author’s name, year of publication, study region and population, duration of follow-up, age of subjects, gender, number of events, participants and person-years for the entire study and for each fish or n-3 PUFA intake category, adjusted covariates, method of dietary assessment, RR or HR with their 95% CIs for each fish or n-3 PUFA intake category, tissue compositions of C22∶6n-3, C20∶5n-3, C18∶3n-3 and total n-3 PUFA in subjects with and without T2D. The greatest degree of adjusted RR or HR from each cohort study was extracted.
Statistical Analysis
RR was taken as the common risk estimate for the associations between fish and n-3 PUFA intake and T2D risk. HR was considered as RR directly. RR from each study was firstly transformed to their natural logarithm and corresponding 95% CIs were used to calculate standard errors. DerSimonian and Laird random-effects model, which took both within- and between-study variation into consideration was used to combine the RRs and they were weighted by the inverse of their variances. The differences in tissue n-3 PUFA compositions in subjects with and without T2D were also analyzed as standardized mean difference (SMD) by pooling the data from case-control and cohort studies.
To further examine the relationship between fish intake and T2D risk, total fish consumption was standardized and categorized into four groups: 1) high (>5 servings/wk); 2) moderate (2–4 servings/wk); 3) low (1 serving/wk); and 4) reference group (<1 serving/wk or 1–3 servings/wk), if both <1 serving/wk and 1–3 servings/wk were available in a study, <1 serving/wk was chosen as reference group. Each RR from included studies was assigned into a corresponding standardized group. If more than one fish intake category fell into the same standardized group, the RRs were combined for further analysis. One portion or serving of fish was regarded as 105 g as described previously [24].
Dose-response analyses for total fish and marine n-3 PUFA intake were conducted according to Greenland and Longnecher [25] and Orsini et al [26], using a method described previously [24]. One study [14] which only reported two fish intake categories and two studies [5], [27] which did not report fish intake dose for each category were excluded from this analysis.
Statistical heterogeneity was assessed with the Q (significant at P<0.1) and I2 statistics [28]. I2 values of 25%, 50% and 75% correspond to cut-off points for low, moderate and high degrees of heterogeneity. Subgroup analyses were conducted to examine the sources of heterogeneity. Influence of individual study on the overall risk estimate or effect size was examined by sensitivity analysis in which one study at a time was excluded. Begg’s funnel plot and Egger’s regression test (significant at P<0.1) were used to evaluate publication bias. All the analyses were performed by using STATA version 11 (StataCorp LP, College Station, TX, USA).
Results
Literature Search
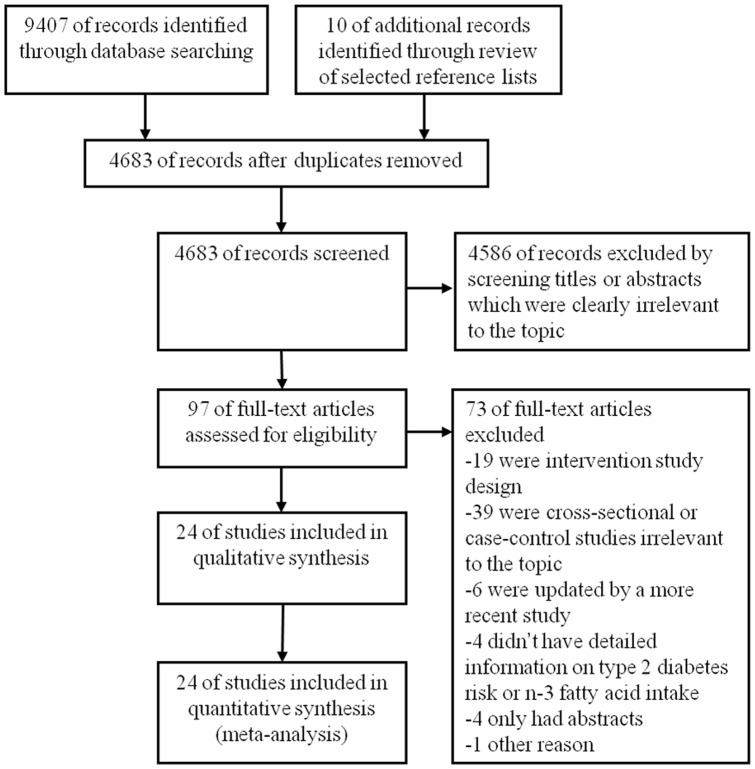
Twenty-four published studies [3], [5]–[11], [14]–[22], [27], [29]–[34] including 24,509 T2D patients and 545,275 participants were identified from a full-text examination of 97 potentially relevant studies (Figure 1), 1 study from Australia [5], 1 study from Cuba [19], 7 studies from Europe [7], [14], [17], [21], [22], [27], [33], 8 studies from Asia [10], [11], [15], [16], [30]–[32], [34], and 7 studies from the US [3], [6], [8], [9], [18], [20], [29]. Among the included studies, 10 studies [3], [5]–[11], [27], [29] reported the association between n-3 PUFA and T2D risk, 7 studies [6]–[10], [14], [15] reported the association between fish intake and T2D risk, and 5 studies [6]–[10] reported both the associations of n-3 PUFA and fish intake with T2D risk. Six studies [5], [8], [9], [11], [27], [29] assessed the association between C18∶3n-3 intake and risk of T2D. Thirteen studies [5], [16]–[22], [30]–[34] reported tissue n-3 PUFA compositions in subjects with and without T2D.
Figure 1. PRISMA flow diagram for selection of studies in the meta-analysis.
Study Characteristics
A total of 23,226 T2D cases among 515,537 subjects were included in the meta-analysis of dietary n-3 PUFA and fish intake and T2D risk, with an average 9.9 years of follow-up (Table 1). For the analysis of different tissue n-3 PUFA compositions between subjects with and without T2D, 1629 T2D cases and 8727 controls were involved (Table 2). For one study [15], the RR for total fish intake was not available, RR for oily fish intake was taken as total fish intake directly. Two studies [10], [15] reported men and women separately, and each study was taken as 2 independent cohort studies of men and women. In another study [5], the relationship between C22∶6n-3 and C20∶5n-3 to T2D risk were reported separately, RRs from C22∶6n-3 and C20∶5n-3 were pooled for further analysis. Fatty acid compositions of plasma or serum PL were used for analysis if multiple tissue n-3 PUFA compositions were reported.
