Abstract
Background
Drought is a major abiotic stress that affects crop productivity worldwide. Sugarcane can withstand periods of water scarcity during the final stage of culm maturation, during which sucrose accumulation occurs. Meanwhile, prolonged periods of drought can cause severe plant losses.
Methodology/Principal Findings
In a previous study, we evaluated the transcriptome of drought-stressed plants to better understand sugarcane responses to drought. Among the up-regulated genes was Scdr1 (sugarcane drought-responsive 1). The aim of the research reported here was to characterize this gene. Scdr1 encodes a putative protein containing 248 amino acids with a large number of proline (19%) and cysteine (13%) residues. Phylogenetic analysis showed that ScDR1is in a clade with homologs from other monocotyledonous plants, separate from those of dicotyledonous plants. The expression of Scdr1 in different varieties of sugarcane plants has not shown a clear association with drought tolerance.
Conclusions/Significance
The overexpression of Scdr1 in transgenic tobacco plants increased their tolerance to drought, salinity and oxidative stress, as demonstrated by increased photosynthesis, water content, biomass, germination rate, chlorophyll content and reduced accumulation of ROS. Physiological parameters, such as transpiration rate (E), net photosynthesis (A), stomatal conductance (gs) and internal leaf CO2 concentration, were less affected by abiotic stresses in transgenic Scdr1 plants compared with wild-type plants. Overall, our results indicated that Scdr1 conferred tolerance to multiple abiotic stresses, highlighting the potential of this gene for biotechnological applications.
Introduction
Crop yield is negatively influenced by a large number of environmental factors. Abiotic stresses are a primary cause of reduced crop growth and productivity, and of these, drought, salinity, temperature, aluminum toxicity, flooding, pollution and radiation are among the most frequent [1]. It is estimated that stresses may reduce productivity by up to 70% [2], [3]. Abiotic stress affects the plant at different levels [4] by reducing CO2 assimilation rates, leaf cell size, rate of transpiration, water potential, plant growth rate and stomatal opening [5], which affect photosynthesis both directly and indirectly by inducing physiological changes that can lead to plant death [6].
There is a constant demand, especially in developing countries, for increased crop production to serve the increasing needs of the population. These needs can be satisfied by increasing the cultivated area (i.e., planting in regions that were not previously used) or by increasing crop productivity. To guarantee a sustainable crop yield, it is necessary to design and develop better crop varieties that can tolerate the harmful effects of constantly changing environmental factors. Thus, it is essential to identify novel and functional candidate genes that may lead to stress tolerance and improved productivity.
Sugarcane is an important tropical and subtropical crop that is used primarily to produce ethanol and sugar; however, there is also important economic activity associated with the production of other products, including rum, animal feed and molasses [7], [8]. In large areas of sugarcane-growing regions, irrigation cannot satisfy plant water requirements during cane formation, which results in low yields [9]. Our understanding of plant responses to stresses has improved significantly due to advances in the related areas of genetics, physiology and molecular biology [10], with increasing evidence that some genes have the potential to reduce the effects of resource limitation imposed on crops [11]. Several studies aiming to understand plant responses to water deficit have been conducted (for reviews see [4], [12], [13]). The currently available data indicate that plant responses to abiotic stress are complex, involving many different genes that produce responses at the biochemical, physiological and molecular levels [4], [14]. These genes are classified into four main categories: genes involved in signaling cascades and transcriptional control, genes that function directly in the protection of membranes and proteins, genes involved in water and ion uptake and transport, and genes of unknown function [15]. The identification and characterization of genes associated with plant responses to stress are crucial to the development of new cultivars with improved tolerance. Towards this end, several stress-induced genes have been overexpressed in transgenic plants, including a gene encoding a hybrid-proline-rich protein from the pigeon pea that confers tolerance to multiple abiotic stress in Arabidopsis; a DREB/CBF factor from wheat, which enhances tolerance to drought and cold stress in barley and wheat; TSRF1, which is an ERF transcription factor from tomato that improves tolerance to drought in rice and a novel sugarcane ethylene responsive factor (ERF), which enhances salt and drought tolerance in tobacco plants [16], [17], [18], [19].
In sugarcane, the expression profiles of 1,545 genes in plants exposed to drought, phosphate starvation, herbivory, methyl jasmonate, abscisic acid and two N2-fixing endophytic bacteria were evaluated by Rocha et al. [20]. More recently, approximately 1,670 genes were found to be differentially expressed in sugarcane plants exposed to water deficit [21]. A wide array of metabolic pathways was reported to be affected by these treatments, based on the numerous genes that were modulated in response [20]. Interestingly, drought was the treatment that caused the most changes in the sugarcane transcriptome. The cDNA array used in these experiments also included several genes of unknown function, some of which were detected as differentially expressed upon drought stress. Here, we have sought to characterize one of these genes. This gene was named Scdr1, for sugarcane drought-related 1. Phylogenetic analysis indicated that this gene was present prior to the divergence between dicots and monocots. The expression pattern of Scdr1 in four sugarcane cultivars showed that it is regulated by drought. The overexpression of Scdr1 in transgenic tobacco plants induced enhanced tolerance to drought, salt and oxidative stress.
Results
Scdr1 Expression in Response to Drought
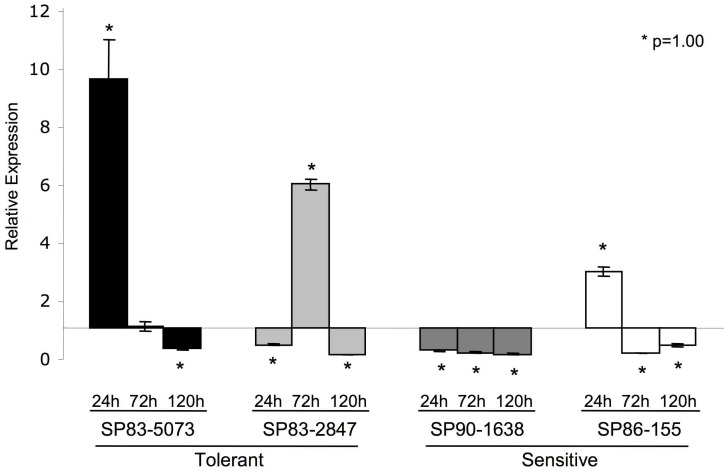
SAS (Sugarcane Assembled Sequence) SCSGSB1009D11.g, which encodes a protein of unknown function, was identified as a drought-repressed gene in a previous experiment using the drought-sensitive variety SP90–1638 (Rocha et al., 2007). To further evaluate the expression pattern of Scdr1, quantitative real-time PCR was conducted using leaves from two drought-tolerant varieties (SP83-5073 and SP83-2847), another drought-sensitive variety (SP86-155) and SP90-1638. Plants were either exposed to drought or to control conditions for 24, 72 and 120 hours, and Scdr1 was found to be differentially expressed between control and stressed plants in the four sugarcane varieties (figure 1). Interestingly, the two drought-tolerant varieties, SP83-5073 and SP83-2847, showed a strong induction of Scdr1. However, the timing of the response was different in these two varieties, peaking after 24 hours in SP83-5073 (9 times more expressed than control plants) and after 72 hours in SP83-2847 (6 times more expressed than control plants). A repression of Scdr1 expression was observed in the other two experimental time points in both plants. In one of the drought-sensitive varieties, SP86-155, Scdr1 was induced after 24 hours of stress but to a much lower level (only four times more expressed in response to drought) compared with the two drought-tolerant varieties and its expression was repressed at later time points. In the other drought-sensitive variety SP90-1638, Scdr1 was down-regulated at all three time points. Although this expression pattern is complex, the higher induction observed in the tolerant varieties suggested that Scdr1 may be associated with drought tolerance. This result prompted us to further characterize the Scdr1 gene.