Table 1. Characteristics of included prospective cohort studies in the meta-analysis of dietary fish and n-3 polyunsaturated fatty acid intake and type 2 diabetes.
| Study source | Duration of follow-up (years) | Age (y) | No. of cases/size of cohort | Fish or n-3 PUFA type | Exposure range (g/d) | Adjusted RR (95% CI) | Adjusted variables |
| Meyer et al, 2001 (3) | 11 | 55–69 | 1890/35988 | LC n-3 PUFA | Highest: 0.39; ref: 0.03 | 1.11 (0.94, 1.30) | Age, total energy, WHR, BMI, physical activity, cigarette smoking, alcohol consumption, education, marital status, residential area, hormone replacement therapy, energy-adjusted dietary magnesium and cereal fiber, dietary protein, saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids and cholesterol. |
| van Dam et al, 2002 (29) | 12 | 40–75 | 1321/42504 | C18∶3n-3 | Highest: 0.671; ref: 0.321 | 0.93 (0.78, 1.11) | Age, total energy intake, time period, physical activity, cigarette smoking, alcohol consumption, hypercholesterolemia, hypertension, family history of type 2 diabetes, intake of cereal fiber and magnesium and BMI |
| Hodge et al, 2007 (5) | 4 | 36–72 | 364/3737 | C22∶6n-3 | Highest: Q5; ref: Q1 | 0.77 (0.52, 1.16) | Age, sex, country of birth, family history of diabetes, physical activity, alcohol intake, BMI, and waist-hip ratio |
| C20∶5n-3 | Highest: Q5; ref: Q1 | 0.68 (0.62, 1.34) | |||||
| C18∶3n-3 | Highest: Q5; ref: Q1 | 1.14 (0.75, 1.73) | |||||
| Kaushik et al_Nurses’ Health Study, 2009 (6) | 18 | 30–55 | 4159/61031 | LC n-3 PUFA | Highest: 0.49; ref: 0.06 | 1.23 (1.11, 1.37) | Smoking, alcohol consumption, physical activity, family history of diabetes mellitus, BMI, intakes of saturated fat, trans fats, linolenic acid, linoleic acid, caffeine, cereal fiber, glycemic index, calories, menopausal status, and postmenopausal hormone use |
| Fish | Highest: ≥5 times/wk; ref: <1 time/mo | 1.29 (1.05, 1.57) | |||||
| Kaushik et al_Nurses’ Health Study 2, 2009 (6) | 14 | 26–46 | 2728/91669 | LC n-3 PUFA | Highest: 0.36; ref: 0.06 | 1.25 (1.10, 1.42) | Smoking, alcohol consumption, physical activity, family history of diabetes mellitus, BMI, intakes of saturated fat, trans fats, linolenic acid, linoleic acid, caffeine, cereal fiber, glycemic index, calories, use of hormone replacement therapy and oral contraceptive use |
| Fish | Highest: ≥5 times/wk; ref: <1 time/mo | 1.32 (0.99, 1.74) | |||||
| Kaushik et al_Health Professionals Follow-up Study, 2009 (6) | 18 | 39–78 | 2493/42504 | LC n-3 PUFA | Highest: 0.62; ref: 0.09 | 1.12 (0.98, 1.28) | Smoking, alcohol consumption, physical activity, family history of diabetes mellitus, BMI, intakes of saturated fat, trans fats, linolenic acid, linoleic acid, caffeine, cereal fiber, glycemic index, and calories |
| Fish | Highest: ≥5 times/wk; ref: <1 time/mo | 1.16 (0.96, 1.41) | |||||
| van Woudenbergh et al, 2009 (7) | 15 | ≥55 | 463/4472 | C22∶6n-3+ C20∶5n-3 | Highest: 0.2368; ref: 0.0238 | 1.05 (0.80, 1.38) | Age, sex, smoking, education level, intake of energy, alcohol, trans fatty acid, fiber, intake of selenium, vitamin D and cholesterol |
| Total fish | Highest: 35.6; ref: 0 | 1.32 (1.02, 1.70) | Age sex, smoking, education level, intake of energy, alcohol, trans fatty acid, and fiber | ||||
| Fatty fish | Highest: ≥15.7; ref: 0 | 0.99 (0.71, 1.38) | Age sex, smoking, education level, intake of energy, alcohol, trans fatty acid, fiber and lean fish | ||||
| Lean fish | Highest: 30.6; ref: 0 | 1.30 (1.01, 1.68) | Age sex, smoking, education level, intake of energy, alcohol, trans fatty acid, fiber and fatty fish | ||||
| Patel et al, 2009 (14) | 10.2 | 40–79 | 725/21984 | Total fish | Highest: ≥1 portion/wk; ref: <1 portion/wk | 0.75 (0.58, 0.96) | Age, sex, family history of diabetes, smoking, education level, physical activity, total energy intake, alcohol intake, plasma vitamin C, BMI, and waist circumference |
| Oily fish | Highest: ≥1 portion/wk; ref: <1 portion/wk | 0.94 (0.78, 1.13) | |||||
| White fish | Highest: ≥1 portion/wk; ref: <1 portion/wk | 0.87 (0.73, 1.03) | |||||
| Shellfish | Highest: ≥1 portion/wk; ref: <1 portion/wk | 1.36 (1.02, 1.81) | |||||
| Djousse et al_Women’s Health Study, 2011 (9) | 12.4 | ≥45 | 2370/36328 | Marine n-3 PUFA | Highest: 0.43; ref: 0.07 | 1.44 (1.25, 1.65) | Age, BMI, parental history of diabetes, smoking, exercise, alcohol intake, menopausal status, red-meat intake, and quintiles of energy intake, linoleic acid, α-linolenic acid, dietary magnesium, trans fat, saturated fat, cereal fiber, and glycemic index |
| C18∶3n-3 | Highest: 1.59; ref: 0.79 | 1.01 (0.85, 1.21) | |||||
| Fish | Highest: 3.93 servings/wk; ref: 0.47 servings/wk | 1.49 (1.30, 1.70) | |||||
| Djousse et al_Cardiovascular Health Study, 2011 (8) | 10.6 | ≥65 | 204/3088 | C22∶6n-3+ C20∶5n-3 | Highest: >0.56; ref: ≤0.17 | 1.04 (0.67, 1.60) | Age, race, sex, clinic site, BMI, alcohol consumption, physical activity, current smoking, LDL cholesterol, and linoleic acid |
| C18∶3n-3 | Highest: >0.18; ref: ≤0.11 | 0.50 (0.24, 1.05) | |||||
| Fish | Highest: ≥5/wk; ref: <1/mo | 1.07 (0.35, 3.30) | Age, race, sex, clinic site, BMI, alcohol consumption, physical activity, current smoking, total energy intake and LDL cholesterol | ||||
| Nanri et al_men, 2011 (15) | 5 | 45–75 | 572/22921 | Oily fish | Highest: 71.2; ref: 10.7 | 0.79 (0.59, 1.05) | Age, study area, BMI, smoking status, alcohol consumption, family history of diabetes mellitus, total physical activity, history of hypertension, total energy intake, coffee consumption, intake of calcium, magnesium, dietary fiber, vegetable, fruit, meat, and rice |
| Lean fish | Highest: 30; ref: 3.3 | 1.05 (0.80, 1.38) | |||||
| Nanri et al_women, 2011 (15) | 5 | 45–75 | 399/29759 | Oily fish | Highest: 68.1; ref: 10.7 | 0.93 (0.67, 1.29) | The same as above |
| Lean fish | Highest: 23.3; ref: 2.7 | 1.02 (0.75, 1.40) | |||||
| Villegas et al_men, 2011 (10) | 4.1 | 40–74 | 900/51963 | LC n-3 PUFA | Highest: 0.2; ref: 0.02 | 0.89 (0.70, 1.12) | Age, energy intake, waist-to-hip ratio, BMI, smoking, alcohol consumption, physical activity, income level, educational level, occupation, family history of diabetes, hypertension and dietary pattern |
| Fish | Highest: 79; ref: 9.7 | 0.94 (0.74, 1.17) | |||||
| shellfish | Highest: 24.3; ref: 1.6 | 0.82 (0.65, 1.02) | |||||
| Villegas et al_women, 2011 (10) | 8.9 | 40–70 | 3034/64193 | LC n-3 PUFA | Highest: 0.2; ref: 0.02 | 0.84 (0.74, 0.95) | The same as above |
| Fish | Highest: 80.2; ref: 9.5 | 0.89 (0.78, 1.01) | |||||
| shellfish | Highest: 23.5; ref: 1.4 | 0.86 (0.76, 0.99) | |||||
| Brostow et al, 2011 (11) | 5.7 | 45–74 | 2252/43176 | C22∶6n-3+ C20∶5n-3 | Highest: 0.6; ref: 0.11 | 0.93 (0.77, 1.11) | Age, sex, dialect, year of interview, educational level, BMI, physical activity, smoking status, alcohol use, hypertension, intakes of omega-6, alternate omega-3, monounsaturated fat, saturated fat, dietary fiber, protein, and total energy |
| C18∶3n-3 | Highest: 1.06; ref: 0.27 | 0.79 (0.67, 0.93) | |||||
| Kroger et al, 2011 (27) | 7 | 35–65 | 673/2724 | LC n-3 PUFA | Highest: 0.59; ref: 0.04 (% of total fat intake) | 1.29 (0.95, 1.75) | Age, sex, BMI, waist circumference, cycling, sports activity, education, smoking status, alcohol intake, occupational activity, coffee intake, fiber intake, total fat intake and energy intake |
| C18∶3n-3 | Highest: 2.6; ref: 1.4 (% of total fat intake) | 1.13 (0.80, 1.59) |
Abbreviations: Q: quintile; ref: reference; LC n-3 PUFA: long-chain n-3 polyunsaturated fatty acids (C22∶6n–3+ C20∶5n-3).
Table 2. Characteristics of included studies in the meta-analysis of different n-3 polyunsaturated fatty acids compositions between subjects with and without type 2 diabetes.
| Study source | Mean age(years) | Study design | No. of cases | No. of controls | Tissue n-3 PUFAs composition (% of totalfatty acids, case vs controls) |
| Faas et al, 1988 (20) | 48.5 | Case-control | 5 | 5 | Plasma C22∶6n-3 (1.6±0.7 vs 1.5±0.5) |
| Red blood cell C22∶6n-3 (4.4±1.1 vs 4.4±1.1) | |||||
| Bohov et al, 1993 (21) | 60.6 | Case-control | 183 | 114 | Serum C22∶6n-3 (1.96±0.05 vs 1.67±0.06) |
| Serum C20∶5n-3 (0.66±0.03 vs 0.62±0.03) | |||||
| Serum C18∶3n-3 (0.42±0.01 vs 0.55±0.01) | |||||
| Serum n-3 PUFA (3.53±0.08 vs 3.31±0.09) | |||||
| Pelikanova et al, 2001 (22) | 40.4 | Case-control | 21 | 24 | Serum PL C22∶6n-3 (3.04±0.87 vs 2.11±0.49) |
| Serum PL C20∶5n-3 (1.04±0.54 vs 0.82±0.41) | |||||
| Serum PL C18∶3n-3 (0.20±0.19 vs 0.29±0.14) | |||||
| Serum PL n-3 PUFA (5.40±1.75 vs 4.67±1.39) | |||||
| Rodriguez et al, 2004 (19) | 53 | Case-control | 13 | 13 | Plasma PL C22∶6n-3 (4.01±0.80 vs 3.47±0.89) |
| Plasma PL C20∶5n-3 (0.64±0.23 vs 0.74±0.35) | |||||
| Plasma PL C18∶3n-3 (0.18±0.13 vs 0.20±0.13) | |||||
| Plasma PL n-3 PUFA (5.96±0.73 vs 5.49±1.17) | |||||
| Red blood cell PL C22∶6n-3 (3.9±1.35 vs 3.91±0.71) | |||||
| Red blood cell PL C20∶5n-3 (0.52±0.23 vs 0.44±0.12) | |||||
| Red blood cell PL C18∶3n-3 (0.15±0.04 vs 0.15±0.06) | |||||
| Red blood cell PL n-3 PUFA (7.18±1.71 vs 5.94±1.15) | |||||
| Bakan et al, 2006 (34) | 56.5 | Case-control | 32 | 20 | Plasma C22∶6n-3 (1.9±0.9 vs 2.7±0.4) |
| Mao et al, 2007 (30) | 57.4 | Case-control | 62 | 53 | Serum PL C22∶6n-3 (5.1±1.3 vs 6.4±1.1) |
| Serum PL C20∶5n-3 (1.9±0.6 vs 1.9±0.5) | |||||
| Serum PL C18∶3n-3 (0.32±0.2 vs 0.33±0.13) | |||||
| Serum PL n-3 PUFA (8.2±1.6 vs 9.6±1.5) | |||||
| Krachler et al, 2008 (17) | 51.6 | Case-control | 159 | 291 | Erythrocyte membrane C22∶6n-3 (4.61±1.01 vs 4.83±1.02) |
| Erythrocyte membrane C20∶5n-3 (1.31±0.45 vs 1.37±0.46) | |||||
| Erythrocyte membrane C18∶3n-3 (0.35±0.10 vs 0.36±0.13) | |||||
| Lou et al, 2010 (31) | 56.1 | Case-control | 60 | 55 | Serum PL C22∶6n-3 (4.11±1.32 vs 6.41±1.26) |
| Serum PL C20∶5n-3 (1.80±0.55 vs 1.90±0.52) | |||||
| Serum PL C18∶3n-3 (0.28±0.08 vs 0.33±0.10) | |||||
| Serum PL n-3 PUFA (6.08±1.66 vs 9.54±1.54) | |||||
| Huang et al, 2010 (16) | 60 | Case-control | 180 | 186 | Plasma PL C22∶6n-3 (2.46±2.2 vs 5.8±2.0) |
| Plasma PL C20∶5n-3 (0.99±0.5 vs 2.12±0.7) | |||||
| Plasma PL C18∶3n-3 (0.36±0.1 vs 0.70±0.2) | |||||
| Plasma PL n-3 PUFA (4.52±2.8 vs 9.22±1.8) | |||||
| Zhang et al, 2011 (32) | 49.2 | Case-control | 241 | 156 | Plasma total n-3 PUFA (5.52±0.77 vs 6.47±2.27) |
| Vessby et al, 1994 (33) | 50 | Prospective cohort | 75 | 1753 | Serum CE C22∶6n-3 (0.68±0.21 vs 0.70±0.21) |
| Serum CE C20∶5n-3 (1.42±0.57 vs 1.35±0.63) | |||||
| Serum CE C18∶3n-3 (0.65±0.18 vs 0.66±0.16) | |||||
| Wang et al, 2003 (18) | 52 | Prospective cohort | 252 | 2657 | Plasma PL C22∶6n-3 (2.71±0.83 vs 2.76±0.84) |
| Plasma PL C20∶5n-3 (0.58±0.33 vs 0.56±0.31) | |||||
| Plasma PL C18∶3n-3 (0.13±0.05 vs 0.15±0.05) | |||||
| Plasma CE C22∶6n-3 (0.43±0.15 vs 0.43±0.15) | |||||
| Plasma CE C20∶5n-3 (0.59±0.36 vs 0.54±0.28) | |||||
| Plasma CE C18∶3n-3 (0.4±0.11 vs 0.42±0.11) | |||||
| Hodge et al, 2007 (5) | 56.2 | Prospective cohort | 346 | 3391 | Plasma PL C22∶6n-3 (4.15±0.99 vs 4.02±1.07) |
| Plasma PL C20∶5n-3 (1.15±0.50 vs 1.05±0.47) | |||||
| Plasma PL C18∶3n-3 (0.17±0.08 vs 0.17±0.08) | |||||
| Plasma PL n-3 PUFA (6.77±1.36 vs 6.55±1.40) |
Abbreviations: n-3 PUFAs: n-3 polyunsaturated fatty acids; PL: phospholipids; CE: cholesterol esters.