Figure 1. Evaluation of Scdr1 gene expression in drought-stressed sugarcane plants.
Scdr1 gene expression was evaluated in four sugarcane varieties (SP83-5073, SP90-1638, SP83-2847 and SP86-155) after 24, 72 and 120 hours of control or drought stress conditions. The poly-ubiquitin gene was used as a reference gene for normalization. The Scdr1 relative expression was normalized to the control condition. Samples with a statistically significant difference in expression level are indicated with asterisks.
ScDR1 Protein Sequence Analysis
The Scdr1 transcript sequence (744 bp; NCBI Acc. No. JN979786) and deduced protein sequence (248 amino acids) are shown in figure 2. Interestingly, the protein has a high content of proline (19%) and cysteine (13%) residues. To evaluate the similarity of ScDR1 with other plant homologs, an alignment using the complete protein sequence was performed (figure 3a). ScDR1 showed high similarity to homologs from monocotyledonous plants, such as Sorghum (89%), maize (84%), rice (70%) and Brachypodium (65%). The similarity with proteins from dicotyledonous species was lower and ranged from 29 to 47% in Arabidopsis thaliana, soybean and medicago. This alignment was used to construct a neighbor-joining tree (figure 3b). ScDR1 was grouped in the same clade as the analyzed monocotyledonous plants (blue circle), while the dicot plants were grouped in another clade (red circle), consistent with the low sequence similarity (figure 3b).These data indicate that the ScDR1 protein arose prior to the divergence between monocots and dicots.
Figure 2. The DNA and deduced protein sequences of Scdr1 (Acc. No JN979786).
The sequence, corresponding to SAS SCSGSB1009D11.g, was obtained from the SUCEST database.
Figure 3. ScDR1 protein sequence analysis.
(A) Alignment of ScDR1 with other homolog proteins; (B) Neighbor-joining tree of sugarcane ScDR1 and its homologs in other monocotyledonous and dicotyledonous plants. All sequences were aligned using the Clustal2W software. Bootstrap values are shown as percentages above each node. Sequence accession numbers: sugarcane (Acc. No JN979786), Sorghum bicolor (XP_002447741.1), Zea mays (ACN37061.1), Brachypodium distachyon (XP_003581156), Oryza sativa (BAG72124.1), Medicago trunculata (ACJ83874.1), Glycine max (AAN03471.1), Arabidopsis thaliana (NP_974559.5), A.lyrata (XP_002870162.1).
Production of Transgenic Plants Overexpressing Scdr1
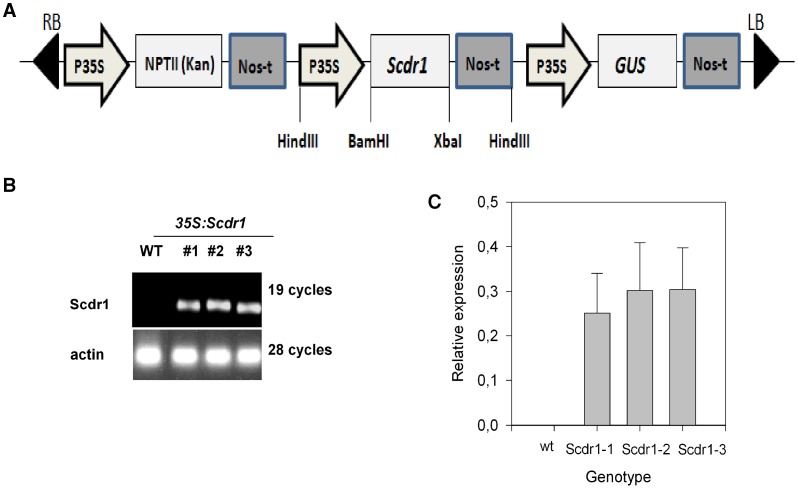
Transgenic tobacco plants were produced to evaluate the functional role of Scdr1. The Scdr1 coding sequence was PCR amplified and cloned under the control of the cauliflower mosaic virus 35S promoter using pRT104 (Töpfer et al., 1987) as an intermediary vector and pCambia 2301 as the final vector (figure 4a). The construct was expressed in Agrobacterium tumefaciens to obtain transgenic tobacco plants. Kanamycin-resistant transformants were further confirmed using an immunohistochemical assay for beta-glucuronidase activity using X-Gluc as a substrate. Seven plants that showed a strong blue staining (data not shown) were selected. Transgenic T0 plants were self-pollinated to obtain homozygous lines. Transformed T1 seedlings were selected using MS medium supplemented with kanamycin (50 mg mL−1) and used to obtain homozygous T3 plants. To confirm the integration of the Scdr1 gene, we performed PCR using genomic DNA as template. Scdr1-specific primers were used to amplify a 744 bp fragment using genomic DNA from T3 transgenic plants. The Scdr1 fragment was detected in transgenic plants; while no amplification was observed in the DNA from wild-type (WT) plants (data not shown). Three independent lines that tested positive for the presence of Scdr1 by PCR and that showed X-Gluc staining were chosen to evaluate the effects of Scdr1 overexpression. The expression of Scdr1in these lines was confirmed by semi-quantitative RT-PCR (figure 4b, c).
Figure 4. Schematic representation of the pCAMBIA2301::Scdr1 construct and PCR confirmation of plant transgene content.
(A) The Scdr1 coding region was cloned between the constitutive CaMV 35S promoter (P35S) and the NOS polyadenylation signal (Nos-t) using pCambia2301 as the backbone. The nptII (kanamycin resistance) gene is also driven by the p35S promoter. LB and RB correspond to the left and right borders of the T-DNA, respectively. The positions of some restriction sites are indicated. (B) Expression of Scdr1 in WT and three T3-generation transgenic lines. Total RNA was extracted from two-week-old seedlings and then analyzed using semi-quantitative RT-PCR. The actin gene was used as an internal standard. (C) Densitometric analysis of the semi-quantitative RT-PCR.
Effects of Scdr1 on Seed Germination
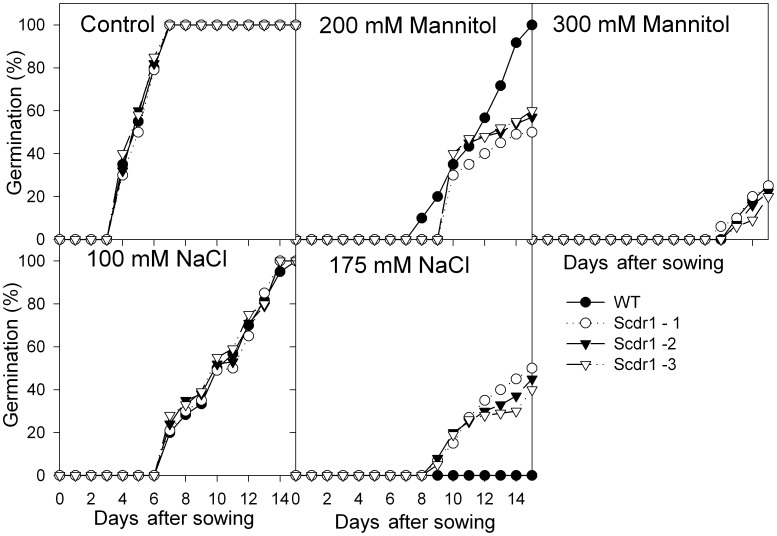
Seeds from WT and Scdr1-transgenic lines were germinated in culture media containing mannitol or NaCl to evaluate the response to drought stress and salt stress, respectively. Under control conditions, similar germination rates were observed between WT and transgenic Scdr1 plants (figure 5a). In the presence of 200 mM mannitol, Scdr1 seeds showed reduced germination rates (60%) compared with WT seeds (100%) (figure 5b). Conversely, at higher mannitol concentration (300 mM), the germination of both transgenic and WT seeds was profoundly reduced, reaching only 20% (figure 5c) or completely inhibited (400 mM, data not shown). When seeds were germinated in 100 mM NaCl, no differences were observed between Scdr1 and WT (figure 5d). However, at a higher salt concentration (175 mM NaCl), while germination was completely inhibited in WT seeds, 50% of the Scdr1 seeds were capable of germination (figure 5e). These results indicate that Scdr1 plays a role in protecting against salt stress, but not drought stress, during seed germination.
Figure 5. The effects of mannitol and NaCl on seed germination.
The percent germination of transgenic (Scdr1) and WT tobacco seeds at different concentrations of mannitol or NaCl was evaluated over 15 days.
Effects of Scdr1 on Plant Growth
To analyze the effects of Scdr1 on plant growth, five-week-old plants were watered with 200 mM mannitol or 175 mM NaCl for 10 days and then allowed to recover for 3 days, during which they were watered with pure water. Under control conditions (watering with pure water for all 13 days), both WT and Scdr1 plants performed equally well (figure 6). Drought and salt stress caused obvious negative effects in WT plants, which exhibited wilted leaves (figure 6). In contrast, drought and salt tolerance were observed in the Scdr1-overexpressing transgenic tobacco plants, indicating thatScdr1 had a protective role against these two abiotic stresses.
Figure 6. The effects of mannitol and NaCl on tobacco plants.
First row: A WT plant and three transformants overexpressing Scdr1 were grown under control conditions for 13 weeks. Middle row: plants watered with 200 mM mannitol for 10 days and then irrigated with water for 3 days. Bottom row: plants irrigated for 10 days with 175 mM NaCl and then irrigated with water for 3 days.
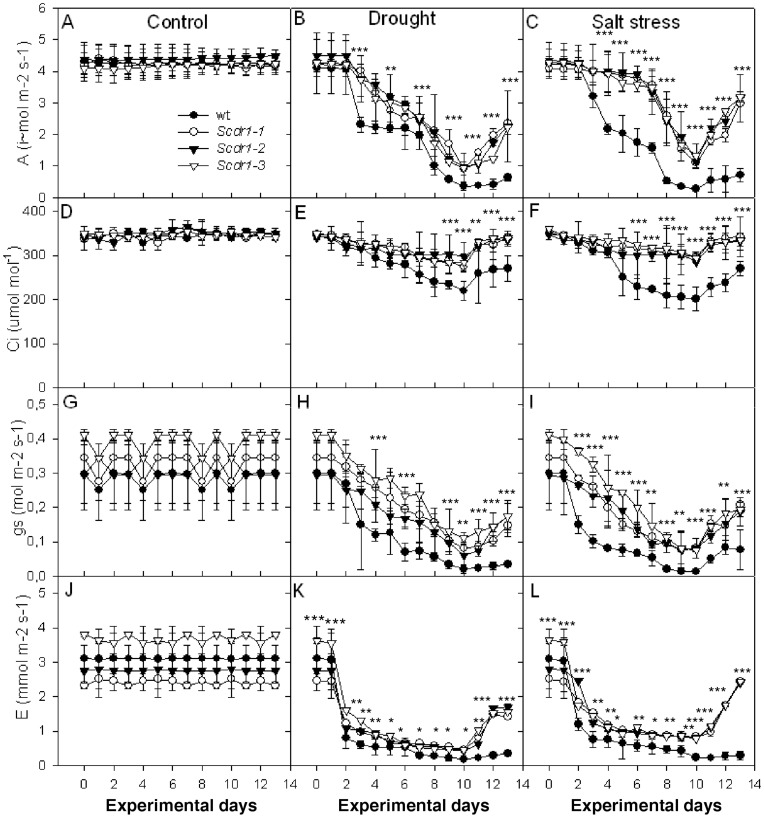
To characterize the performance of the transgenic plants at the physiological level, WT plants and the three independent lines containing the Scdr1 gene were analyzed using an infrared gas analyzer (IRGA). The obtained transpiration rate (E), net photosynthesis (A), stomatal conductance (gs), internal leaf CO2 concentration (Ci) and respiration (R) data are presented in figure 7. Photosynthesis, which is one of the most important parameters involved in plant productivity, was affected in both transgenic and WT plants. In the initial days, no differences between transgenic and WT plants were observed. Beginning on the third day of stress, transgenic plants maintained higher photosynthesis levels than WTs. Following the recovery period, transgenic plants that had been exposed to drought recovered 50% of their initial net photosynthesis (figure 7b), and 75% recovery was observed in previously salt-stressed plants (figure 7c).The WT and scdr1 transgenic plants exhibited no significant differences in net photosynthesis under control growth conditions (figure 7a).
Figure 7. The effects of stress on gas exchange parameters in WT and Scdr1 transgenic plants.
Thirty-day-old plants were exposed for 10 days to 200 mM mannitol or 175 mM NaCl and then allowed to recover for three days by watering with pure water, as described for figure 7. A–C: Net photosynthesis (A); D–F: Internal leaf CO2 concentration (Ci); G–I: Stomatal conductance (gs); J–K: Transpiration rate (E). A, D, G and J: Control treatment; B, E, H and K: 200 mM mannitol (drought); C, F, I and L: 175 mM NaCl (salt). Asterisks (***, ** and *) indicate significant differences compared with WT plants in each treatment and each time point (P<0.0001, P<0.001 and P<0.01, respectively, n = 5).
The Ci was similar in WT and transgenic plants under control conditions (figure 7d); however, a slight difference of around 20% was observed during drought (figure 7e), and a greater difference of around 50% was observed during salt stress (figure 7f). The gs showed a similar pattern to that of photosynthesis, with Scdr1 plants less affected by drought and salt stress (figure 7h, i). Similar transpiration rate (E) values were found between the plant lines under normal growth conditions (figure 7j), and both drought and salt stress caused a strong decrease in E values in both lines. Scdr1 overexpression allowed plants to recover E values after re-watering (figure 7k, l).