Fish Intake and Risk of T2D
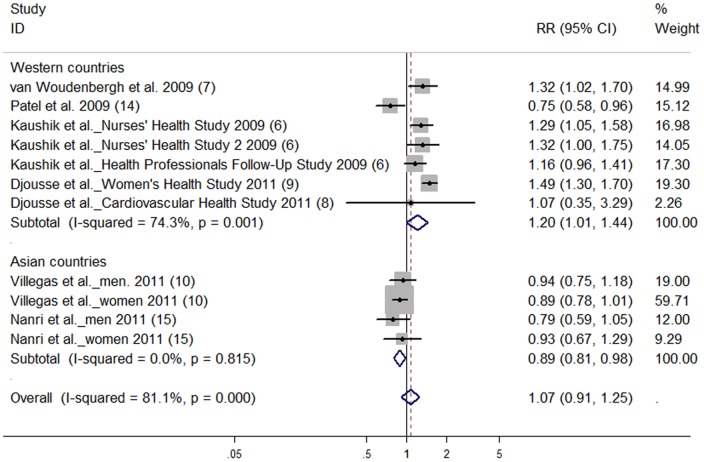
There was no significant association between total fish intake (highest vs lowest category) and risk of T2D (RR: 1.07; 95% CI: 0.91, 1.25) (Figure 2). High degree of heterogeneity was observed (P for heterogeneity <0.001, I2 = 81.1). No publication bias was observed from Begg’s funnel plot and Egger’s test (P = 0.60).
Figure 2. Relative risk of type 2 diabetes for highest vs lowest categories of total fish intake.
The combined relative risk was achieved using random-effects model. Grey square represents relative risk in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The diamond indicates summary risk estimate.
The summary RR of T2D was 1.03 (95% CI: 0.90, 1.19), 1.06 (95% CI: 0.90, 1.25), and 1.05 (95% CI: 0.97, 1.13) for high (>5 per wk), moderate (2–4 per wk), and low (1 per wk) fish intake respectively (Table 3). No dose-response association for total fish intake and risk of T2D was observed (RR: 1.02; 95% CI: 0.98, 1.06).
Table 3. Subgroup analyses for the associations of fish intake with risk of type 2 diabetes according to standardized fish intake categories (high, moderate and low).
| Fish intake classification | No. of cohorts | RR (95% CI) | P-heterogeneity | I 2 (%) |
| High fish intake (>5 per wk) | 8 | 1.03 (0.9, 1.19) | 0.011 | 61.6 |
| Regions | ||||
| Asian countries | 4 | 0.89 (0.81, 0.98) | 0.815 | 0 |
| Western countries | 4 | 1.24 (1.09, 1.40) | 0.834 | 0 |
| US | 4 | 1.24 (1.09, 1.40) | 0.834 | 0 |
| Follow-up duration | ||||
| >9.9 years | 4 | 1.24 (1.09, 1.40) | 0.834 | 0 |
| ≤9.9 years | 4 | 0.89 (0.81, 0.98) | 0.815 | 0 |
| Gender | ||||
| Men | 3 | 0.97 (0.78, 1.21) | 0.077 | 61.1 |
| Women | 4 | 1.08 (0.86, 1.36) | 0.005 | 77 |
| Both | 1 | 1.07 (0.35, 3.29) | ||
| Moderate fish intake (2–4 per wk) | 10 | 1.06 (0.90, 1.25) | <0.001 | 89 |
| Regions | ||||
| Asian countries | 4 | 0.83 (0.78, 0.89) | 0.934 | 0 |
| Western countries | 6 | 1.25 (1.12, 1.40) | 0.067 | 51.4 |
| Europe | 1 | 1.32 (1.02, 1.70) | ||
| US | 5 | 1.24 (1.09, 1.42) | 0.038 | 60.7 |
| Follow-up duration | ||||
| >9.9 years | 6 | 1.25 (1.12, 1.40) | 0.067 | 51.4 |
| ≤9.9 years | 4 | 0.83 (0.78, 0.89) | 0.934 | 0 |
| Gender | ||||
| Men | 3 | 0.94 (0.78, 1.13) | 0.038 | 69.4 |
| Women | 5 | 1.10 (0.84, 1.44) | <0.001 | 94.2 |
| Both | 2 | 1.28 (1.00, 1.64) | 0.434 | 0 |
| Low fish intake (1 per wk) | 10 | 1.05 (0.97, 1.13) | 0.016 | 55.8 |
| Regions | ||||
| Asian countries | 4 | 0.96 (0.88, 1.04) | 0.92 | 0 |
| Western countries | 6 | 1.11 (1.03, 1.20) | 0.121 | 42.6 |
| Europe | 1 | 1.19 (0.92, 1.54) | ||
| US | 5 | 1.10 (1.01, 1.20) | 0.073 | 53.2 |
| Follow-up duration | ||||
| >9.9 years | 6 | 1.11 (1.03, 1.20) | 0.121 | 42.6 |
| ≤9.9 years | 4 | 0.96 (0.88, 1.04) | 0.92 | 0 |
| Gender | ||||
| Men | 3 | 0.97 (0.88, 1.06) | 0.812 | 0 |
| Women | 5 | 1.08 (0.98, 1.20) | 0.01 | 69.8 |
| Both | 2 | 1.17 (0.92, 1.48) | 0.646 | 0 |
The summary risk estimates for the highest vs lowest categories of fatty fish, lean fish and shellfish intake were 0.91 (95% CI: 0.80, 1.04), 1.03 (95% CI: 0.86, 1.24) and 0.96 (95% CI: 0.75, 1.25) respectively. No publication bias was observed for all the analyses of fatty fish, lean fish and shellfish intake (data not shown).