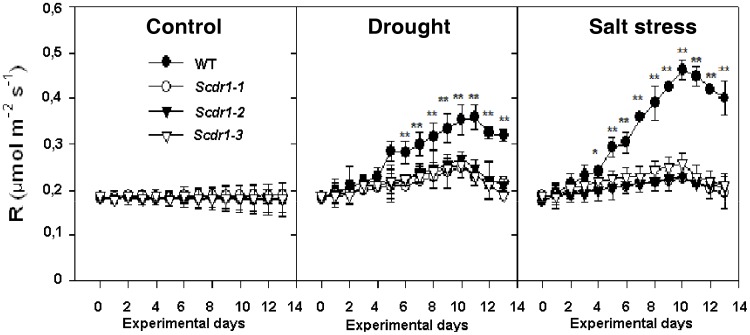
R in the Scdr1 and WT plants was similar under control conditions. In both WT and Scdr1 plants, R increased in response to drought and salt stress, but Scdr1-transgenic plants were less affected (figure 8). WT plants showed a two-fold increase in R under salt stress compared with control conditions, while Scdr1 plants showed only a 25% increase in R. These results suggest that WT plants increase R as a strategy to maintain homeostasis under stress. In contrast, R did not increase in Scdr1 plants, due to their higher tolerance.
Figure 8. Respiration in tobacco leaves exposed to drought and salt stress.
Thirty-day-old WT and transgenic plants were exposed for 10 days to 200 mM mannitol or 175 mM NaCl and were then allowed to recover for three days by watering with pure water. The data shown represent the means of three replicate measurements. Asterisks (** and*) indicate significant differences relative to WT plants in each treatment and each time point (P<0.0001 and P<0.01, respectively, n = 5).
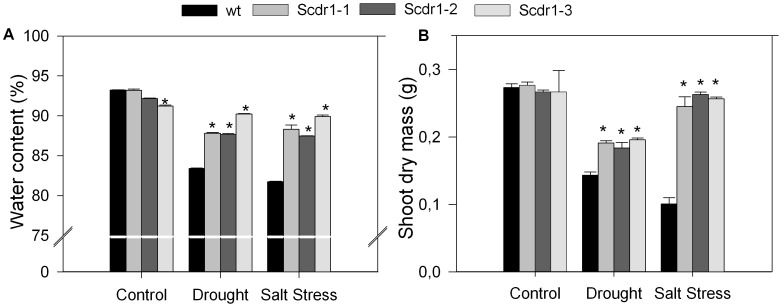
Drought and salt stress reduced the water content in both WT and Scdr1-transgenic plants. While in the WT plants, water content was reduced from 94% to 83% due to drought stress and to 82% in salt-stressed plants, in Scdr1 plants these values ranged from 88 to 90%, indicating that a smaller reduction was observed in the transgenic plants (figure 9a). These results indicate that plants overexpressing Scdr1 were capable of maintaining turgidity under stress, which suggests the occurrence of osmotic adjustment and/or the activation of another mechanism that prevents cellular dehydration. Shoot dry matter was evaluated as an additional parameter to compare WT and Scdr1 transgenic plants (figure 9b). Under control conditions, WT and Scdr1 plants had a dry mass of around 0.27 g. Drought and salt stress decreased dry mass in WT plants to 0.15 g and 0.1 g, respectively. Scdr1 plants were less affected due to drought (dry mass on average of 0.2 g in the three events) and salt-stress (0.26 g). Therefore, although both stresses affected the amount of shoot dry matter in transgenic and WT plants, the reduction was more severe in WT plants than in Scdr1 plants.
Figure 9. Water content and biomass in WT and Scdr1 transgenic plants.
Thirty-day-old plants were exposed to 200 mM mannitol or 175 mM NaCl for 10 days and then allowed to recover for 3 days by watering with pure water. Control plants were irrigated with water only. The water content in leaves (A) and shoot dry matter (B) were evaluated. Bars represent the means of three independent experiments. Asterisk (*) indicates significant differences compared with WT plants in each treatment (P<0.001, n = 5).
Analysis of Resistance to Oxidative Stress in Scdr1 Transgenic Plants
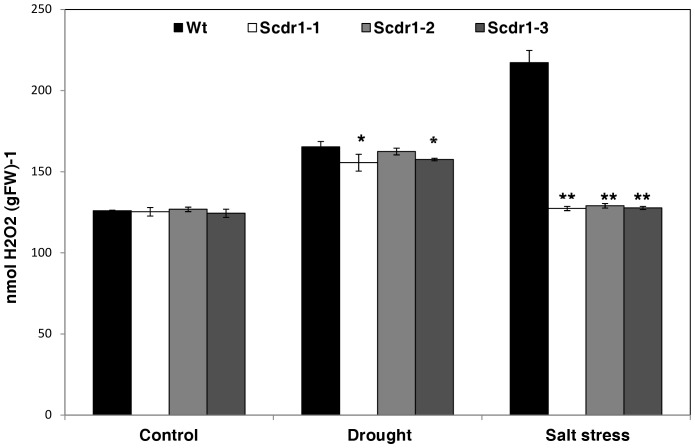
To further characterize the role of Scdr1 on tolerance to abiotic stress, we evaluated the accumulation of H2O2 in the leaves of transgenic and WT seedlings. Under control conditions, both transgenic and WT plants showed similar H2O2 levels (figure 10). Following 10 days of drought stress, H2O2 levels increased similarly in both transgenic and WT plants. Under salt stress, H2O2 levels increased dramatically in WT plants, reaching 217 nmol H2O2/gFW, while Scdr1 transgenic plants were substantially less affected, presenting 127 to 129 nmol H2O2/gFW in the three independent transgenic events (figure 10). Because H2O2 levels are correlated with ROS production, we can infer that Scdr1 plants produced less ROS than WT plants under salt stress.
Figure 10. Quantification of hydrogen peroxide in tobacco leaves.
Thirty-day-old plants were exposed for 10 days to 200 mM mannitol or 175 mM NaCl. Control plants were irrigated with water. H2O2 levels in WT and Scdr1 transgenic lines were determined using Fe-Xylenol orange. Data are represented as the mean±standard deviation from three independent experiments (n = 5). Asterisks (** and *) indicate significant differences compared with WT plants in each treatment (P<0.0001 and P<0.001, respectively).
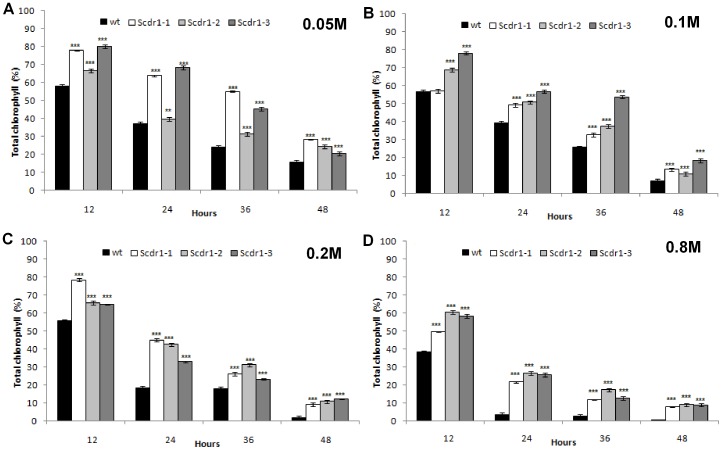
To further evaluate the role of Scdr1 in oxidative stress, leaf discs were exposed to different concentrations of H2O2 for up to 48 hours (figure 11). Even when the lowest H2O2 concentration was used (0.05 M), Scdr1 plants showed a higher percentage of total chlorophyll at all time points (figure 11a). As the H2O2 concentration was raised to 0.1 and 0.2 M, the differences between WT and Scdr1 plants increased. The highest concentration (0.8 M) affected WT plants severely, with almost no chlorophyll noted after 48 hours, while Scdr1 transgenic plants maintained approximately 10% of the initial levels (figure 11d). These results indicate that constitutive overexpression of the Scdr1 gene in transgenic tobacco plants enhanced tolerance to oxidative stress, allowing the preservation of a higher percentage of chlorophyll.