N-3 PUFA Intake and Risk of T2D
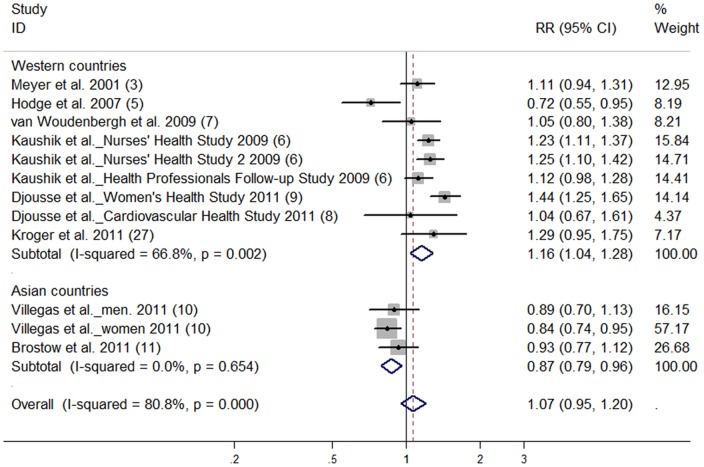
There was no significant association between marine n-3 PUFA intake and risk of T2D (highest vs lowest category; RR: 1.07; 95% CI: 0.95, 1.20) (Figure 3). High degree of heterogeneity was found (P for heterogeneity <0.001, I2 = 80.8). Overall, no publication bias was observed from Begg’s funnel plot and Egger’s test (P = 0.338). No significant dose-response association between marine n-3 PUFA and risk of T2D was found (RR: 1.01; 95% CI: 0.98, 1.05).
Figure 3. Relative risk of type 2 diabetes for highest vs lowest categories of marine n-3 polyunsaturated fatty acids intake.
The combined relative risk was achieved using random-effects model. Grey square represents relative risk in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The diamond indicates summary risk estimate.
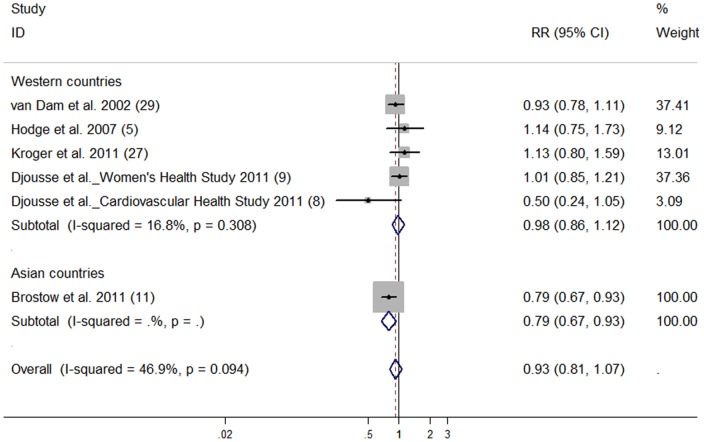
The pooled RR of T2D was 0.93 (95% CI: 0.81, 1.07) for the highest vs lowest C18∶3n-3 intake (Figure 4). No publication bias was observed (data not shown).
Figure 4. Relative risk of type 2 diabetes for highest vs lowest categories of alpha-linolenic acid intake.
The combined relative risk was achieved using random-effects model. Grey square represents relative risk in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The diamond indicates summary risk estimate.
Differences in Tissue n-3 PUFA Compositions between Subjects with and without T2D
Subjects with T2D had significantly lower tissue compositions of C18∶3n-3 (SMD: −0.48; 95% CI: -0.86, −0.11) compared with subjects without T2D. No publication bias was observed (data not shown). No significant differences were observed for the other n-3 PUFA compositions (Table 4).
Table 4. Subgroup analyses on different tissue n-3 polyunsaturated fatty acid compositions in subjects with and without type 2 diabetes.
| N-3 PUFAs | No. of studies | SMD (95% CI) | P-heterogeneity | I 2 (%) |
| C22∶6n-3 | 12 | −0.31 (−0.70, 0.07) | <0.001 | 96.4 |
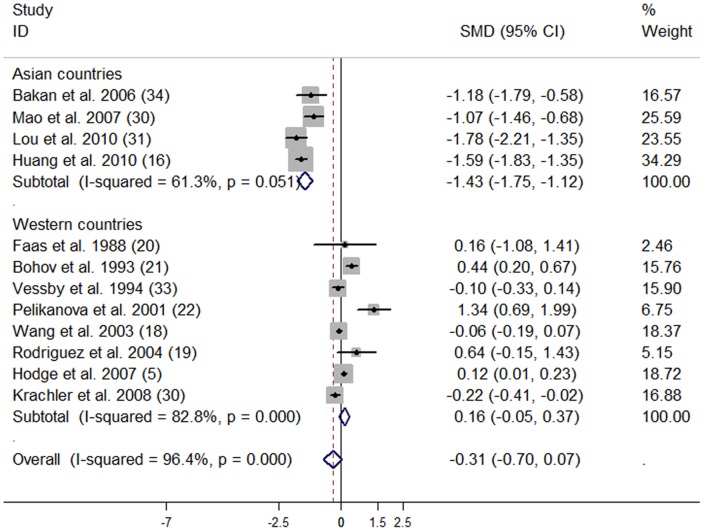
| Asian countries | 4 | −1.43 (−1.75, −1.12) | 0.051 | 61.3 |
| Western countries | 8 | 0.16 (−0.05, 0.37) | <0.001 | 82.8 |
| C20∶5n-3 | 10 | −0.16 (−0.53, 0.21) | <0.001 | 96.3 |
| Asian countries | 4 | −0.69 (−1.96, 0.59) | <0.001 | 97.9 |
| Western countries | 6 | 0.09 (−0.03, 0.20) | 0.055 | 51.3 |
| C18∶3n-3 | 10 | −0.48 (−0.86, −0.11) | <0.001 | 96.5 |
| Asian countries | 3 | −0.92 (−2.27, 0.42) | <0.001 | 98.0 |
| Western countries | 7 | −0.27 (−0.50, −0.05) | <0.001 | 87.2 |
| Total n-3 PUFA | 8 | −0.56 (−1.22, 0.09) | <0.001 | 98.0 |
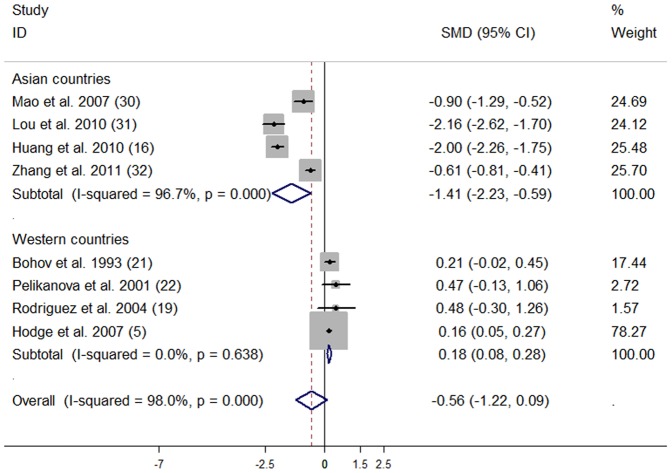
| Asian countries | 4 | −1.41 (−2.23, −0.59) | <0.001 | 96.7 |
| Western countries | 4 | 0.18 (0.08, 0.28) | 0.638 | 0 |
Abbreviations: n-3 PUFAs: n-3 polyunsaturated fatty acids; SMD: standardized mean difference.
Sensitivity Analyses
Subgroup analyses (Table 5) revealed that summary RR of T2D for the highest vs lowest total fish intake categories was 0.89 (95% CI: 0.81, 0.98) for studies in Asian populations, and 1.20 (95% CI: 1.01, 1.44) for studies in Western populations. Exclusion of two case-cohort studies produced similar results (RR: 1.10; 95% CI: 0.98, 1.23) as the overall risk estimate. Exclusion of one study [15] in which oily fish intake was considered as total fish intake directly did not greatly change the pooled RR (1.12; 95% CI: 0.94, 1.33). Subgroup analyses (Table 3) for high, moderate and low fish consumption models produced similar results as those derived from the relationship between fish intake and T2D risk (highest vs lowest category). Subgroup analyses (Table 5) found that for marine n-3 PUFA intake, summary RR of T2D for studies conducted in the Western populations (1.16; 95% CI: 1.04, 1.28) was higher than that in Asian populations (0.87; 95% CI: 0.79, 0.96).
Table 5. Subgroup analyses on fish and marine n-3 polyunsaturated fatty acids intake and risk of type 2 diabetes.
| Fish intake and risk of T2D | Marine n-3 PUFA (C22∶6n-3+ C20∶5n-3) and risk of T2D | |||||||
| Group | No. of cohorts | RR (95% CI) | P-heterogeneity | I 2 | No. of cohorts | RR (95% CI) | P-heterogeneity | I 2 (%) |
| All studies | 11 | 1.07 (0.91, 1.25) | <0.001 | 81.1 | 12 | 1.07 (0.95, 1.20) | <0.001 | 80.8 |
| Regions | ||||||||
| Asian countries | 4 | 0.89 (0.81, 0.98) | 0.815 | 0 | 3 | 0.87 (0.79, 0.96) | 0.654 | 0 |
| Western countries | 7 | 1.20 (1.01, 1.44) | 0.001 | 74.3 | 9 | 1.16 (1.04, 1.28) | 0.002 | 66.8 |
| Europe | 2 | 1.00 (0.57, 1.73) | 0.002 | 89.5 | 2 | 1.15 (0.94, 1.41) | 0.324 | 0 |
| US | 5 | 1.33 (1.20, 1.48) | 0.302 | 17.6 | 6 | 1.22 (1.13, 1.33) | 0.109 | 44.5 |
| Follow-up duration | ||||||||
| >9.9 years | 7 | 1.20 (1.01, 1.44) | 0.001 | 74.3 | 7 | 1.21 (1.12, 1.31) | 0.116 | 41.2 |
| ≤9.9 years | 4 | 0.89 (0.81, 0.98) | 0.815 | 0 | 5 | 0.90 (0.78, 1.04) | 0.061 | 55.5 |
| Gender | ||||||||
| Men | 3 | 0.97 (0.78, 1.21) | 0.077 | 61.1 | 2 | 1.02 (0.82, 1.27) | 0.096 | 64 |
| Women | 5 | 1.17 (0.91, 1.49) | <0.001 | 87.9 | 5 | 1.16 (0.97, 1.38) | <0.001 | 89.4 |
| Both | 3 | 1.00 (0.63, 1.61) | 0.008 | 79.1 | 5 | 0.97 (0.81, 1.17) | 0.075 | 53 |
Abbreviations: n-3 PUFA: n-3 polyunsaturated fatty acids.
Subgroup analyses (Table 4 ) showed that Asian subjects with T2D compared with those without T2D, tissue compositions of C22∶6n-3 (SMD: −1.43; 95% CI: −1.75, −1.12) and total n-3 PUFA (SMD: −1.41; 95% CI: −2.23, −0.59) were significantly lower. (Figure 5, Figure 6). In Western populations, tissue compositions of total n-3 PUFA (SMD: 0.18; 95% CI: 0.08, 0.28) were significantly higher in subjects with T2D compared with controls; but tissue C18∶3n-3 was significantly lower (SMD: −0.27; 95% CI: −0.50, −0.05) in subjects with T2D compared with controls.
Figure 5. Effect of type 2 diabetes on tissue C22∶6n-3 composition compared with controls.
The combined standardized mean difference (SMD) was achieved using random-effects model. Grey square represents SMD in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The diamond indicates summary SMD.
Figure 6. Effect of type 2 diabetes on tissue total n-3 PUFA composition compared with controls.
The combined standardized mean difference (SMD) was achieved using random-effects model. Grey square represents SMD in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The diamond indicates summary SMD.
Discussion
Overall, there were no significant associations between fish and n-3 PUFA intake and risk of T2D. However, subgroup analyses revealed that study regions remarkably affected the summary risk estimates and reduced the study heterogeneity. Marine n-3 PUFA and fish intake was inversely associated with risk of T2D only in Asian populations. Tissue C22∶6n-3 and total n-3 PUFA compositions were significantly lower in T2D subjects compared with controls in Asian but not Western populations.
One possible explanation was the influence of genetics on incidence of T2D as suggested by the high levels of differentiation between different populations for the ensemble of T2D loci [35] and the influences of gene-diet interaction [36], [37]. Klimentidis et al. [35] suggested that East Asians and sub-Saharan Africans experienced natural selection at loci associated with T2D, and there might be an evolutionary genetic basis for population differences in T2D. Compared with other common diseases, T2D risk alleles showed extreme directional differentiation across different populations, and the frequencies of T2D risk alleles showed a consistent decrease from Sub-Saharan African, through European, to East Asian populations. The predicted genetic risks of T2D also showed a significant difference across different populations, with lower risk in the Asian and higher risk in the African populations [38]. The disparities in T2D rates may reflect the adaptation of humans to the local environments along with human migration [39]. This is further supported by genome-wide association studies (GWAS)-identified genetic loci associated with T2D were substantially different between East Asian and European populations [40]. For example, genetic variants at some genes (ADAMTS9, BCL11A, DCD, CENTD2, CHCHD2P9, DUSP9, FTO, JAZF1, PPARG, etc.) associated with T2D only in the GWAS of European populations, while genetic variants at some others genes (ANK1, CMIP, CR2, GCC1, GLIS3, HUNK, etc.) associated with T2D only in the GWAS of East Asian populations [40]. This suggests that genetic backgrounds for the risk of T2D are different between different populations. Given different T2D loci or frequencies of risk alleles between East Asian and European populations, these two populations may respond differently to the same marine n-3 PUFA exposure in relation to the incidence of T2D. It is biologically plausible. Marine n-3 PUFA were known to affect gene expression through regulation of two group of transcription factors, including sterol regulatory element binding proteins and peroxisome proliferator-activated receptors (PPAR), both are crucial for the modulation of numerous gene expressions, including genes involved in the inflammation, lipid metabolism, energy utilization and insulin signaling [41], [42]. For example, genetic variants at PPARG associated with T2D only in the GWAS of Europeans, but not East Asians [40]. Expression of the PPARG can be regulated by n-3 PUFA [41], [42], and it is biologically plausible for the interaction of n-3 PUFA with PPARG variants to influence T2D risk in the European populations, but not in the East Asian populations. Therefore, as most of our included Asian studies are from East Asia, it is reasonable to postulate that n-3 PUFA intake may interact with a number of T2D-related genes for the risk of T2D, and there may be a different interaction pattern for n-3 PUFA and the T2D-related genes for the risk of T2D in the East Asian and Caucasian populations [35], [38]. However, genetic information in relation to the T2D-related variants was not available in most of these prospective studies, making it difficult to examine this hypothesis. In Asia, Huang et al. found that plasma n-3 PUFA were inversely associated with insulin sensitivity and metabolic syndrome in Chinese populations [16], [43]. Prospective studies also supported that for Chinese populations in Shanghai and in Singapore [10], [11], n-3 PUFA was inversely associated with incidence of T2D. In contrast, many studies from the US and Europe showed null [29] or even positive [3], [6], [9] association between n-3 PUFA and incidence of T2D. All these differences between Asian and US or European studies shed light on the possibility that gene-diet interaction might be an important explanation for the observed inconsistent association between n-3 PUFA and T2D incidence.