Figure 11. Spectrophotometric quantification of the chlorophyll content in WT and Scdr1 plants exposed to oxidative stress.
Leaf discs (1 mm) from three independent Scdr1 transgenic lines (Scdr1-1, Scdr1-2, Scdr1-3) and non-transgenic control plants were treated with water (control) or with different concentrations of H2O2: A) 0.05 M, B) 0.1 M, C) 0.2 M and D) 0.8 M. The total chlorophyll content in acetone extracts of H2O2-treated leaf discs was evaluated spectrophotometrically. Error bars were calculated from three independent experiments (n = 5). Asterisks (*** and **) indicate significant differences compared with WT plants in each treatment (P<0.0001 and P<0.001 respectively).
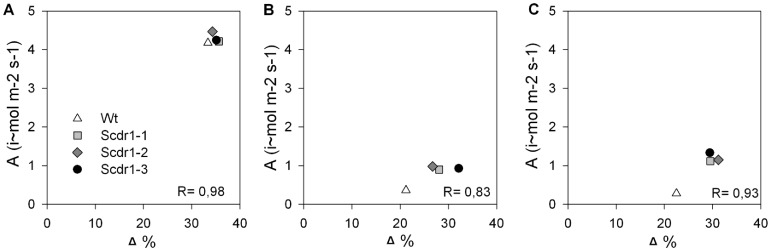
Carbon Isotope Discrimination and Photosynthesis in Stressed Plants
Under environmental stress conditions, there is variation in carbon assimilation that affects photosynthesis (A), mainly due to stomatal limitations. To detect this variation, the relationship between carbon isotopic discrimination (CID) and photosynthesis (A) under well-irrigated, drought or salt-stress conditions was investigated. Under well-irrigated conditions, no significant differences were observed between Scdr1 and WT plants, because both had A rates around 4 i∼mol m−2 s−1and CID around 34% (figure 12a). CID decreased to 21–22% due to drought and salt stress in WT plants, while in Scdr1 plants this decrease was smaller (up to 30%). Although A had a strong decrease in both plants, WT plants had values around 0.5 i∼mol m−2 s−1 and Scdr1 plants had A rates in the range from 1–1.3 i∼mol m−2 s−1 (figure 12b–c). The higher CID and A levels in Scdr1 plants correlated with lower levels of plant stress (figure 6).
Figure 12. The relationship between net photosynthetic rate (A) and carbon isotope discrimination (Δ) in transgenic Scdr1 and WT plants following 10 days of stress.
(A) control, (B) drought, (C) salt stress.
Discussion
Major environmental stresses, such as drought and salinity, contribute to the gap between actual and potential crop yields. To guarantee a sustainable crop yield, it is imperative to design and develop better crop varieties with inbuilt tolerance to the harmful effects of constantly changing environmental factors. Many novel sugarcane stress-induced genes putatively linked to drought and salt stress have been identified [20], [21], but their function in the stress response remains unknown. In this study, a novel drought stress-responsive sugarcane gene (Scdr1) was characterized functionally.
Different responses to abiotic stresses are the result of cooperative interactions between multiple physiological, biochemical and morphological features. These interactions may vary between species and even varieties, as observed for drought stress in other plant species [22], [23], [24] and for the Scdr1 gene in this study in sugarcane (figure 1). The expression of Scdr1 was different between sensitive and tolerant sugarcane varieties exposed to drought stress. As shown in figure 1, drought-induced Scdr1 expression was high in two drought-tolerant varieties, down-regulated in one sensitive variety and exhibited only one peak of induction in another sensitive variety. Although we are currently unable to explain the highly complex regulation of this gene, the overexpression of Scdr1 in transgenic tobacco plants supported the hypothesis that Scdr1 is involved in sugarcane defense against drought stress. These data raise the question of how gene expression patterns can be used to select genes that are amenable to biotechnological improvement. Certainly, several genes with complex expression patterns, such as Scdr1, may have been ignored in functional assays.
The deduced amino acid sequence of the Scdr1 sugarcane protein (figure 1) has similarity to several proteins, all of which are of unknown function (figure 2). The ScDR1 protein has 19% proline and 13% cysteine residues, and no conserved domains were found in this protein. The amino acid sequence of ScDR1 was similar to that of other monocot proteins, and it appeared in a separate clade from that of the dicotyledonous plants (figure 3). This separation suggests an early evolutionary origin for this gene, prior to the divergence of monocots and dicots and followed by an independent evolution inside each clade that might reflect the differences found between the species evaluated. However, the fact that the sugarcane ScDR1 has conferred drought and salt tolerance to tobacco plants demonstrated that the metabolic pathway in which this gene operates is conserved between monocots and dicots.
In numerous crop plants, the stages of seed germination and early seedling growth are the most susceptible to abiotic stresses [25], [26]. For example, some environmental factors, such low temperature, high concentrations of salt or water deficit, significantly delay the onset of germination and reduce the rate of seed germination events [27], [28], [29], [30]. Transgenic plants overexpressing Scdr1 showed a greater than 20% germination rate under high salt conditions, while WT seeds did not germinate at all. This result agrees with several previous studies [27], [31], [32], [33] and shows that salt stress is an important limiting factor for germination in different crop species. Here, we showed that transgenic tobacco plants that overexpress Scdr1 exhibit increased tolerance to salt stress during seed germination and early seedling development (figure 5).
Three independent homozygous Scdr1 transgenic tobacco lines subjected to multiple stresses, including mannitol (drought), NaCl (salt stress) or H2O2 (oxidative stress), developed healthy seedlings, in contrast to WT plants, which developed injured and debilitated seedlings (figure 6). Photosynthesis parameters, including rates of transpiration, net photosynthesis, stomatal conductance and internal leaf CO2 concentration, were less affected by salt or drought stress in transgenic plants (figure 7). These results demonstrate the unequivocal contribution of Scdr1 in affording abiotic stress tolerance at the whole-plant level.
The effects of drought stress on plant respiration vary according to the severity of the stress and between species [34], [35]. The percentage of fixed carbon that is respired is predicted to be higher in water-stressed plants because drought typically causes a relatively greater inhibition of photosynthesis than of plant respiration [36]. The increase in respiration observed in WT plants could reflect a plant strategy to increase ATP levels to repair the damage caused by drought and salt stress, as we [37] have shown in drought-stressed tobacco leaves (reviewed by Atkin and Macherel [38]). Because Scdr1 overexpression reduced the harmful effects of environmental stresses, the need for higher respiration rates was reduced (figure 8).
Environmental stresses affect photosynthetic parameters directly (net photosynthesis) or indirectly (stomatal closure) due primarily to the increased production of oxidative stress-induced ROS [35], [39]. As shown in figure 10, under drought or salt stress, transgenic Scdr1 tobacco plants showed lower levels of H2O2 than WT plants. Under optimal conditions, ROS are produced at a low level in organelles with specialized compartments (mitochondria, chloroplasts and peroxisomes) in which a variety of metabolic activities takes place. However, a dramatic increase in ROS levels occurs when cells are submitted to drought, salinity or osmotic stress [35], [40]. Thus, some researchers have suggested that high tolerance to environmental stresses, and drought and salinity in particular, may be associated with a strong defense against oxidative stress at the subcellular and cellular levels [41]. To achieve this tolerance, the whole plant may employ several different strategies [42], [43]. One of these is to control ROS production.
During oxidative stress, Scdr1 transgenic plants showed higher total chlorophyll content than WT plants (figure 11). All pathways that activate mechanisms leading to ROS scavenging have been shown to play an important role in protecting plants against different abiotic stresses [35], [44]. Because the overexpression of Scdr1 in transgenic tobacco plants enhanced tolerance to drought, salt and oxidative stress, a putative role for the ScDR1 protein is the avoidance of ROS production under harmful conditions. The elucidation of the exact pathway leading to this protection mechanism requires further study.
During photosynthesis, plants discriminate against 13C. Under stressful conditions, 13C discrimination is affected by stomatal limitations and the enzymatic processes of photosynthesis [45]. Different studies [46], [47], [48] have demonstrated that carbon isotope discrimination (CID) is highly correlated with plant water-use efficiency. Measurements of CID in C3 and C4 plants have also been used as integrated measures of the photosynthetic gas exchange response to environmental variables, such as drought [49], [50], [51], [52] and salinity [53], [54], [55]. As shown in figure 12, WT plants showed less CID and A during drought and salt stress, which correlated with lower stomatal conductance and consequently reduced absorption of CO2, as reviewed by Chaves et al. [39].
It is worth to note that although Scdr1 overexpression had a positive impact in several plant parameters either under drought or salt stress, major impacts were observed under salt stress. Interestingly, in WT plants the increase in the H2O2 levels was much higher due to salt stress compared to drought stress, and ScDR1 overexpression decreased oxidative stress at a higher level in salt-stressed plants compared to the drought stressed ones (Figure 10). Therefore, we believe that this action on oxidative stress could explain the better effect of ScDR1 in protecting plants from salt stress.
In summary, our data shed light on the role of a novel sugarcane gene in drought and salt stress response. Scdr1 encodes a protein of unknown function, and our data suggest that Scdr1 is involved in protecting cells and the whole plant against the stress-induced accumulation of ROS. Our study highlights the relevance of the group of genes that encode unknown proteins, which make up a large portion of most genomes. Scdr1 has the potential to be used in biotechnological applications to produce sugarcane varieties with greater tolerance to both drought and salt stress. Future work will focus on understanding the pathways controlled by this gene.
Materials and Methods
Plant Material and Growth Conditions
Seeds of wild-type (WT) tobacco (Nicotiana tabacum, var. SR1) were germinated in Petri dishes containing Murashige-Skoog (MS) medium with 0.9% (w/v) agar. Seedlings were transplanted to soil consisting of Bacto (Michigan Peat Co., Houston) and sand (4∶1, v/v) in a 325 mL pot. Plants with 10 to 12 leaves were transplanted to 1-L containers of the same mixture and maintained in a growth chamber with a 16/8 h light/dark cycle (300–400 µmol photons m−2 s−1) at 25°C and a relative humidity of 75–80%. Under these conditions, WT tobacco remains in the rosette stage until at least 5 weeks of age.
Sugarcane plants from two drought-tolerant (SP83-5073 and SP83-2847) and two drought-sensitive varieties (SP90-1638 and SP86-155) were grown in greenhouses as described by Rocha et al. [20]. Briefly, plants were grown in pots containing moist sand and watered with Hoagland’s solution [56] for 5 weeks. Water was then withheld, and leaves were collected after 24, 72 and 120 h. Control plants were irrigated normally. Leaf samples were stored at −80°C.
Quantitative Real-time PCR
Quantitative real-time PCR conducted as described by Rocha et al. [20]. Total RNA was isolated from leaf samples taken from drought-stressed and non-stressed plants. Poly-ubiquitin was used as the reference gene [20]. Relative expression (experimental/control) was determined using the 2−ΔΔC t method [57], and a sample not subjected to stress was used as a control. For the statistical analysis of relative gene expression, we assumed a log-normal model that calculates the probability Pr (sample> reference) and Pr (sample <reference) for up- and down-regulated genes, respectively. The expression profile was considered validated when P≥0.95. Leaves from six plants were used for each time point per treatment.
Construction of Plant Expression Vectors
The complete coding sequence of Scdr1 (Acc. No JN979786) was cloned from SAS SCSGSB1009D11.g, which was obtained from the Brazilian Clone Collection Center (BCCCENTER, Brazil). The template DNA was amplified using PCR with gene-specific primers (Forward: 5′-GGATCCCTCATCGCCAGCTCCCAT-3′ and reverse: 5′-TCTAGACCTGTGCAGTGTCGGATTATTC-3′), cloned into pGEMT-Easy (Promega, USA) and then introduced between the BamHI and XbaI sites of the pRT104 vector [58]. The resulting expression cassette, under the control of the 35S promoter and using the NOS terminator, was transferred as a HindIII fragment into the pCAMBIA 2301 vector (Cambia, Australia). The resulting construct (pCAMBIA2301::Scdr1) was introduced into Agrobacterium tumefaciens strain GV3101 (Clontech, USA).
Semi-quantitative RT-PCR
Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, USA), and first-strand cDNA was synthesized from 2 µg of total RNA using the Superscript III Kit (Invitrogen, USA) with oligo d(T)18 primers according to the manufacturer’s instructions. Semi-quantitative RT-PCR was performed with 1 µl of cDNA in a 25 µl reaction volume. The PCR conditions for the amplification of Scdr1 were as follows: 1 min at 94°C, followed by 30 cycles of 45 s at 94°C, 60 s at 53°C and 75 s at 72°C. The same conditions were used for the amplification of the WT tobacco actin gene, except that the number of PCR cycles were decreased to 28 and the Tm used was 60°C. The PCR products were examined using electrophoresis on a 1% agarose gel. The experiment was repeated three times, and relative densitometric ratios were determined using ImageJ (http://rsbweb.nih.gov/ij/).
Transformation of Tobacco Plants
Leaves from WT tobacco plants were surface-sterilized, cut into small discs and incubated with A. tumefaciens suspensions for 5–10 min. Plant tissues were then transferred to MS medium (supplied with 2 mg L−1 benzyladenine and 0.1 mg L−1 naphthylacetic acid) for 3 days. Selection was conducted using a selection medium (MS salts, 2 mg L−1 benzyladenine, 0.1 mg L−1 naphthylacetic acid, and either 100 mg L−1 kanamycin and 500 mg L−1 carbenicillin), and developing shoots were then transferred to MS medium containing 0.1 mg L−1 indole-3-acetic acid and either 100 mg L−1 kanamycin and 500 mg L−1carbenicillin. Plants with roots were then transferred to soil and maintained in a growth chamber as described above.
Seed Germination Assays
Seeds from transgenic and WT tobacco plants were surface-sterilized with 70% (v/v) ethanol for 1 min, incubated in a 2% (v/v) NaClO solution for 30 min and rinsed 5–6 times in sterile distilled water. Seeds were sown in Petri dishes (30 seedlings/dish) containing MS medium and incubated in a chamber at 23°C with a 16/8 h light/dark cycle (300–400 µmol photons m−2 s−1). Different concentrations of mannitol (0, 200, 300 and 400 mM) or NaCl (0, 100 and 175 mM) were used to induce drought and salt stress, respectively. The number of germinated seeds was measured daily.