In addition to the influence of gene-diet interaction, differences in dietary patterns between Asian and Western populations might be another explanation for the inconsistent findings. Western dietary pattern is characterized by high intakes of sugar, red meat and fried food; while Asian dietary pattern, especially Chinese and Japanese, also known as a prudent dietary pattern, includes high intake of fruit, vegetable, fish and tofu. Western dietary pattern score was positively associated with risk of T2D, while a prudent dietary pattern score was inversely associated with risk of T2D [44], [45]. Fish intake is only part of the respective dietary pattern in different regions. Fried fish may be more popular in the Western populations, while Asians prefers boiled or steamed fish. Therefore, the different dietary patterns and their corresponding cooking methods may have been related to the inconsistent association of fish intake with T2D risk in the Western and Asian populations.
The potential benefit of marine n-3 PUFA is their incorporation into cell membranes, subsequently increasing insulin sensitivity [46]. Animal studies also supported the biological effect of marine n-3 PUFA on insulin resistance [4], [47]. However, in a meta-analysis of 26 randomized controlled trials [48], fish oil supplementation only marginally increased fasting blood glucose by 0.43 mmol/L in T2D patients. Another recent meta-analysis [49] found that n-3 PUFA intervention had no effects on insulin sensitivity compared to placebo. Most controlled trials [50], [51] did not find significant associations between fish oil treatment and parameters of glucose metabolism; some controlled trials [52], [53] even reported mild adverse effects of fish oil on glucose metabolism. However most of these studies were conducted in Western countries; and in the light of the previous hypothesis, genetic backgrounds and gene-diet interaction of populations in these countries may contribute to the contradictory results, and more randomized controlled trials with regard to fish oil supplement and glucose homeostasis in Asian populations are needed to confirm the hypothesis. In addition, the different response of tissue n-3 PUFA compositions to T2D in Asian and Western populations also indicated the existence of possible gene-diet interaction and genetic differences in the two regions.
The effects of fish consumption on T2D were highly consistent with that of marine n-3 PUFA in the present meta-analysis. Fish are rich sources of marine n-3 PUFA and the effects of fish on T2D might be attributed to the effects of C22∶6n-3 and C20∶5n-3. However, oily fish and lean fish, which contained quite different concentrations of C22∶6n-3 and C20∶5n-3, did not exert significantly different effects on risk of T2D compared with each other, and with total fish consumption in the present study; it might be that the study number for fatty fish and lean fish intake with T2D risk was small [7], [14], [15] and more research in this field is needed in future.
In contrast to marine n-3 PUFA, only a few cohort studies reported the association between C18∶3n-3 intake and T2D risk; further, C18∶3n-3 was lower in T2D subjects compared with controls, especially in Western populations. It might be that C18∶3n-3, the vegetable fatty acid, might affect the risk of T2D through different mechanisms compared with marine n-3 PUFA, which merits further investigations [54].
There are several strengths in the present study. First, large sample size of the included studies makes this meta-analysis more powerful to examine the associations between fish and n-3 PUFA intake and risk of T2D than any individual study. Second, although randomized controlled trials are the best way to achieve causal inference, however, there is no data for the association of fish and n-3 PUFA intake with T2D risk available in the literatures. Therefore, we systematic reviewed results from available cohort studies, and prospective nature of the included cohort studies (fish and marine n-3 PUFAs intake and T2D risk) makes the results less likely to be affected by recall and selection biases. Third, both the associations of marine and non-marine n-3 PUFA with risk of T2D were examined; and the associations of total fish, fatty fish, lean fish and shellfish intake with risk of T2D were all analyzed in this study, thus giving a relatively complete view of the current available evidence in relation to the associations of fish, n-3 PUFA intake with risk of T2D. Lastly, tissue n-3 PUFA compositions in subjects with and without T2D were compared, which supported the results from cohort studies.
Some limitations presented in this meta-analysis. First, classifications of fish and n-3 PUFA intake amounts were inconsistent among studies, which might have influenced the results. However, we used RRs of the highest vs lowest categories of fish or n-3 PUFA intake, which might reduce the bias caused by misclassification. Second, observational studies could not avoid residual confounders. Potential confounders always existed although most of the included studies had adjusted a wide range of confounders.
In conclusion, this systematic review and meta-analysis provided evidence that marine n-3 PUFA consumption had protective associations with risk of T2D in Asian populations, but was positively associated with risk of T2D in Western populations. T2D subjects in Asian but not in Western countries had lower tissue C22∶6n-3 and total n-3 PUFA compositions than controls. These findings have important public health implications. The prevalence of T2D has been steadily increasing around the world; dietary marine n-3 PUFA intake has proven to be protective for cardiovascular disease, but its effects on T2D risk were inconsistent among different populations. Influences of genetics and gene-diet interaction on T2D within different populations should be further explored to understand the association between marine n-3 PUFA intake and risk of T2D.
Supporting Information
PRISMA checklist.
(DOC)
Study protocol for systematic review and meta-analysis to determine the effects of n-3 polyunsaturated fatty acids on risk of type 2 diabetes.
(DOC)
Funding Statement
The project was funded by the National Natural Science Foundation of China (NSFC, No. 30972464) and the National Basic Research Program of China (973 Program: 2011CB504002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 2. Feskens EJ, Virtanen SM, Rasanen L, Tuomilehto J, Stengard J, et al. (1995) Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 18: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 3. Meyer KA, Kushi LH, Jacobs DR Jr, Folsom AR (2001) Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 24: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 4. Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, et al. (1987) Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885–888. [DOI] [PubMed] [Google Scholar]
- 5. Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, et al. (2007) Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 86: 189–197. [DOI] [PubMed] [Google Scholar]
- 6. Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, et al. (2009) Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 90: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Woudenbergh GJ, van Ballegooijen AJ, Kuijsten A, Sijbrands EJ, van Rooij FJ, et al. (2009) Eating fish and risk of type 2 diabetes: A population-based, prospective follow-up study. Diabetes Care 32: 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Djousse L, Biggs ML, Lemaitre RN, King IB, Song X, et al. (2011) Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr 94: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Djousse L, Gaziano JM, Buring JE, Lee IM (2011) Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr 93: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villegas R, Xiang YB, Elasy T, Li HL, Yang G, et al. (2011) Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr 94: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brostow DP, Odegaard AO, Koh WP, Duval S, Gross MD, et al. (2011) Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr 94: 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nkondjock A, Receveur O (2003) Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab 29: 635–642. [DOI] [PubMed] [Google Scholar]
- 13. Feskens EJM, Bowles CH, Kromhout D (1991) Inverse association between fish intake and risk of glucose-intolerance in normoglycemic elderly men and women. Diabetes Care 14: 935–941. [DOI] [PubMed] [Google Scholar]
- 14. Patel PS, Sharp SJ, Luben RN, Khaw KT, Bingham SA, et al. (2009) Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: the European prospective investigation of cancer (EPIC)-Norfolk cohort study. Diabetes Care 32: 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nanri A, Mizoue T, Noda M, Takahashi Y, Matsushita Y, et al. (2011) Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 94: 884–891. [DOI] [PubMed] [Google Scholar]
- 16. Huang T, Wahlqvist ML, Xu TC, Xu A, Zhang AZ, et al. (2010) Increased plasma n-3 polyunsaturated fatty acid is associated with improved insulin sensitivity in type 2 diabetes in China. Mol Nutr Food Res 54: S112–S119. [DOI] [PubMed] [Google Scholar]
- 17. Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, et al. (2008) Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 18: 503–510. [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH, et al. (2003) Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 78: 91–98. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez Y, Christophe AB (2004) Effect of diabetes mellitus and different treatments on plasma and erythrocyte phospholipid fatty acid composition in type 2 diabetics. Ann Nutr Metab 48: 335–342. [DOI] [PubMed] [Google Scholar]
- 20. Faas FH, Dang AQ, Kemp K, Norman J, Carter WJ (1988) Red Blood-Cell and Plasma Fatty-Acid Composition in Diabetes-Mellitus. Metabolism 37: 711–713. [DOI] [PubMed] [Google Scholar]
- 21. Bohov P, Gelienova K, Sebokova E, Klimes I (1993) Abnormal serum fatty acid composition in non-insulin-dependent diabetes mellitus. Ann N Y Acad Sci 683: 367–370. [DOI] [PubMed] [Google Scholar]
- 22. Pelikanova T, Kazdova L, Chvojkova S, Base J (2001) Serum phospholipid fatty acid composition and insulin action in type 2 diabetic patients. Metabolism 50: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 24. Zheng J, Huang T, Yu Y, Hu X, Yang B, et al. (2011) Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutr 15: 725–737. [DOI] [PubMed] [Google Scholar]
- 25. Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with application to meta-analysis. Am J Epidemiol 135: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 26. Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6: 40–57. [Google Scholar]
- 27. Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, et al. (2011) Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 93: 127–142. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB (2002) Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 25: 417–424. [DOI] [PubMed] [Google Scholar]
- 30. Mao XY, Zhang AZ, Li D (2007) Serum phospholipids fatty acid spectrum and its correclation with plasma lipid in patients with type 2 diabetes (in Chinese). Chinese General Practice 10: 1508–1510. [Google Scholar]
- 31. Lou DJ, Zhu LQ, Si XW, Guan LL, You QY, et al. (2010) Correlation between phospholipid fatty acid profile and high-sensitivity C reactive protein in patients with newly diagnosed type 2 diabetes mellitus (in Chinese). Chin Med J 18: 464–466. [Google Scholar]
- 32. Zhang B, Wang ZZ, Song HP (2011) The change of plasma long-chain polyunsaturated fatty acids in type 2 diabetes (in Chinese). Journal of China Traditional Chinese Medicine Information 3: 353–354. [Google Scholar]
- 33. Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, et al. (1994) The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 43: 1353–1357. [DOI] [PubMed] [Google Scholar]
- 34. Bakan E, Yildirim A, Kurtul N, Polat MF, Dursun H, et al. (2006) Effects of type 2 diabetes mellitus on plasma fatty acid composition and cholesterol content of erythrocyte and leukocyte membranes. Acta Diabetol 43: 109–113. [DOI] [PubMed] [Google Scholar]
- 35. Klimentidis YC, Abrams M, Wang J, Fernandez JR, Allison DB (2011) Natural selection at genomic regions associated with obesity and type-2 diabetes: East Asians and sub-Saharan Africans exhibit high levels of differentiation at type-2 diabetes regions. Hum Genet 129: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dedoussis GV, Kaliora AC, Panagiotakos DB (2007) Genes, diet and type 2 diabetes mellitus: a review. Rev Diabet Stud 4: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YC, Lai CQ, Ordovas JM, Parnell LD (2011) A Database of Gene-Environment Interactions Pertaining to Blood Lipid Traits, Cardiovascular Disease and Type 2 Diabetes. J Data Mining Genomics Proteomics 2. [DOI] [PMC free article] [PubMed]
- 38. Chen R, Corona E, Sikora M, Dudley JT, Morgan AA, et al. (2012) Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases. Plos Genetics 8: 100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai CQ (2012) Adaptive genetic variation and population differences. Prog Mol Biol Transl Sci 108: 461–489. [DOI] [PubMed] [Google Scholar]
- 40. Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, et al. (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106: 9362–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F (2009) Cross-Talk between PPAR gamma and Insulin Signaling and Modulation of Insulin Sensitivity. Ppar Res. [DOI] [PMC free article] [PubMed]
- 42. Deckelbaum RJ, Worgall TS, Seo T (2006) n-3 fatty acids and gene expression. Am J Clin Nutr 83: 1520S–1525S. [DOI] [PubMed] [Google Scholar]
- 43. Huang T, Bhulaidok S, Cai ZZ, Xu TC, Xu F, et al. (2010) Plasma phospholipids n-3 polyunsaturated fatty acid is associated with metabolic syndrome. Mol Nutr Food Res 54: 1628–1635. [DOI] [PubMed] [Google Scholar]
- 44. Montonen J, Knekt P, Harkanen T, Jarvinen R, Heliovaara M, et al. (2005) Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol 161: 219–227. [DOI] [PubMed] [Google Scholar]
- 45. van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB (2002) Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 136: 201–209. [DOI] [PubMed] [Google Scholar]
- 46. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, et al. (1993) The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 328: 238–244. [DOI] [PubMed] [Google Scholar]
- 47. Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, et al. (1991) Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40: 280–289. [DOI] [PubMed] [Google Scholar]
- 48. Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE (1998) Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care 21: 494–500. [DOI] [PubMed] [Google Scholar]
- 49.Akinkuolie AO, Ngwa JS, Meigs JB, Djousse L (2011) Omega-3 polyunsaturated fatty acid and insulin sensitivity: A meta-analysis of randomized controlled trials. Clin Nutr. [DOI] [PMC free article] [PubMed]
- 50. Egert S, Fobker M, Andersen G, Somoza V, Erbersdobler HF, et al. (2008) Effects of dietary alpha-linolenic acid, eicosapentaenoic acid or docosahexaenoic acid on parameters of glucose metabolism in healthy volunteers. Ann Nutr Metab 53: 182–187. [DOI] [PubMed] [Google Scholar]
- 51. Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T (1995) Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med 123: 911–918. [DOI] [PubMed] [Google Scholar]
- 52. Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V (2006) Effects of n-3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 84: 540–550. [DOI] [PubMed] [Google Scholar]
- 53. Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, et al. (2002) Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 76: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 54. Feskens EJM (2011) The prevention of type 2 diabetes: should we recommend vegetable oils instead of fatty fish? Am J Clin Nutr 94: 369–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Study protocol for systematic review and meta-analysis to determine the effects of n-3 polyunsaturated fatty acids on risk of type 2 diabetes.
(DOC)