Drought and Salt Stress Assays in Tobacco Plants
Seeds from WT Nicotiana tabacum and three independent and homozygous T3-generation transgenic lines (Scdr1-1, Scdr1-2 and Scdr1-3) were allowed to germinate for 16 days and were then grown in pots filled with Plantmax HT (Eucatex, Brazil). The pots were placed in a growth chamber at 22°C with an 18-hour light period/day. Plants were irrigated with 70 ml of water daily for 4 weeks prior to stress treatments. For drought or salt stress, seedlings were irrigated with 70 ml of 200 mM mannitol or 175 mM NaCl, respectively, for 10 days and were then allowed to recover with pure water irrigation for 3 days, as described by Zhang et al. [59]. Five plants were used for each treatment.
We used completely expanded leaves at the same positions on the tobacco plants to estimate the stomatal conductance of CO2 (gs), transpiration rate (E) and net photosynthetic rate (A). Measurements were taken with an Infrared Gas Analyzer (IRGA, LCpro+; ADC Bioscientific, UK) at a CO2 concentration of 360 µL L−1, a saturating light intensity of 1000 µmol m−2 s−1 and a gas flow rate of 200 mL min−1. The temperature inside the leaf chamber was 25°C [60].
To perform respiration (R) measurements, 5-week-old plants were grown in a chamber at 25°C with a 16/8 h light/dark photoperiod. To avoid artifacts caused by transient metabolic activities following darkening, known as light-enhanced dark respiration, measurements of night respiration were performed after 3 hours of acclimation to darkness. Carbon dioxide production was measured with an IRGA, as described by Begcy et al. [37].
Hydrogen Peroxide Determination
A modified ferrous ammonium sulphate/xylenol orange (FOX) method was used to quantify H2O2 [61]. Briefly, 300 mg of leaves (fresh material) from WT and Scdr1 transgenic plants that were well irrigated and treated with mannitol or salt for 10 days, as described previously, was subjected to methanol extraction (1∶5 w/v mg sample/mL) at 0°C. The lead samples were ground in a mortar and then centrifuged at 10,000×g for 5 min. We then mixed 100 µL of supernatant, 500 µL of 1 mM Fe(NH4)2(SO4)2 and 200 µL of 250 mM HSO4. The reaction mixture was incubated in the dark for 5 min. Xylenol orange (100 µL, 1 mM) was then added, and the mixture was again incubated in the dark for 20 min. H2O2 donates electrons to Fe, which in turn binds to xylenol to form a purple compound. A standard curve of known concentrations of H2O2 (0, 2.5, 5, 7.5, 10, 12.5 and 15 µM H2O2) was generated and used to quantify the contents of the sample.
Carbon Isotope Discrimination
We used leaves from the same plants that were used for H2O2 quantification. The carbon isotope composition of dry leaf samples was determined using ratio mass spectrometry in the Laboratório de Isótopos Estáveis, Centro de Energia Nuclear na Agricultura (CENA), Universidade de São Paulo (Piracicaba, Brazil),as described by Chandra and Bhatt [62]. Δ13C was calculated according to the protocol of Williams et al. [51] from plant Δ13C values measured under drought, salinity or control conditions (n = 3).
H2O2 Treatment and Total Chlorophyll Determination
Oxidative stress was induced as described by Brandalise et al. [63]. Fully-expanded leaves from WT and three independent transgenic Scdr1 plants were grown for 5 weeks in a growth chamber at 23°C with a 16/8 h light/dark cycle (300–400 µmol photons m−2 s−1). Leaf discs (1 cm in diameter) were cut and floated on 0, 0.05, 0.1, 0.2, 0.4 or 0.8 M H2O2 for 12, 24, 36 or 48 h at 25°C under constant light in three independent experiments. The degree of oxidative stress in treated leaf tissues was determined spectrophotometrically as the total chlorophyll content in leaf discs following extraction in acetone at 4°C, as described by Arnon [64].
Statistical Analysis
For statistical calculations, the mean values, standard deviation and t-test values were computed using pre-loaded software in Excel (http://www.Physics.csbsju.edu/stats/t-test.html).
Acknowledgments
We thank Éder Bedani for assistance with the tobacco transformation.
Funding Statement
KB received a fellowship from National Council for Scientific and Technological Development (CNPq). This research was developed as part of a research network involving grants 2008/5798-6 and 2008/57908-6 from Fundação de Amparo à Pesquisa de São Paulo (FAPESP), 574002/2008-1 and 552802/2007-7 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and 815/07 from Financiadora de Estudos e Projetos (FINEP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25: 275–294. [DOI] [PubMed] [Google Scholar]
- 2. Boyer JS (1982) Plant productivity and environment. Science 218: 443–448. [DOI] [PubMed] [Google Scholar]
- 3. Maybank J, Bonsal BR, Jones J, Lawford RG, O’Brien EG, et al. (1995) Drought as a natural disaster. Atmosphere-Ocean 33: 195–222. [Google Scholar]
- 4. Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solari LI, Johnson S, DeJong TM (2006) Relationship of water status to vegetative growth and leaf gas exchange of peach (Prunus persica) trees on different rootstocks. Tree Physiol 26: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 6. Affenzeller MJ, Darehshouri A, Andosch A, Lutz C, Lutz-Meindl U (2009) Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata . J Exp Bot 60: 939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM (2010) Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotechnol J 8: 263–276. [DOI] [PubMed] [Google Scholar]
- 8. Lakshmanan P, Geijskes RJ, Aitken KS, Grof CPL, Bonnett GD, et al. (2005) Sugarcane biotechnology: The challenges and opportunities. In Vitro Cell Dev -Pl 41: 345–363. [Google Scholar]
- 9. Ahmad S, Ahmad N, Khaliq A (1999) relation studies in water stressed sugarcane (Saccharum officinarum L.). Int J Agr Biol 2: 1–4. [Google Scholar]
- 10. Gao JP, Chao DY, Lin HX (2007) Understanding abiotic stress tolerance mechanisms: Recent studies on stress response in rice. J.Integr Plant Biol 49: 742–750. [Google Scholar]
- 11. Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotech 17: 113–122. [DOI] [PubMed] [Google Scholar]
- 12. Chaves M, Davies B (2010) Drought effects and water use efficiency: improving crop production in dry environments foreword. Funct Plant Biol 37: Iii–Vi. [Google Scholar]
- 13. Bray EA (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana . J Exp Bot 55: 2331–2341. [DOI] [PubMed] [Google Scholar]
- 14. Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14. [DOI] [PubMed] [Google Scholar]
- 15. Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3: 217–223. [PubMed] [Google Scholar]
- 16. Priyanka B, Sekhar K, Reddy VD, Rao KV (2010) Expression of pigeonpea hybrid-proline-rich protein encoding gene (CcHyPRP) in yeast and Arabidopsis affords multiple abiotic stress tolerance. Plant Biotechnol J 8: 76–87. [DOI] [PubMed] [Google Scholar]
- 17. Morran S, Eini O, Pyvovarenko T, Parent B, Singh R, et al. (2011) Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol J 9: 230–249. [DOI] [PubMed] [Google Scholar]
- 18. Quan R, Hu S, Zhang Z, Zhang H, Huang R (2010) Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J 8: 476–488. [DOI] [PubMed] [Google Scholar]
- 19. Trujillo LE, Sotolongo M, Menendez C, Ochogavia ME, Coll Y, et al. (2008) SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when overexpressed in tobacco plants. Plant Cell Physiol 49: 512–525. [DOI] [PubMed] [Google Scholar]
- 20. Rocha FR, Papini-Terzi FS, Nishiyama MY, Vencio RZN, Vicentini R, et al. (2007) Signal transduction-related responses to phytohormones and environmental challenges in sugarcane. BMC Genomics 8: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues FA, Da Graca JP, De Laia ML, Nhani A, Galbiati JA, et al. (2011) Sugarcane genes differentially expressed during water deficit. Biol Plantarum 55: 43–53. [Google Scholar]
- 22. Jia JP, Fu JJ, Zheng J, Zhou X, Huai JL, et al. (2006) Annotation and expression profile analysis of 2073 full-length cDNAs from stress-induced maize (Zea mays L.) seedlings. Plant J 48: 710–727. [DOI] [PubMed] [Google Scholar]
- 23. Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2: 48–54. [Google Scholar]
- 24. Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sekhar K, Priyanka B, Reddy VD, Rao KV (2010) Isolation and characterization of a pigeonpea cyclophilin (CcCYP) gene, and its over-expression in Arabidopsis confers multiple abiotic stress tolerance. Plant Cell Environ 33: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 26. Mito T, Seki M, Shinozaki K, Ohme-Takagi M, Matsui K (2010) Generation of chimeric repressors that confer salt tolerance in Arabidopsis and rice. Plant Biotechnol J. [DOI] [PubMed] [Google Scholar]
- 27. Bradford KJ (1990) A water relations analysis of seed-germination rates. Plant Physiol 94: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foolad MR, Lin GY (1998) Genetic analysis of low-temperature tolerance during germination in tomato, Lycopersicon esculentum Mill. Plant Breeding 117: 171–176. [Google Scholar]
- 29. Jones RA (1986) High salt tolerance potential in Lycopersicon species during germination. Euphytica 35: 575–582. [Google Scholar]
- 30. Liptay A, Schopfer P (1983) Effect of water-stress, seed coat restraint, and abscisic-acid upon different dermination capabilities of two tomato lines at low-temperature. Plant Physiol 73: 935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bliss RD, Plattaloia KA, Thomson WW (1986) Osmotic sensitivity in relation to salt sensitivity in germinating barley seeds. Plant Cell Environ 9: 721–725. [Google Scholar]
- 32. Haigh AM, Barlow EWR (1987) Water relations of tomato seed germination. Australian J Plant Physiol 14: 485–492. [Google Scholar]
- 33. Ungar IA (1978) Halophyte seed germination. Bot Rev 44: 233–264. [Google Scholar]
- 34. Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62: 869–882. [DOI] [PubMed] [Google Scholar]
- 35. Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467. [DOI] [PubMed] [Google Scholar]
- 36. Flexas J, Bota J, Galmes J, Medrano H, Ribas-Carbo M (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plantarum 127: 343–352. [Google Scholar]
- 37. Begcy K, Mariano ED, Mattiello L, Nunes AV, Mazzafera P, et al. (2011) An Arabidopsis mitochondrial uncoupling protein confers tolerance to drought and salt stress in transgenic tobacco plants. PLoS One 6: e23776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atkin OK, Macherel D (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot 103: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giraud E, Ho LH, Clifton R, Carroll A, Estavillo G, et al. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: Relationships in green cells. Physiol Plant 100: 224–233. [Google Scholar]
- 42. Pastore D, Trono D, Laus MN, Di Fonzo N, Flagella Z (2007) Possible plant mitochondria involvement in cell adaptation to drought stress. A case study: durum wheat mitochondria. J Exp Bot 58: 195–210. [DOI] [PubMed] [Google Scholar]
- 43. Moller IM (2001) Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591. [DOI] [PubMed] [Google Scholar]
- 44. Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, et al. (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283: 34197–34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Monti A, Brugnoli E, Scartazza A, Amaducci MT (2006) The effect of transient and continuous drought on yield, photosynthesis and carbon isotope discrimination in sugar beet (Beta vulgaris L.). J Exp Bot 57: 1253–1262. [DOI] [PubMed] [Google Scholar]
- 46. Farquhar GD, Gan KS (2003) On the progressive enrichment of the oxygen isotopic composition of water along a leaf. Plant Cell Environ 26: 801–819. [PubMed] [Google Scholar]
- 47. Cumbie WP, Eckert A, Wegrzyn J, Whetten R, Neale D, et al. (2011) Association genetics of carbon isotope discrimination, height and foliar nitrogen in a natural population of Pinus taeda L. Heredity. 107: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ebdon JS, Kopp KL (2004) Relationships between water use efficiency, carbon isotope discrimination, and turf performance in genotypes of Kentucky bluegrass during drought. Crop Sci 44: 1754–1762. [Google Scholar]
- 49. Yang H, Auerswald K, Bai Y, Wittmer MH, Schnyder H (2011) Variation in carbon isotope discrimination in Cleistogenes squarrosa (Trin.) Keng: patterns and drivers at tiller, local, catchment, and regional scales. J Exp Bot 62: 4143–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fravolini A, Williams DG, Thompson TL (2002) Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply. J Exp Bot 53: 2261–2269. [DOI] [PubMed] [Google Scholar]
- 51. Williams DG, Gempko V, Fravolini A, Leavitt SW, Wall GW, et al. (2001) Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytol 150: 285–293. [Google Scholar]
- 52. Ghannoum O, von Caemmerer S, Conroy JP (2002) The effect of drought on plant water use efficiency of nine NAD-ME and nine NADP-ME Australian C4 grasses. Funct Plant Biol 29: 1337–1348. [DOI] [PubMed] [Google Scholar]
- 53. Meinzer FC, Plaut Z, Saliendra NZ (1994) Carbon isotope discrimination, gas exchange, and growth of sugarcane cultivars under salinity. Plant Physiol 104: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poss JA, Grattan SR, Suarez DL, Grieve CM (2000) Stable carbon isotope discrimination: an indicator of cumulative salinity and boron stress in Eucalyptus camaldulensis . Tree Physiol 20: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 55. Brugnoli E, Lauteri M (1991) Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol 95: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoagland D, Arnon D (1950) The water culture method for growing plants without soil. California Agricultural Experimental Station 347: 1–32. [Google Scholar]
- 57. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-Δ Δ C T method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 58. Topfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res 15: 5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang XX, Liu SK, Takano T (2008) Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68: 131–143. [DOI] [PubMed] [Google Scholar]
- 60. Guo QF, Zhang J, Gao Q, Xing SC, Li F, et al. (2008) Drought tolerance through overexpression of monoubiquitin in transgenic tobacco. J. Plant Physiol 165: 1745–1755. [DOI] [PubMed] [Google Scholar]
- 61. Gay C, Collins J, Gebicki JM (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273: 149–155. [DOI] [PubMed] [Google Scholar]
- 62. Chandra A, Bhatt RK (2008) Carbon isotope discrimination function analysis and drought tolerance of stylo species grown under rain-fed environment. Acta Phys Plant 30: 63–69. [Google Scholar]
- 63. Brandalise M, Maia IG, Borecky J, Vercesi AE, Arruda P (2003) Overexpression of plant uncoupling mitochondrial protein in transgenic tobacco increases tolerance to oxidative stress. J Bioenerg Biomembr 35: 203–209. [DOI] [PubMed] [Google Scholar]
- 64. Arnon DI (1949) Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris . Plant Physiol 24: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]